 Found 5534 hits with Last Name = 'bhat' and Initial = 's'
Found 5534 hits with Last Name = 'bhat' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50527134
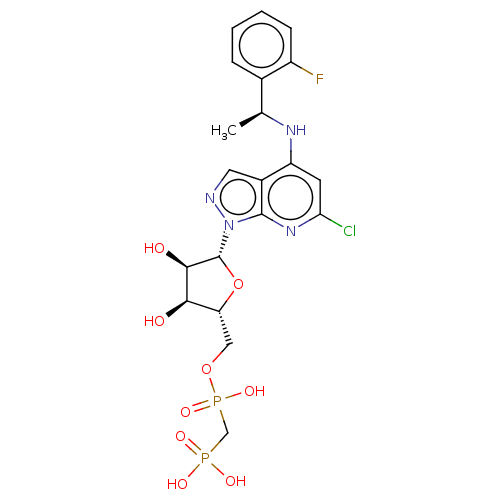
(CHEMBL4471306 | US20230295213, Compound a)Show SMILES C[C@H](Nc1cc(Cl)nc2n(ncc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O)c1ccccc1F |r| Show InChI InChI=1S/C20H24ClFN4O9P2/c1-10(11-4-2-3-5-13(11)22)24-14-6-16(21)25-19-12(14)7-23-26(19)20-18(28)17(27)15(35-20)8-34-37(32,33)9-36(29,30)31/h2-7,10,15,17-18,20,27-28H,8-9H2,1H3,(H,24,25)(H,32,33)(H2,29,30,31)/t10-,15+,17+,18+,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human CD73 |
J Med Chem 63: 2941-2957 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01611
BindingDB Entry DOI: 10.7270/Q2NS0ZBH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163573

((R)-3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-2...)Show SMILES Oc1c2CN([C@@H](c2nc2ccccc12)c1ccc2OCCc2c1)c1ncc(cn1)-c1ccccn1 Show InChI InChI=1S/C28H21N5O2/c34-27-20-5-1-2-7-23(20)32-25-21(27)16-33(26(25)18-8-9-24-17(13-18)10-12-35-24)28-30-14-19(15-31-28)22-6-3-4-11-29-22/h1-9,11,13-15,26H,10,12,16H2,(H,32,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50397944
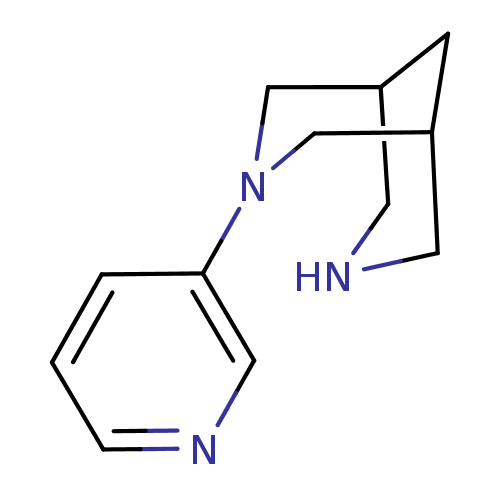
(CHEMBL2177512)Show InChI InChI=1S/C12H17N3/c1-2-12(7-13-3-1)15-8-10-4-11(9-15)6-14-5-10/h1-3,7,10-11,14H,4-6,8-9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398821
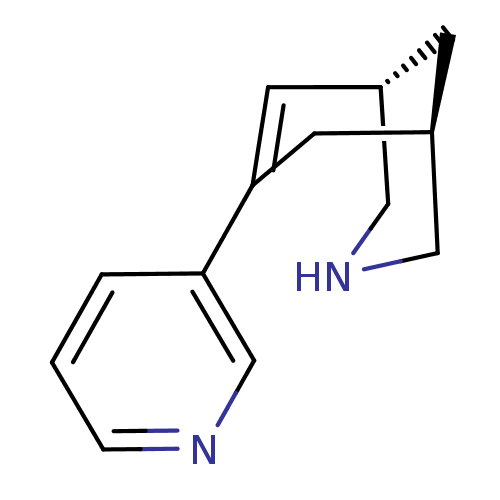
(CHEMBL2177537)Show InChI InChI=1S/C13H16N2/c1-2-12(9-14-3-1)13-5-10-4-11(6-13)8-15-7-10/h1-3,5,9-11,15H,4,6-8H2/t10-,11+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163576
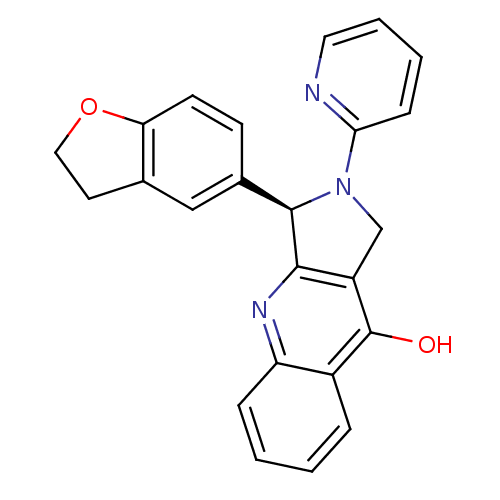
((R)-3-(2,3-Dihydro-benzofuran-5-yl)-2-pyridin-2-yl...)Show SMILES Oc1c2CN([C@@H](c2nc2ccccc12)c1ccc2OCCc2c1)c1ccccn1 Show InChI InChI=1S/C24H19N3O2/c28-24-17-5-1-2-6-19(17)26-22-18(24)14-27(21-7-3-4-11-25-21)23(22)16-8-9-20-15(13-16)10-12-29-20/h1-9,11,13,23H,10,12,14H2,(H,26,28)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122970

(3-Benzo[1,3]dioxol-5-yl-2-(5-pyridin-3-yl-furan-2-...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccc(o1)-c1cccnc1 Show InChI InChI=1S/C28H19N3O5/c32-27-18-5-1-2-6-20(18)30-25-19(27)14-31(26(25)16-7-8-22-24(12-16)35-15-34-22)28(33)23-10-9-21(36-23)17-4-3-11-29-13-17/h1-13,26H,14-15H2,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163581

(2-[2,3'']Bipyridinyl-6''-yl-3-(2,3-dihydro-benzofu...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCCc2c1)c1ccc(cn1)-c1ccccn1 Show InChI InChI=1S/C29H22N4O2/c34-29-21-5-1-2-7-24(21)32-27-22(29)17-33(28(27)19-8-10-25-18(15-19)12-14-35-25)26-11-9-20(16-31-26)23-6-3-4-13-30-23/h1-11,13,15-16,28H,12,14,17H2,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163570

(2-[5-(3-Benzyl-3H-imidazol-4-yl)-pyridin-2-yl]-3-(...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCCc2c1)c1ccc(cn1)-c1cncn1Cc1ccccc1 Show InChI InChI=1S/C34H27N5O2/c40-34-26-8-4-5-9-28(26)37-32-27(34)20-39(33(32)24-10-12-30-23(16-24)14-15-41-30)31-13-11-25(17-36-31)29-18-35-21-38(29)19-22-6-2-1-3-7-22/h1-13,16-18,21,33H,14-15,19-20H2,(H,37,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM312188
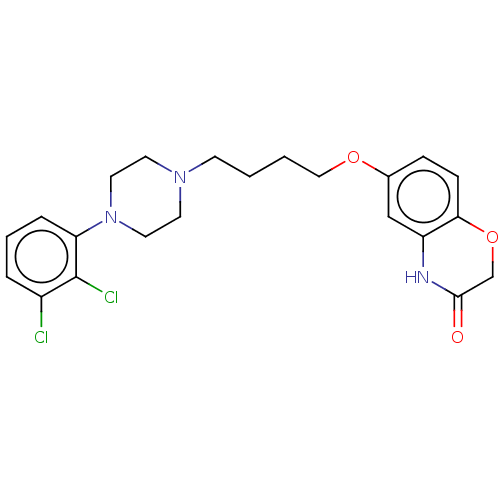
(6-(4-(4-(2,3-Dichlorophenyl)piperazin-1-yl)butoxy)...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4OCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C22H25Cl2N3O3/c23-17-4-3-5-19(22(17)24)27-11-9-26(10-12-27)8-1-2-13-29-16-6-7-20-18(14-16)25-21(28)15-30-20/h3-7,14H,1-2,8-13,15H2,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Reviva Pharmaceuticals, Inc.
US Patent
| Assay Description
Serotonin, 5HT1A: Materials and Methods:Receptor Source: Human recombinant 5-HT1A expressed mammalian cellsRadioligand: [3H]-8-OH-DPAT (221 Ci/mmol)C... |
US Patent US10441590 (2019)
BindingDB Entry DOI: 10.7270/Q2959KXP |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163577

(3-Benzofuran-5-yl-2-(5-pyridin-2-yl-pyrimidin-2-yl...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2occc2c1)c1ncc(cn1)-c1ccccn1 Show InChI InChI=1S/C28H19N5O2/c34-27-20-5-1-2-7-23(20)32-25-21(27)16-33(26(25)18-8-9-24-17(13-18)10-12-35-24)28-30-14-19(15-31-28)22-6-3-4-11-29-22/h1-15,26H,16H2,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398849
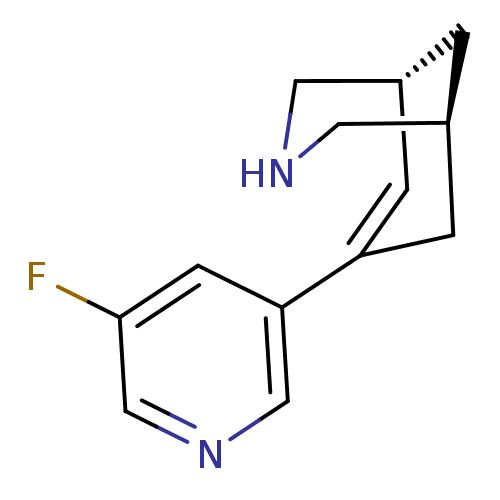
(CHEMBL2177553)Show SMILES Fc1cncc(c1)C1=C[C@@H]2CNC[C@@H](C2)C1 |r,t:8| Show InChI InChI=1S/C13H15FN2/c14-13-4-12(7-16-8-13)11-2-9-1-10(3-11)6-15-5-9/h2,4,7-10,15H,1,3,5-6H2/t9-,10+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398850
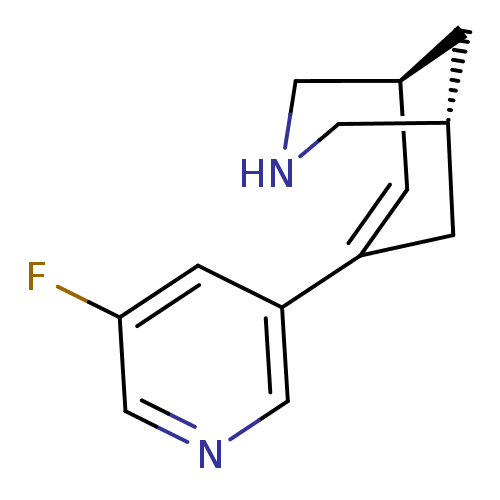
(CHEMBL2177552)Show SMILES Fc1cncc(c1)C1=C[C@H]2CNC[C@H](C2)C1 |r,t:8| Show InChI InChI=1S/C13H15FN2/c14-13-4-12(7-16-8-13)11-2-9-1-10(3-11)6-15-5-9/h2,4,7-10,15H,1,3,5-6H2/t9-,10+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398820

(CHEMBL2177538)Show InChI InChI=1S/C13H16N2/c1-2-12(9-14-3-1)13-5-10-4-11(6-13)8-15-7-10/h1-3,5,9-11,15H,4,6-8H2/t10-,11+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122969
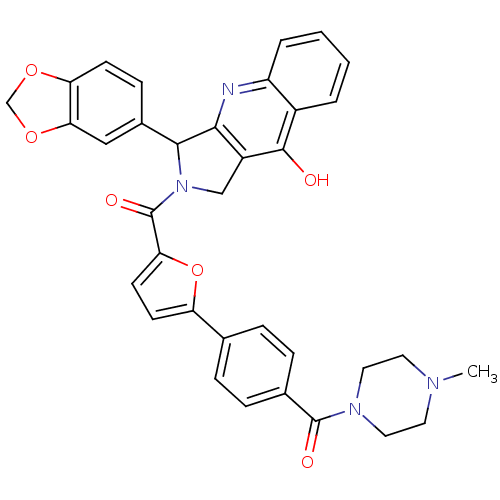
(3-Benzo[1,3]dioxol-5-yl-2-{5-[4-(4-methyl-piperazi...)Show SMILES CN1CCN(CC1)C(=O)c1ccc(cc1)-c1ccc(o1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C35H30N4O6/c1-37-14-16-38(17-15-37)34(41)22-8-6-21(7-9-22)27-12-13-29(45-27)35(42)39-19-25-31(36-26-5-3-2-4-24(26)33(25)40)32(39)23-10-11-28-30(18-23)44-20-43-28/h2-13,18,32H,14-17,19-20H2,1H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398820

(CHEMBL2177538)Show InChI InChI=1S/C13H16N2/c1-2-12(9-14-3-1)13-5-10-4-11(6-13)8-15-7-10/h1-3,5,9-11,15H,4,6-8H2/t10-,11+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50401333
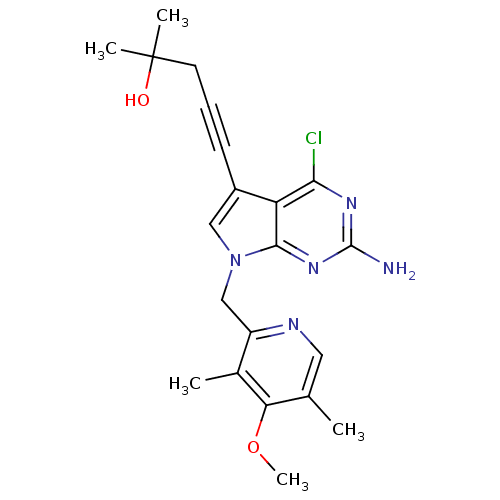
(CHEMBL1230584)Show SMILES COc1c(C)cnc(Cn2cc(C#CCC(C)(C)O)c3c(Cl)nc(N)nc23)c1C Show InChI InChI=1S/C21H24ClN5O2/c1-12-9-24-15(13(2)17(12)29-5)11-27-10-14(7-6-8-21(3,4)28)16-18(22)25-20(23)26-19(16)27/h9-10,28H,8,11H2,1-5H3,(H2,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Binding affinity at recombinant Hsp90alpha incubated for 16 hrs by fluorescence polarization competition assay |
J Med Chem 55: 7786-95 (2012)
Article DOI: 10.1021/jm300810x
BindingDB Entry DOI: 10.7270/Q2V125Z3 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50397944

(CHEMBL2177512)Show InChI InChI=1S/C12H17N3/c1-2-12(7-13-3-1)15-8-10-4-11(9-15)6-14-5-10/h1-3,7,10-11,14H,4-6,8-9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to alpha4beta2 nAChR |
J Med Chem 55: 9181-94 (2012)
Article DOI: 10.1021/jm3006542
BindingDB Entry DOI: 10.7270/Q2W0972W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398832
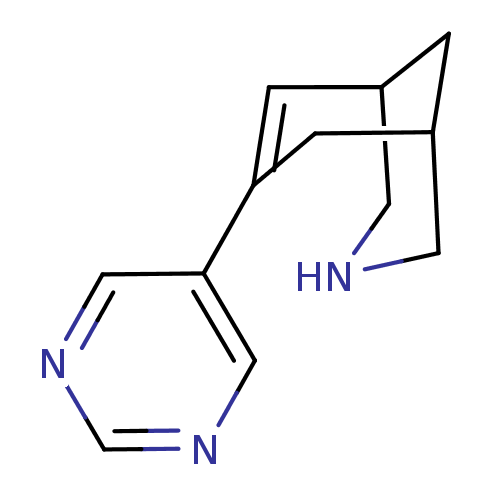
(CHEMBL2177518)Show SMILES C1NCC2CC1CC(=C2)c1cncnc1 |c:8,TLB:9:7:0.1.2:4| Show InChI InChI=1S/C12H15N3/c1-9-2-11(3-10(1)5-13-4-9)12-6-14-8-15-7-12/h2,6-10,13H,1,3-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50370143
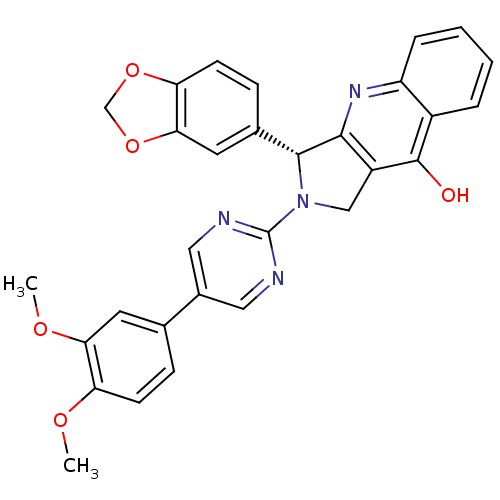
(CHEMBL1744059)Show SMILES COc1ccc(cc1OC)-c1cnc(nc1)N1Cc2c(nc3ccccc3c2O)[C@H]1c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C30H24N4O5/c1-36-23-9-7-17(11-25(23)37-2)19-13-31-30(32-14-19)34-15-21-27(33-22-6-4-3-5-20(22)29(21)35)28(34)18-8-10-24-26(12-18)39-16-38-24/h3-14,28H,15-16H2,1-2H3,(H,33,35)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118249
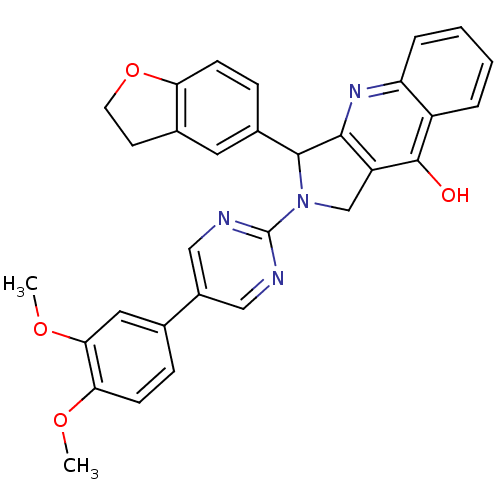
(3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(3,4-dimethox...)Show SMILES COc1ccc(cc1OC)-c1cnc(nc1)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCCc2c1 Show InChI InChI=1S/C31H26N4O4/c1-37-26-10-7-18(14-27(26)38-2)21-15-32-31(33-16-21)35-17-23-28(34-24-6-4-3-5-22(24)30(23)36)29(35)20-8-9-25-19(13-20)11-12-39-25/h3-10,13-16,29H,11-12,17H2,1-2H3,(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138930
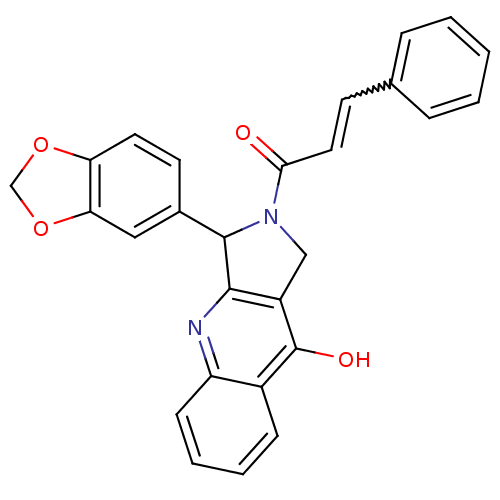
(3-Benzo[1,3]dioxol-5-yl-2-(3-phenyl-acryloyl)-1,2,...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)C=Cc1ccccc1 |w:26.31| Show InChI InChI=1S/C27H20N2O4/c30-24(13-10-17-6-2-1-3-7-17)29-15-20-25(28-21-9-5-4-8-19(21)27(20)31)26(29)18-11-12-22-23(14-18)33-16-32-22/h1-14,26H,15-16H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163578

(3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-4-yl-...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCCc2c1)c1ncc(cn1)-c1ccncc1 Show InChI InChI=1S/C28H21N5O2/c34-27-21-3-1-2-4-23(21)32-25-22(27)16-33(26(25)19-5-6-24-18(13-19)9-12-35-24)28-30-14-20(15-31-28)17-7-10-29-11-8-17/h1-8,10-11,13-15,26H,9,12,16H2,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138939
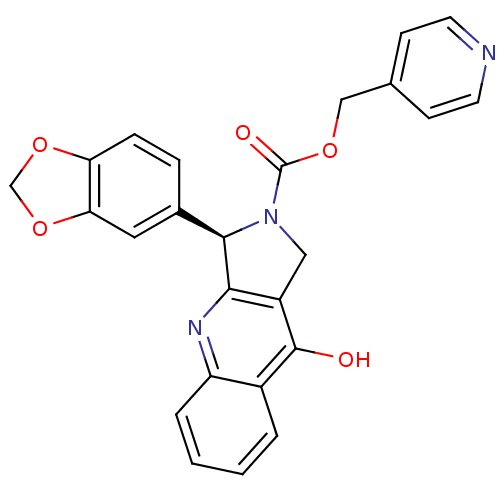
((R)-3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahyd...)Show SMILES Oc1c2CN([C@@H](c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)OCc1ccncc1 Show InChI InChI=1S/C25H19N3O5/c29-24-17-3-1-2-4-19(17)27-22-18(24)12-28(25(30)31-13-15-7-9-26-10-8-15)23(22)16-5-6-20-21(11-16)33-14-32-20/h1-11,23H,12-14H2,(H,27,29)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118248
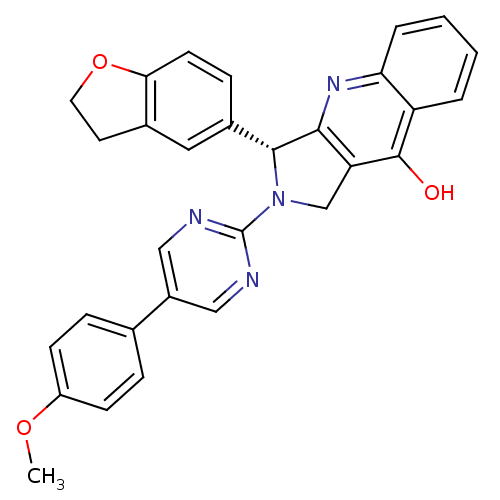
(3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(4-methoxy-ph...)Show SMILES COc1ccc(cc1)-c1cnc(nc1)N1Cc2c(nc3ccccc3c2O)[C@H]1c1ccc2OCCc2c1 Show InChI InChI=1S/C30H24N4O3/c1-36-22-9-6-18(7-10-22)21-15-31-30(32-16-21)34-17-24-27(33-25-5-3-2-4-23(25)29(24)35)28(34)20-8-11-26-19(14-20)12-13-37-26/h2-11,14-16,28H,12-13,17H2,1H3,(H,33,35)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163574
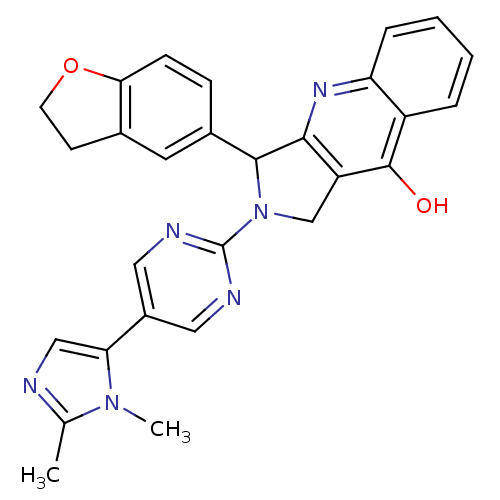
(3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(2,3-dimethyl...)Show SMILES Cc1ncc(-c2cnc(nc2)N2Cc3c(nc4ccccc4c3O)C2c2ccc3OCCc3c2)n1C Show InChI InChI=1S/C28H24N6O2/c1-16-29-14-23(33(16)2)19-12-30-28(31-13-19)34-15-21-25(32-22-6-4-3-5-20(22)27(21)35)26(34)18-7-8-24-17(11-18)9-10-36-24/h3-8,11-14,26H,9-10,15H2,1-2H3,(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163571
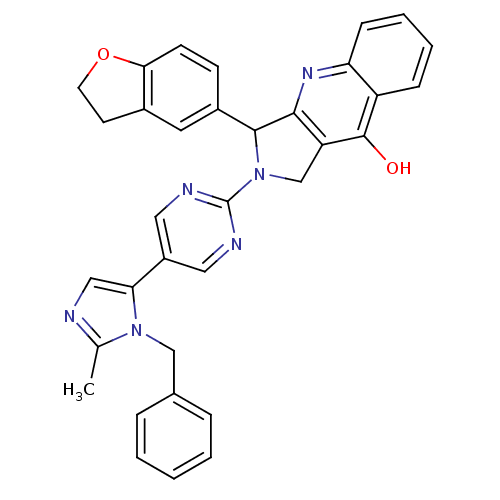
(2-[5-(3-Benzyl-2-methyl-3H-imidazol-4-yl)-pyrimidi...)Show SMILES Cc1ncc(-c2cnc(nc2)N2Cc3c(nc4ccccc4c3O)C2c2ccc3OCCc3c2)n1Cc1ccccc1 Show InChI InChI=1S/C34H28N6O2/c1-21-35-18-29(39(21)19-22-7-3-2-4-8-22)25-16-36-34(37-17-25)40-20-27-31(38-28-10-6-5-9-26(28)33(27)41)32(40)24-11-12-30-23(15-24)13-14-42-30/h2-12,15-18,32H,13-14,19-20H2,1H3,(H,38,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122990
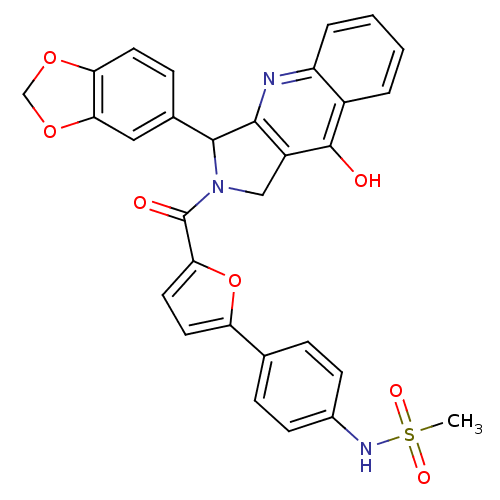
(CHEMBL342159 | N-{4-[5-(3-Benzo[1,3]dioxol-5-yl-9-...)Show SMILES CS(=O)(=O)Nc1ccc(cc1)-c1ccc(o1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C30H23N3O7S/c1-41(36,37)32-19-9-6-17(7-10-19)23-12-13-25(40-23)30(35)33-15-21-27(31-22-5-3-2-4-20(22)29(21)34)28(33)18-8-11-24-26(14-18)39-16-38-24/h2-14,28,32H,15-16H2,1H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398815
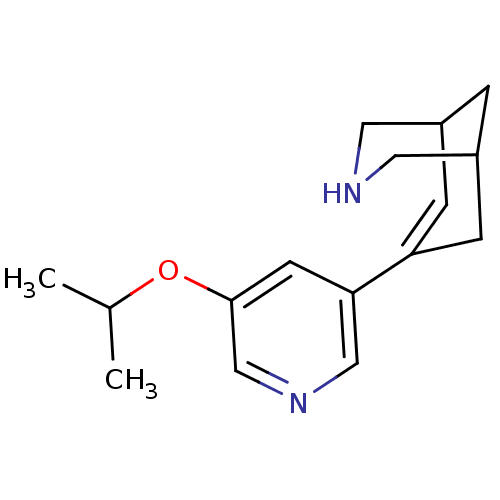
(CHEMBL2177543)Show SMILES CC(C)Oc1cncc(c1)C1=CC2CNCC(C2)C1 |t:11,TLB:8:10:15.14.13:17| Show InChI InChI=1S/C16H22N2O/c1-11(2)19-16-6-15(9-18-10-16)14-4-12-3-13(5-14)8-17-7-12/h4,6,9-13,17H,3,5,7-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50135597
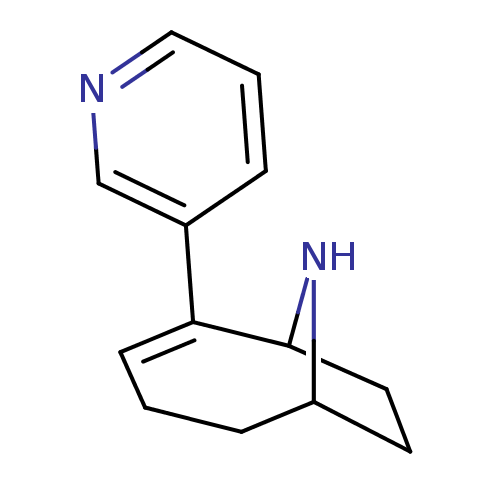
(2-Pyridin-3-yl-9-aza-bicyclo[4.2.1]non-2-ene | CHE...)Show InChI InChI=1S/C13H16N2/c1-4-11-6-7-13(15-11)12(5-1)10-3-2-8-14-9-10/h2-3,5,8-9,11,13,15H,1,4,6-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to alpha4beta2 nAChR |
J Med Chem 55: 9181-94 (2012)
Article DOI: 10.1021/jm3006542
BindingDB Entry DOI: 10.7270/Q2W0972W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138929

(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahydro-p...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)OCc1ccncc1 Show InChI InChI=1S/C25H19N3O5/c29-24-17-3-1-2-4-19(17)27-22-18(24)12-28(25(30)31-13-15-7-9-26-10-8-15)23(22)16-5-6-20-21(11-16)33-14-32-20/h1-11,23H,12-14H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163579
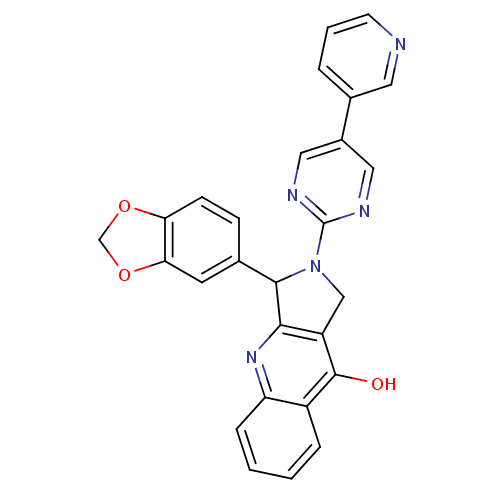
(3-Benzo[1,3]dioxol-5-yl-2-(5-pyridin-3-yl-pyrimidi...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)c1ncc(cn1)-c1cccnc1 Show InChI InChI=1S/C27H19N5O3/c33-26-19-5-1-2-6-21(19)31-24-20(26)14-32(25(24)16-7-8-22-23(10-16)35-15-34-22)27-29-12-18(13-30-27)17-4-3-9-28-11-17/h1-13,25H,14-15H2,(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118250
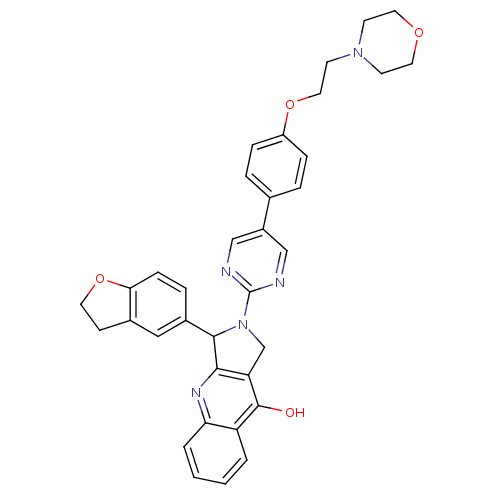
(3-(2,3-Dihydro-benzofuran-5-yl)-2-{5-[4-(2-morphol...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCCc2c1)c1ncc(cn1)-c1ccc(OCCN2CCOCC2)cc1 Show InChI InChI=1S/C35H33N5O4/c41-34-28-3-1-2-4-30(28)38-32-29(34)22-40(33(32)25-7-10-31-24(19-25)11-15-44-31)35-36-20-26(21-37-35)23-5-8-27(9-6-23)43-18-14-39-12-16-42-17-13-39/h1-10,19-21,33H,11-18,22H2,(H,38,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398817
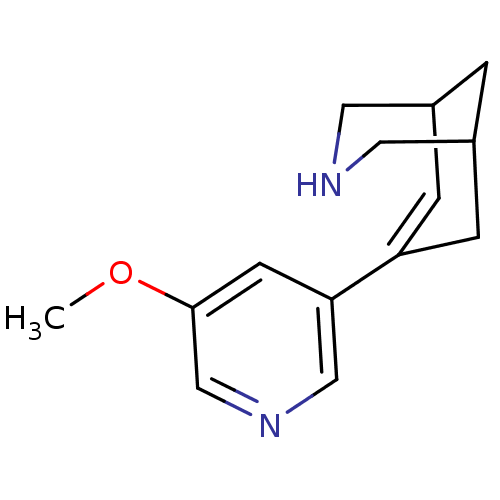
(CHEMBL2177541)Show SMILES COc1cncc(c1)C1=CC2CNCC(C2)C1 |t:9,TLB:6:8:13.12.11:15| Show InChI InChI=1S/C14H18N2O/c1-17-14-5-13(8-16-9-14)12-3-10-2-11(4-12)7-15-6-10/h3,5,8-11,15H,2,4,6-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM312156

(6-(4-(4-(2-Methoxyphenyl)piperazin-1-yl)butoxy)-2H...)Show SMILES COc1ccccc1N1CCN(CCCCOc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C23H29N3O4/c1-28-22-7-3-2-6-20(22)26-13-11-25(12-14-26)10-4-5-15-29-18-8-9-21-19(16-18)24-23(27)17-30-21/h2-3,6-9,16H,4-5,10-15,17H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Reviva Pharmaceuticals, Inc.
US Patent
| Assay Description
Dopamine, D2s: Radioligand: [3H]Spiperone (20-60 Ci/mmol) or [3H]-7-hydroxy DPAT, 1.0 nMControl Compound: Haloperidol or ChlorpromazineIncubation Con... |
US Patent US10441590 (2019)
BindingDB Entry DOI: 10.7270/Q2959KXP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398848
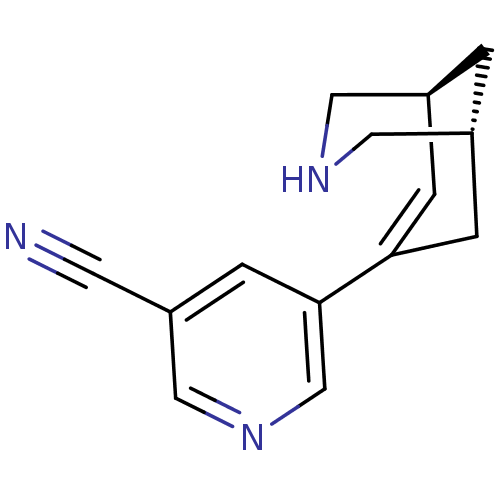
(CHEMBL2178156)Show SMILES N#Cc1cncc(c1)C1=C[C@H]2CNC[C@H](C2)C1 |r,t:9| Show InChI InChI=1S/C14H15N3/c15-5-12-4-14(9-17-8-12)13-2-10-1-11(3-13)7-16-6-10/h2,4,8-11,16H,1,3,6-7H2/t10-,11+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398849

(CHEMBL2177553)Show SMILES Fc1cncc(c1)C1=C[C@@H]2CNC[C@@H](C2)C1 |r,t:8| Show InChI InChI=1S/C13H15FN2/c14-13-4-12(7-16-8-13)11-2-9-1-10(3-11)6-15-5-9/h2,4,7-10,15H,1,3,5-6H2/t9-,10+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398847
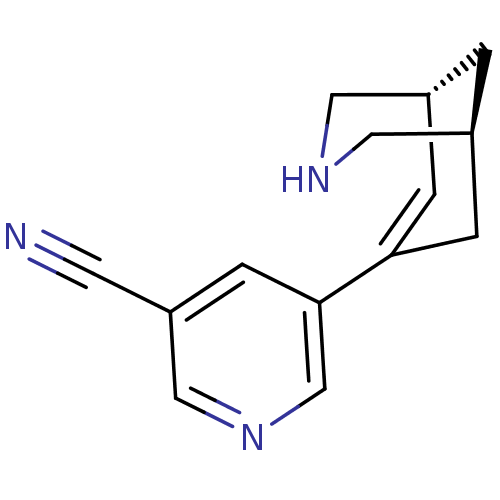
(CHEMBL2177535)Show SMILES N#Cc1cncc(c1)C1=C[C@@H]2CNC[C@@H](C2)C1 |r,t:9| Show InChI InChI=1S/C14H15N3/c15-5-12-4-14(9-17-8-12)13-2-10-1-11(3-13)7-16-6-10/h2,4,8-11,16H,1,3,6-7H2/t10-,11+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398841
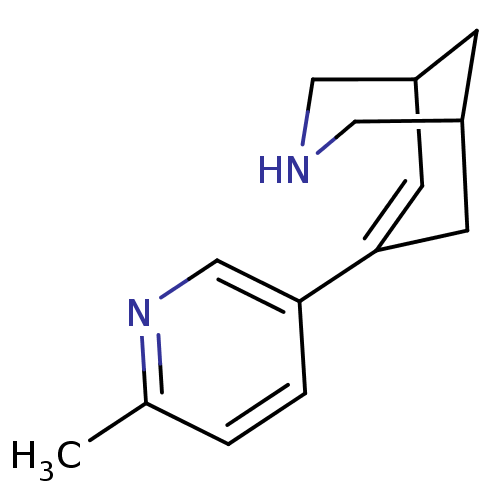
(CHEMBL2177528)Show SMILES Cc1ccc(cn1)C1=CC2CNCC(C2)C1 |t:8,TLB:4:7:12.11.10:14| Show InChI InChI=1S/C14H18N2/c1-10-2-3-13(9-16-10)14-5-11-4-12(6-14)8-15-7-11/h2-3,5,9,11-12,15H,4,6-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122974
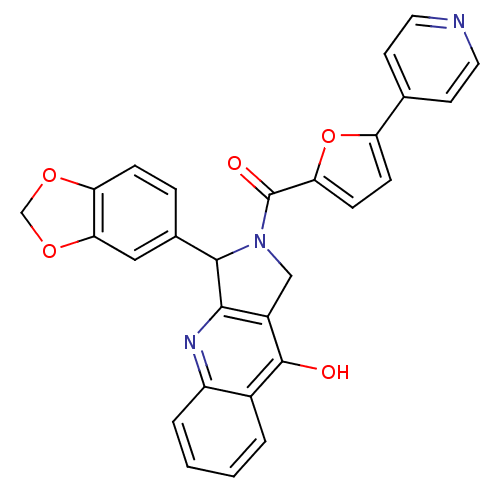
(3-Benzo[1,3]dioxol-5-yl-2-(5-pyridin-4-yl-furan-2-...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccc(o1)-c1ccncc1 Show InChI InChI=1S/C28H19N3O5/c32-27-18-3-1-2-4-20(18)30-25-19(27)14-31(26(25)17-5-6-22-24(13-17)35-15-34-22)28(33)23-8-7-21(36-23)16-9-11-29-12-10-16/h1-13,26H,14-15H2,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118252
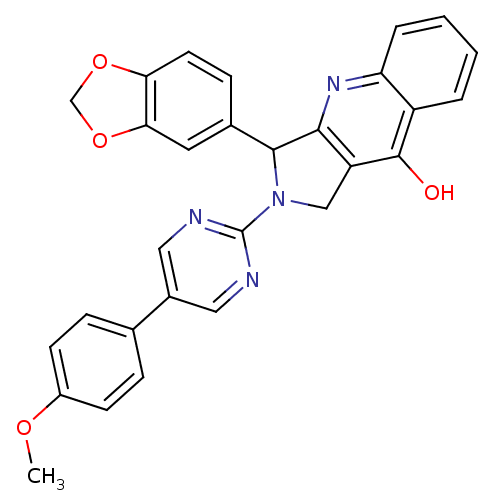
(3-Benzo[1,3]dioxol-5-yl-2-[5-(4-methoxy-phenyl)-py...)Show SMILES COc1ccc(cc1)-c1cnc(nc1)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C29H22N4O4/c1-35-20-9-6-17(7-10-20)19-13-30-29(31-14-19)33-15-22-26(32-23-5-3-2-4-21(23)28(22)34)27(33)18-8-11-24-25(12-18)37-16-36-24/h2-14,27H,15-16H2,1H3,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50527135
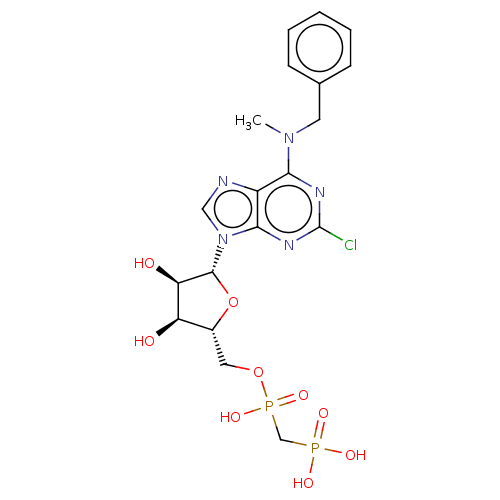
(CHEMBL4452072)Show SMILES CN(Cc1ccccc1)c1nc(Cl)nc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H24ClN5O9P2/c1-24(7-11-5-3-2-4-6-11)16-13-17(23-19(20)22-16)25(9-21-13)18-15(27)14(26)12(34-18)8-33-36(31,32)10-35(28,29)30/h2-6,9,12,14-15,18,26-27H,7-8,10H2,1H3,(H,31,32)(H2,28,29,30)/t12-,14-,15-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.318 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human CD73 |
J Med Chem 63: 2941-2957 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01611
BindingDB Entry DOI: 10.7270/Q2NS0ZBH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163572
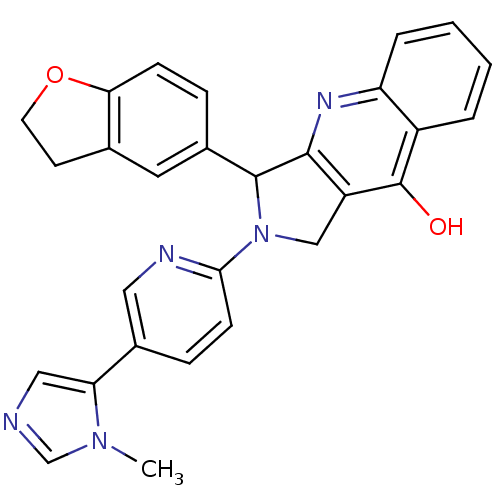
(3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(3-methyl-3H-...)Show SMILES Cn1cncc1-c1ccc(nc1)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCCc2c1 Show InChI InChI=1S/C28H23N5O2/c1-32-16-29-14-23(32)19-7-9-25(30-13-19)33-15-21-26(31-22-5-3-2-4-20(22)28(21)34)27(33)18-6-8-24-17(12-18)10-11-35-24/h2-9,12-14,16,27H,10-11,15H2,1H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138936
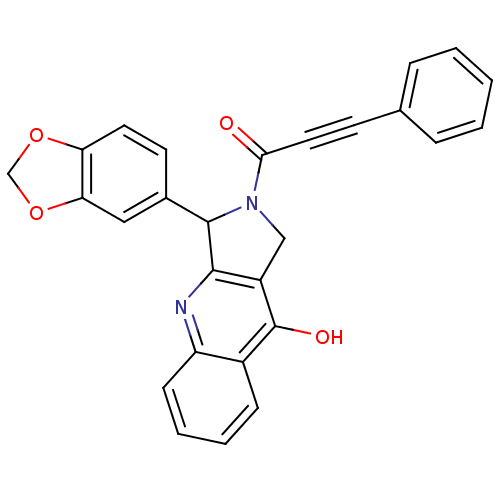
(3-Benzo[1,3]dioxol-5-yl-2-(3-phenyl-propynoyl)-1,2...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)C#Cc1ccccc1 Show InChI InChI=1S/C27H18N2O4/c30-24(13-10-17-6-2-1-3-7-17)29-15-20-25(28-21-9-5-4-8-19(21)27(20)31)26(29)18-11-12-22-23(14-18)33-16-32-22/h1-9,11-12,14,26H,15-16H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122964

(3-Benzo[1,3]dioxol-5-yl-2-(6-hydroxy-benzofuran-2-...)Show SMILES Oc1ccc2cc(oc2c1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C27H18N2O6/c30-16-7-5-14-9-23(35-21(14)11-16)27(32)29-12-18-24(28-19-4-2-1-3-17(19)26(18)31)25(29)15-6-8-20-22(10-15)34-13-33-20/h1-11,25,30H,12-13H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
Appetite-regulating hormone
(Homo sapiens (Human)) | BDBM50359261
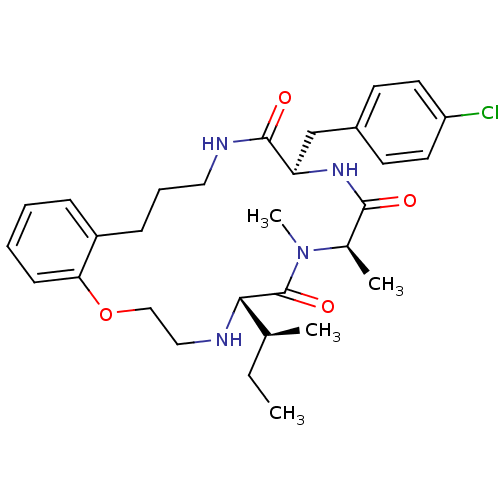
(CHEMBL1923617)Show SMILES CC[C@H](C)[C@@H]1NCCOc2ccccc2CCCNC(=O)[C@@H](Cc2ccc(Cl)cc2)NC(=O)[C@@H](C)N(C)C1=O |r| Show InChI InChI=1S/C30H41ClN4O4/c1-5-20(2)27-30(38)35(4)21(3)28(36)34-25(19-22-12-14-24(31)15-13-22)29(37)33-16-8-10-23-9-6-7-11-26(23)39-18-17-32-27/h6-7,9,11-15,20-21,25,27,32H,5,8,10,16-19H2,1-4H3,(H,33,37)(H,34,36)/t20-,21+,25+,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tranzyme Pharma Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I-His9]-ghrelin from human GRLN receptor by radioligand binding assay |
J Med Chem 54: 8305-20 (2011)
Article DOI: 10.1021/jm2007062
BindingDB Entry DOI: 10.7270/Q2930TK0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163568

(3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-3-yl-...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCCc2c1)c1ncc(cn1)-c1cccnc1 Show InChI InChI=1S/C28H21N5O2/c34-27-21-5-1-2-6-23(21)32-25-22(27)16-33(26(25)18-7-8-24-17(12-18)9-11-35-24)28-30-14-20(15-31-28)19-4-3-10-29-13-19/h1-8,10,12-15,26H,9,11,16H2,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
Appetite-regulating hormone
(Homo sapiens (Human)) | BDBM50359239
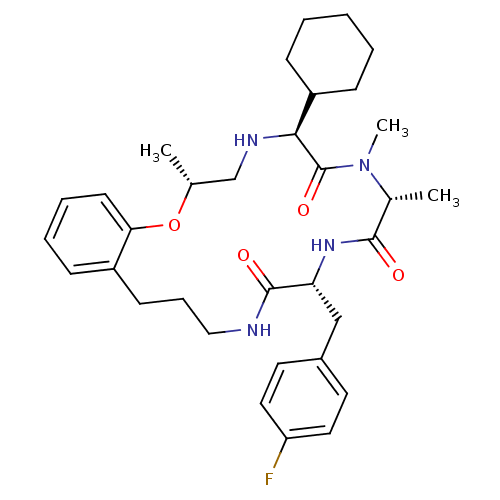
(CHEMBL1923629)Show SMILES C[C@@H]1CN[C@@H](C2CCCCC2)C(=O)N(C)[C@H](C)C(=O)N[C@H](Cc2ccc(F)cc2)C(=O)NCCCc2ccccc2O1 |r| Show InChI InChI=1S/C33H45FN4O4/c1-22-21-36-30(26-11-5-4-6-12-26)33(41)38(3)23(2)31(39)37-28(20-24-15-17-27(34)18-16-24)32(40)35-19-9-13-25-10-7-8-14-29(25)42-22/h7-8,10,14-18,22-23,26,28,30,36H,4-6,9,11-13,19-21H2,1-3H3,(H,35,40)(H,37,39)/t22-,23-,28-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tranzyme Pharma Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I-His9]-ghrelin from human GRLN receptor by radioligand binding assay |
J Med Chem 54: 8305-20 (2011)
Article DOI: 10.1021/jm2007062
BindingDB Entry DOI: 10.7270/Q2930TK0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118255
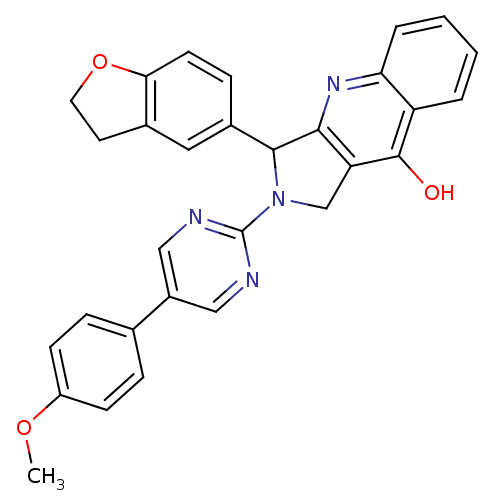
(3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(4-methoxy-ph...)Show SMILES COc1ccc(cc1)-c1cnc(nc1)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCCc2c1 Show InChI InChI=1S/C30H24N4O3/c1-36-22-9-6-18(7-10-22)21-15-31-30(32-16-21)34-17-24-27(33-25-5-3-2-4-23(25)29(24)35)28(34)20-8-11-26-19(14-20)12-13-37-26/h2-11,14-16,28H,12-13,17H2,1H3,(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM529895

(US11203594, Example 5)Show SMILES CC(=O)c1c(C)c2cnc(N[C@@H]3CCOC[C@H]3O)cc2n(C2CCCC2)c1=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The purpose CDK4/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitors by us... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34GC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398852
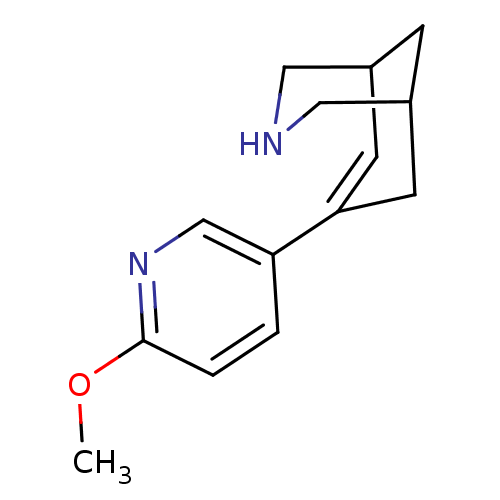
(CHEMBL2177526)Show SMILES COc1ccc(cn1)C1=CC2CNCC(C2)C1 |t:9,TLB:5:8:13.12.11:15| Show InChI InChI=1S/C14H18N2O/c1-17-14-3-2-12(9-16-14)13-5-10-4-11(6-13)8-15-7-10/h2-3,5,9-11,15H,4,6-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data