 Found 76 hits with Last Name = 'biagini' and Initial = 'ga'
Found 76 hits with Last Name = 'biagini' and Initial = 'ga' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50113463
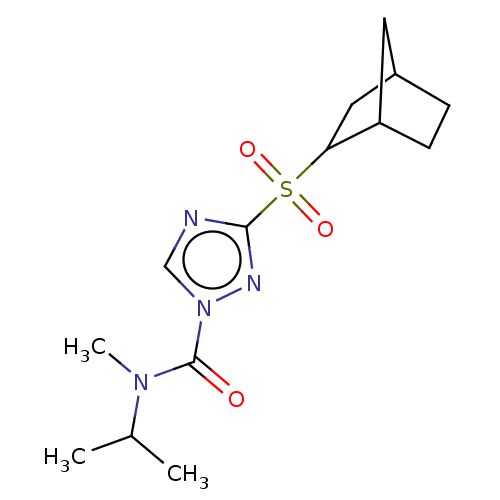
(CHEMBL537586)Show SMILES CC(C)N(C)C(=O)n1cnc(n1)S(=O)(=O)C1CC2CCC1C2 Show InChI InChI=1S/C14H22N4O3S/c1-9(2)17(3)14(19)18-8-15-13(16-18)22(20,21)12-7-10-4-5-11(12)6-10/h8-12H,4-7H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
J Med Chem 58: 6448-55 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00434
BindingDB Entry DOI: 10.7270/Q2N018BP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM151585

(US11739089, Compound Ketoconazole | US8987315, Ket...)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OCC2COC(Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes assessed as testosterone 6beta-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysis |
Bioorg Med Chem 26: 2996-3005 (2018)
Article DOI: 10.1016/j.bmc.2018.05.006
BindingDB Entry DOI: 10.7270/Q2K076SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466327
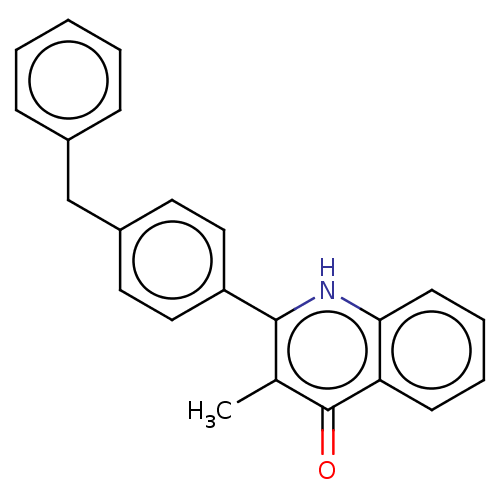
(US10799494, Compound CK-2-88)Show InChI InChI=1S/C23H19NO/c1-16-22(24-21-10-6-5-9-20(21)23(16)25)19-13-11-18(12-14-19)15-17-7-3-2-4-8-17/h2-14H,15H2,1H3,(H,24,25) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM151585

(US11739089, Compound Ketoconazole | US8987315, Ket...)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OCC2COC(Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes assessed as midazolam 1'-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysis |
Bioorg Med Chem 26: 2996-3005 (2018)
Article DOI: 10.1016/j.bmc.2018.05.006
BindingDB Entry DOI: 10.7270/Q2K076SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes assessed as dextromethorphan O-demethylation after 4 to 40 mins in presence of NADPH by LCMS analysis |
Bioorg Med Chem 26: 2996-3005 (2018)
Article DOI: 10.1016/j.bmc.2018.05.006
BindingDB Entry DOI: 10.7270/Q2K076SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM79214
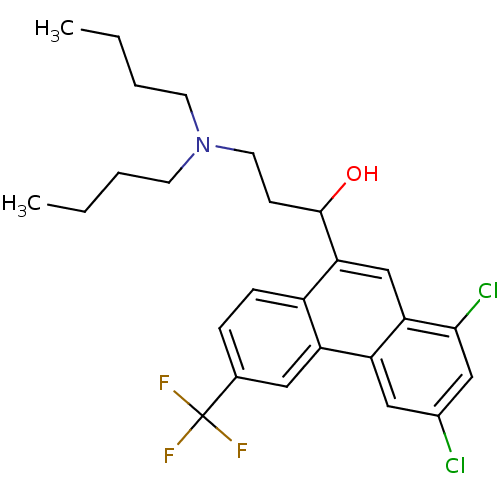
(1-[1,3-bis(chloranyl)-6-(trifluoromethyl)phenanthr...)Show SMILES CCCCN(CCCC)CCC(O)c1cc2c(Cl)cc(Cl)cc2c2cc(ccc12)C(F)(F)F Show InChI InChI=1S/C26H30Cl2F3NO/c1-3-5-10-32(11-6-4-2)12-9-25(33)23-16-22-21(14-18(27)15-24(22)28)20-13-17(26(29,30)31)7-8-19(20)23/h7-8,13-16,25,33H,3-6,9-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human cloned ERG |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466330

(US10799494, Compound PG-203)Show SMILES Cc1[nH]c2ccccc2c(=O)c1-c1ccc(Oc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C23H16F3NO3/c1-14-21(22(28)19-4-2-3-5-20(19)27-14)15-6-8-16(9-7-15)29-17-10-12-18(13-11-17)30-23(24,25)26/h2-13H,1H3,(H,27,28) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466340
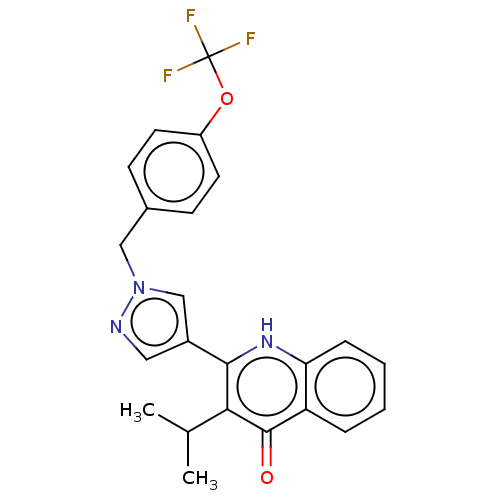
(US10799494, Example WDH-2G-6)Show SMILES CC(C)c1c([nH]c2ccccc2c1=O)-c1cnn(Cc2ccc(OC(F)(F)F)cc2)c1 Show InChI InChI=1S/C23H20F3N3O2/c1-14(2)20-21(28-19-6-4-3-5-18(19)22(20)30)16-11-27-29(13-16)12-15-7-9-17(10-8-15)31-23(24,25)26/h3-11,13-14H,12H2,1-2H3,(H,28,30) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466333
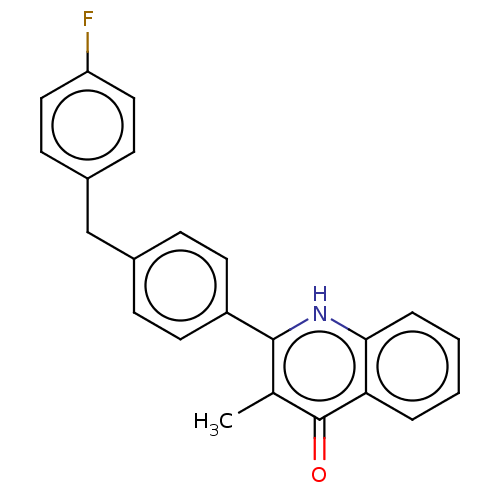
(US10799494, Compound LT-9)Show SMILES Cc1c([nH]c2ccccc2c1=O)-c1ccc(Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C23H18FNO/c1-15-22(25-21-5-3-2-4-20(21)23(15)26)18-10-6-16(7-11-18)14-17-8-12-19(24)13-9-17/h2-13H,14H2,1H3,(H,25,26) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466310
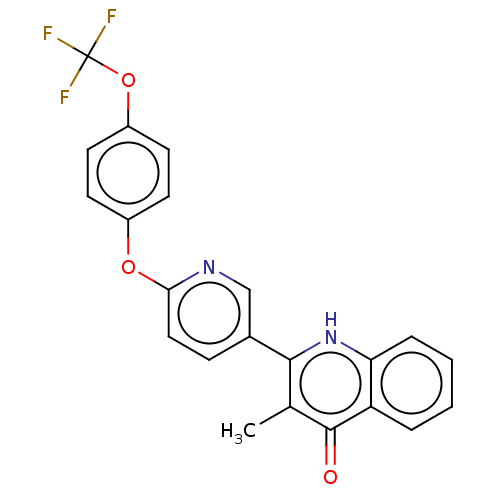
(US10799494, Compound CK-3-22)Show SMILES Cc1c([nH]c2ccccc2c1=O)-c1ccc(Oc2ccc(OC(F)(F)F)cc2)nc1 Show InChI InChI=1S/C22H15F3N2O3/c1-13-20(27-18-5-3-2-4-17(18)21(13)28)14-6-11-19(26-12-14)29-15-7-9-16(10-8-15)30-22(23,24)25/h2-12H,1H3,(H,27,28) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466334
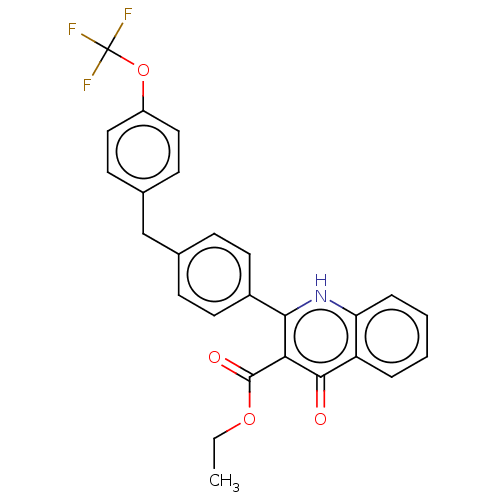
(US10799494, Compound GN-1710)Show SMILES CCOC(=O)c1c([nH]c2ccccc2c1=O)-c1ccc(Cc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C26H20F3NO4/c1-2-33-25(32)22-23(30-21-6-4-3-5-20(21)24(22)31)18-11-7-16(8-12-18)15-17-9-13-19(14-10-17)34-26(27,28)29/h3-14H,2,15H2,1H3,(H,30,31) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466326
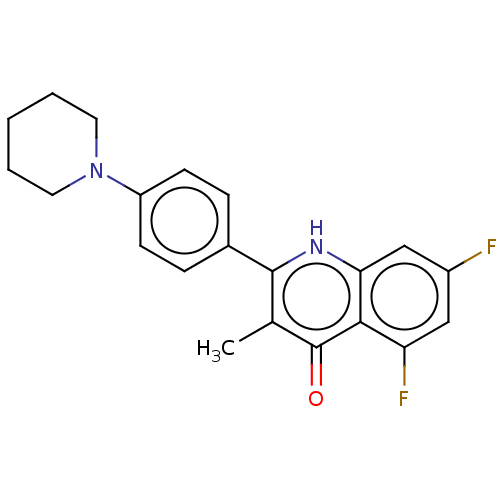
(US10799494, Compound MTD-403)Show SMILES Cc1c([nH]c2cc(F)cc(F)c2c1=O)-c1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C21H20F2N2O/c1-13-20(24-18-12-15(22)11-17(23)19(18)21(13)26)14-5-7-16(8-6-14)25-9-3-2-4-10-25/h5-8,11-12H,2-4,9-10H2,1H3,(H,24,26) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466336
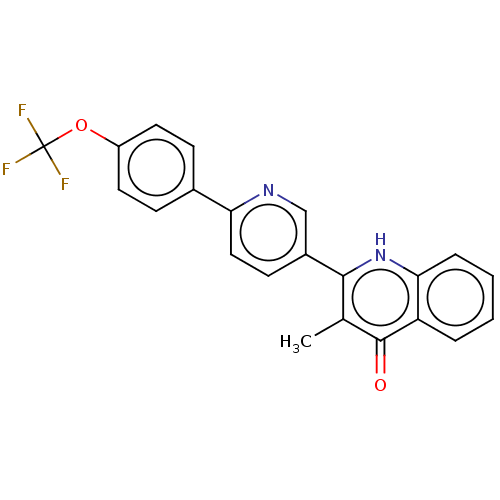
(US10799494, Compound SL-2-25)Show SMILES Cc1c([nH]c2ccccc2c1=O)-c1ccc(nc1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C22H15F3N2O2/c1-13-20(27-19-5-3-2-4-17(19)21(13)28)15-8-11-18(26-12-15)14-6-9-16(10-7-14)29-22(23,24)25/h2-12H,1H3,(H,27,28) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466332
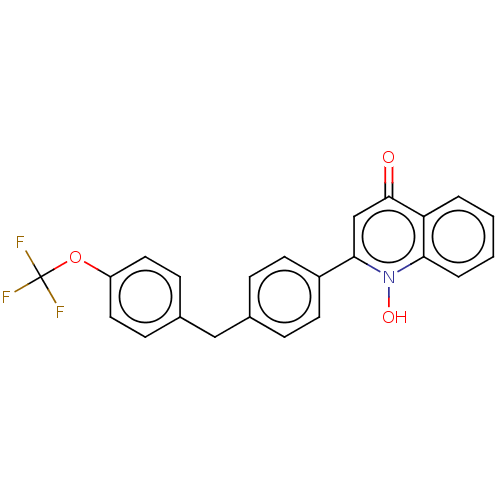
(US10799494, Compound RKA-73)Show SMILES On1c(cc(=O)c2ccccc12)-c1ccc(Cc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C23H16F3NO3/c24-23(25,26)30-18-11-7-16(8-12-18)13-15-5-9-17(10-6-15)21-14-22(28)19-3-1-2-4-20(19)27(21)29/h1-12,14,29H,13H2 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466341

(US10799494, Example WDH-2R-4)Show SMILES CC(C)c1c([nH]c2ccccc2c1=O)-c1cnn(CCc2ccc(OC(F)(F)F)cc2)c1 Show InChI InChI=1S/C24H22F3N3O2/c1-15(2)21-22(29-20-6-4-3-5-19(20)23(21)31)17-13-28-30(14-17)12-11-16-7-9-18(10-8-16)32-24(25,26)27/h3-10,13-15H,11-12H2,1-2H3,(H,29,31) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466316

(US10799494, Compound RKA-307)Show InChI InChI=1S/C21H22N2O/c1-15-20(22-19-8-4-3-7-18(19)21(15)24)16-9-11-17(12-10-16)23-13-5-2-6-14-23/h3-4,7-12H,2,5-6,13-14H2,1H3,(H,22,24) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50273689
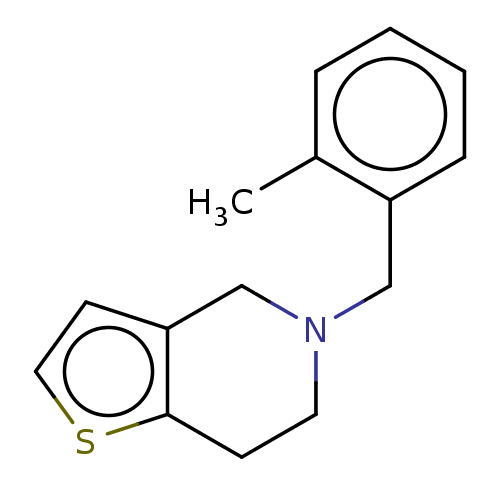
(CHEMBL4128999)Show InChI InChI=1S/C15H17NS/c1-12-4-2-3-5-13(12)10-16-8-6-15-14(11-16)7-9-17-15/h2-5,7,9H,6,8,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes assessed as (S)-mephenytoin 4'-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysis |
Bioorg Med Chem 26: 2996-3005 (2018)
Article DOI: 10.1016/j.bmc.2018.05.006
BindingDB Entry DOI: 10.7270/Q2K076SB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50090677
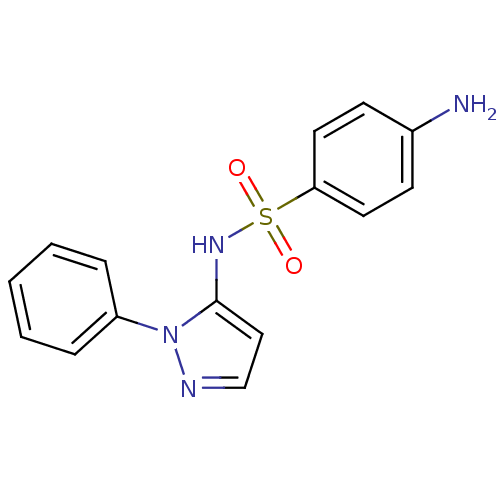
(4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...)Show InChI InChI=1S/C15H14N4O2S/c16-12-6-8-14(9-7-12)22(20,21)18-15-10-11-17-19(15)13-4-2-1-3-5-13/h1-11,18H,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes assessed as tolbutamide methylhydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysis |
Bioorg Med Chem 26: 2996-3005 (2018)
Article DOI: 10.1016/j.bmc.2018.05.006
BindingDB Entry DOI: 10.7270/Q2K076SB |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466331

(US10799494, Compound RKA-70)Show SMILES FC(F)(F)Oc1ccc(Cc2ccc(cc2)-c2cc(=O)c3ccccc3[nH]2)cc1 Show InChI InChI=1S/C23H16F3NO2/c24-23(25,26)29-18-11-7-16(8-12-18)13-15-5-9-17(10-6-15)21-14-22(28)19-3-1-2-4-20(19)27-21/h1-12,14H,13H2,(H,27,28) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466320

(US10799494, Compound RKA-310)Show SMILES COc1ccc2c(c1)[nH]c(-c1ccc(cc1)N1CCCCC1)c(C)c2=O Show InChI InChI=1S/C22H24N2O2/c1-15-21(23-20-14-18(26-2)10-11-19(20)22(15)25)16-6-8-17(9-7-16)24-12-4-3-5-13-24/h6-11,14H,3-5,12-13H2,1-2H3,(H,23,25) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466342

(US10799494, Example RKA 142)Show SMILES Cc1c([nH]c2ccccc2c1=O)-c1ccc(nc1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H15F3N2O/c1-13-20(27-19-5-3-2-4-17(19)21(13)28)15-8-11-18(26-12-15)14-6-9-16(10-7-14)22(23,24)25/h2-12H,1H3,(H,27,28) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human cloned ERG |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM22985
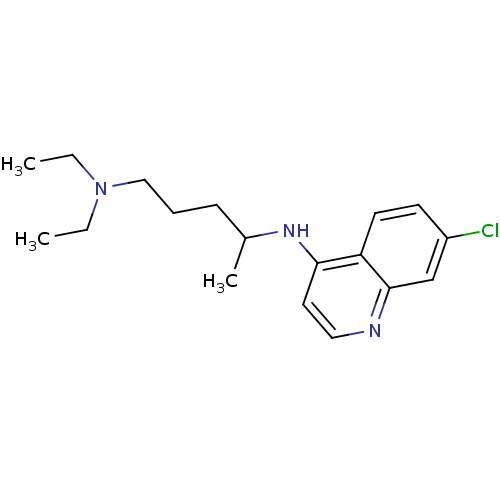
(Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chloro...)Show InChI InChI=1S/C18H26ClN3/c1-4-22(5-2)12-6-7-14(3)21-17-10-11-20-18-13-15(19)8-9-16(17)18/h8-11,13-14H,4-7,12H2,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human cloned ERG |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C8 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50134936
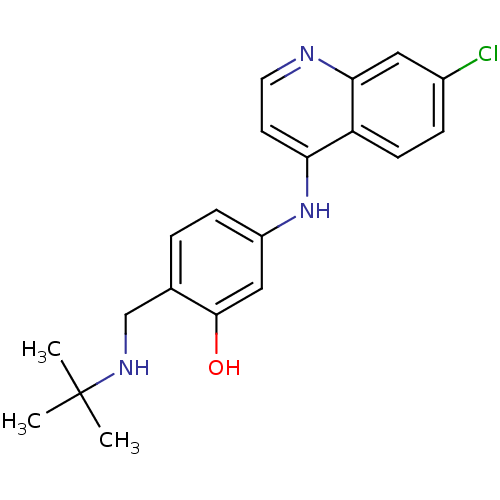
(2-(tert-Butylamino-methyl)-5-(7-chloro-quinolin-4-...)Show InChI InChI=1S/C20H22ClN3O/c1-20(2,3)23-12-13-4-6-15(11-19(13)25)24-17-8-9-22-18-10-14(21)5-7-16(17)18/h4-11,23,25H,12H2,1-3H3,(H,22,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50134934
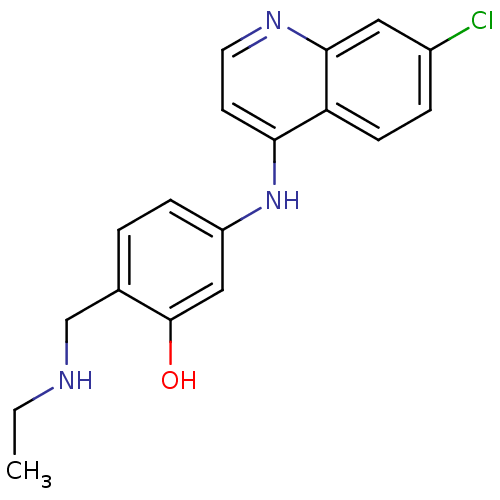
(5-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-3-5-14(10-18(12)23)22-16-7-8-21-17-9-13(19)4-6-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50236897
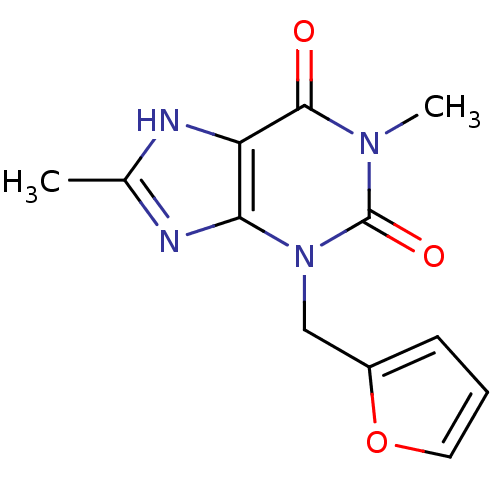
(3-(furan-2-ylmethyl)-1,8-dimethyl-1H-purine-2,6(3H...)Show InChI InChI=1S/C12H12N4O3/c1-7-13-9-10(14-7)16(6-8-4-3-5-19-8)12(18)15(2)11(9)17/h3-5H,6H2,1-2H3,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes assessed as phenacetin O-deethylation after 4 to 40 mins in presence of NADPH by LCMS analysis |
Bioorg Med Chem 26: 2996-3005 (2018)
Article DOI: 10.1016/j.bmc.2018.05.006
BindingDB Entry DOI: 10.7270/Q2K076SB |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466314
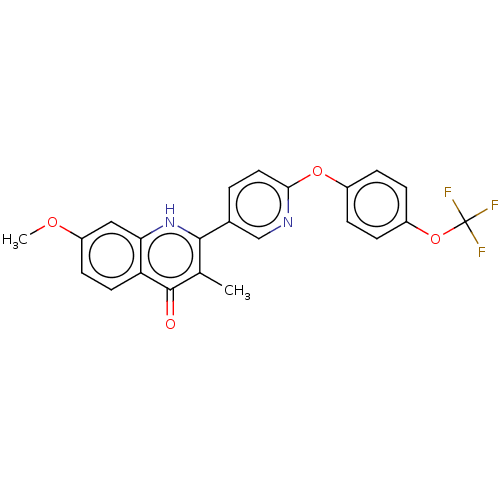
(US10799494, Compound RKA-259)Show SMILES COc1ccc2c(c1)[nH]c(-c1ccc(Oc3ccc(OC(F)(F)F)cc3)nc1)c(C)c2=O Show InChI InChI=1S/C23H17F3N2O4/c1-13-21(28-19-11-17(30-2)8-9-18(19)22(13)29)14-3-10-20(27-12-14)31-15-4-6-16(7-5-15)32-23(24,25)26/h3-12H,1-2H3,(H,28,29) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466328
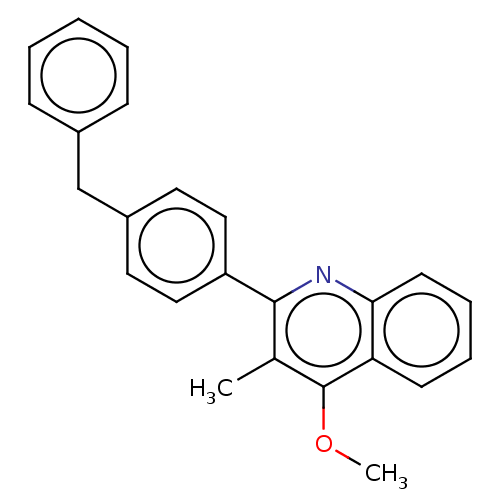
(US10799494, Compound CK-3-23)Show InChI InChI=1S/C24H21NO/c1-17-23(25-22-11-7-6-10-21(22)24(17)26-2)20-14-12-19(13-15-20)16-18-8-4-3-5-9-18/h3-15H,16H2,1-2H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50134931
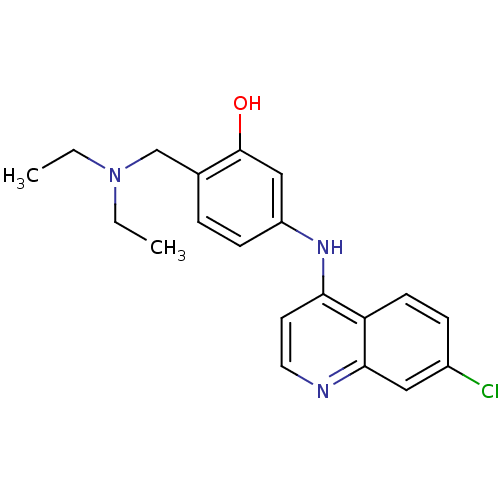
(5-(7-Chloro-quinolin-4-ylamino)-2-diethylaminometh...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human cloned ERG |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466335

(US10799494, Compound PG-128)Show SMILES Cc1c([nH]c2ccccc2c1=O)-c1ccc(nc1)-c1ccc(nc1)C(F)(F)F Show InChI InChI=1S/C21H14F3N3O/c1-12-19(27-17-5-3-2-4-15(17)20(12)28)14-6-8-16(25-11-14)13-7-9-18(26-10-13)21(22,23)24/h2-11H,1H3,(H,27,28) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50056190
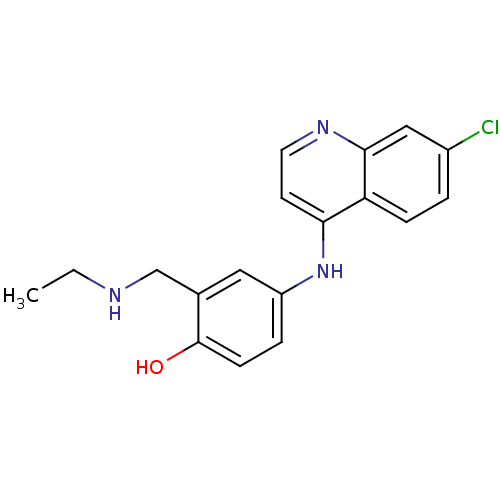
(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466329
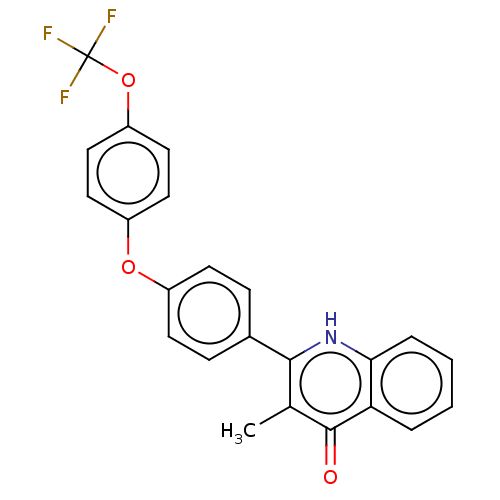
(US10799494, Compound CK-2-63)Show SMILES Cc1c([nH]c2ccccc2c1=O)-c1ccc(Oc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C23H16F3NO3/c1-14-21(27-20-5-3-2-4-19(20)22(14)28)15-6-8-16(9-7-15)29-17-10-12-18(13-11-17)30-23(24,25)26/h2-13H,1H3,(H,27,28) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466337

(US10799494, Compound WDH-1U-10)Show SMILES CCOC(=O)c1c([nH]c2ccccc2c1=O)-c1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H18ClNO3/c1-2-29-24(28)21-22(26-20-6-4-3-5-19(20)23(21)27)17-9-7-15(8-10-17)16-11-13-18(25)14-12-16/h3-14H,2H2,1H3,(H,26,27) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466339

(US10799494, Compound WDH-2A-9)Show SMILES Cc1c([nH]c2ccccc2c1=O)-c1cnn(Cc2ccc(OC(F)(F)F)cc2)c1 Show InChI InChI=1S/C21H16F3N3O2/c1-13-19(26-18-5-3-2-4-17(18)20(13)28)15-10-25-27(12-15)11-14-6-8-16(9-7-14)29-21(22,23)24/h2-10,12H,11H2,1H3,(H,26,28) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50134931

(5-(7-Chloro-quinolin-4-ylamino)-2-diethylaminometh...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50134936

(2-(tert-Butylamino-methyl)-5-(7-chloro-quinolin-4-...)Show InChI InChI=1S/C20H22ClN3O/c1-20(2,3)23-12-13-4-6-15(11-19(13)25)24-17-8-9-22-18-10-14(21)5-7-16(17)18/h4-11,23,25H,12H2,1-3H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human cloned ERG |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50134934

(5-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-3-5-14(10-18(12)23)22-16-7-8-21-17-9-13(19)4-6-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50056190

(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human cloned ERG |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466311
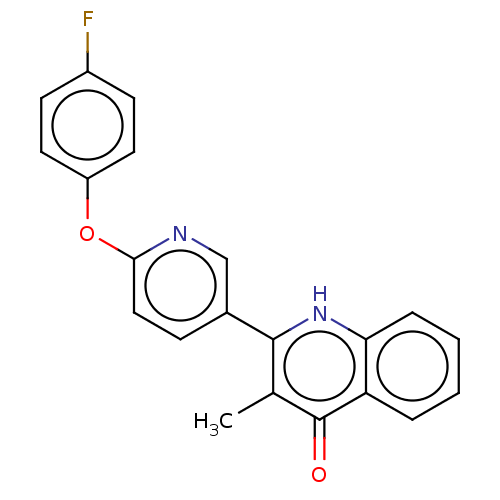
(US10799494, Compound CK-3-14)Show SMILES Cc1c([nH]c2ccccc2c1=O)-c1ccc(Oc2ccc(F)cc2)nc1 Show InChI InChI=1S/C21H15FN2O2/c1-13-20(24-18-5-3-2-4-17(18)21(13)25)14-6-11-19(23-12-14)26-16-9-7-15(22)8-10-16/h2-12H,1H3,(H,24,25) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50134931

(5-(7-Chloro-quinolin-4-ylamino)-2-diethylaminometh...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C8 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II)
() | BDBM466338

(US10799494, Compound WDH-1W-5)Show SMILES FC(F)(F)Oc1ccc(Cn2cc(cn2)-c2cc(=O)c3ccccc3[nH]2)cc1 Show InChI InChI=1S/C20H14F3N3O2/c21-20(22,23)28-15-7-5-13(6-8-15)11-26-12-14(10-24-26)18-9-19(27)16-3-1-2-4-17(16)25-18/h1-10,12H,11H2,(H,25,27) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
The Mtb cytochrome bd inhibition assay described in accompanying Example section, or elsewhere in the literature, may be used to measure the pharmaco... |
US Patent US10799494 (2020)
BindingDB Entry DOI: 10.7270/Q20C4ZVQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50273688

(CHEMBL4127539)Show SMILES C(N1CCOCC1)c1ccc(cc1)C1CCC2(CC1)OOC1(OO2)C2CC3CC(C2)CC1C3 |TLB:32:31:27.26.25:29,THB:32:26:29:21.30.31,27:26:21:28.29.30,27:28:21:26.25.32,(41.94,-38.08,;42.71,-39.42,;41.93,-40.75,;42.69,-42.09,;44.24,-42.1,;45.02,-40.76,;44.25,-39.41,;40.39,-38.08,;39.61,-39.42,;38.07,-39.41,;37.31,-38.07,;38.08,-36.74,;39.62,-36.74,;35.77,-38.06,;34.99,-39.39,;33.46,-39.39,;32.69,-38.05,;33.46,-36.72,;35,-36.73,;31.92,-39.38,;30.39,-39.38,;29.63,-38.04,;30.39,-36.71,;31.93,-36.72,;28.73,-39.28,;27.39,-38.74,;26.6,-37.56,;24.87,-37.89,;26.34,-38.44,;27.15,-39.72,;27.26,-37.14,;28.84,-36.75,;27.46,-36.35,)| Show InChI InChI=1S/C27H37NO5/c1-3-22(4-2-19(1)18-28-9-11-29-12-10-28)23-5-7-26(8-6-23)30-32-27(33-31-26)24-14-20-13-21(16-24)17-25(27)15-20/h1-4,20-21,23-25H,5-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes assessed as midazolam 1'-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysis |
Bioorg Med Chem 26: 2996-3005 (2018)
Article DOI: 10.1016/j.bmc.2018.05.006
BindingDB Entry DOI: 10.7270/Q2K076SB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50273688

(CHEMBL4127539)Show SMILES C(N1CCOCC1)c1ccc(cc1)C1CCC2(CC1)OOC1(OO2)C2CC3CC(C2)CC1C3 |TLB:32:31:27.26.25:29,THB:32:26:29:21.30.31,27:26:21:28.29.30,27:28:21:26.25.32,(41.94,-38.08,;42.71,-39.42,;41.93,-40.75,;42.69,-42.09,;44.24,-42.1,;45.02,-40.76,;44.25,-39.41,;40.39,-38.08,;39.61,-39.42,;38.07,-39.41,;37.31,-38.07,;38.08,-36.74,;39.62,-36.74,;35.77,-38.06,;34.99,-39.39,;33.46,-39.39,;32.69,-38.05,;33.46,-36.72,;35,-36.73,;31.92,-39.38,;30.39,-39.38,;29.63,-38.04,;30.39,-36.71,;31.93,-36.72,;28.73,-39.28,;27.39,-38.74,;26.6,-37.56,;24.87,-37.89,;26.34,-38.44,;27.15,-39.72,;27.26,-37.14,;28.84,-36.75,;27.46,-36.35,)| Show InChI InChI=1S/C27H37NO5/c1-3-22(4-2-19(1)18-28-9-11-29-12-10-28)23-5-7-26(8-6-23)30-32-27(33-31-26)24-14-20-13-21(16-24)17-25(27)15-20/h1-4,20-21,23-25H,5-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes assessed as phenacetin O-deethylation after 4 to 40 mins in presence of NADPH by LCMS analysis |
Bioorg Med Chem 26: 2996-3005 (2018)
Article DOI: 10.1016/j.bmc.2018.05.006
BindingDB Entry DOI: 10.7270/Q2K076SB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50273688

(CHEMBL4127539)Show SMILES C(N1CCOCC1)c1ccc(cc1)C1CCC2(CC1)OOC1(OO2)C2CC3CC(C2)CC1C3 |TLB:32:31:27.26.25:29,THB:32:26:29:21.30.31,27:26:21:28.29.30,27:28:21:26.25.32,(41.94,-38.08,;42.71,-39.42,;41.93,-40.75,;42.69,-42.09,;44.24,-42.1,;45.02,-40.76,;44.25,-39.41,;40.39,-38.08,;39.61,-39.42,;38.07,-39.41,;37.31,-38.07,;38.08,-36.74,;39.62,-36.74,;35.77,-38.06,;34.99,-39.39,;33.46,-39.39,;32.69,-38.05,;33.46,-36.72,;35,-36.73,;31.92,-39.38,;30.39,-39.38,;29.63,-38.04,;30.39,-36.71,;31.93,-36.72,;28.73,-39.28,;27.39,-38.74,;26.6,-37.56,;24.87,-37.89,;26.34,-38.44,;27.15,-39.72,;27.26,-37.14,;28.84,-36.75,;27.46,-36.35,)| Show InChI InChI=1S/C27H37NO5/c1-3-22(4-2-19(1)18-28-9-11-29-12-10-28)23-5-7-26(8-6-23)30-32-27(33-31-26)24-14-20-13-21(16-24)17-25(27)15-20/h1-4,20-21,23-25H,5-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes assessed as testosterone 6beta-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysis |
Bioorg Med Chem 26: 2996-3005 (2018)
Article DOI: 10.1016/j.bmc.2018.05.006
BindingDB Entry DOI: 10.7270/Q2K076SB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50273688

(CHEMBL4127539)Show SMILES C(N1CCOCC1)c1ccc(cc1)C1CCC2(CC1)OOC1(OO2)C2CC3CC(C2)CC1C3 |TLB:32:31:27.26.25:29,THB:32:26:29:21.30.31,27:26:21:28.29.30,27:28:21:26.25.32,(41.94,-38.08,;42.71,-39.42,;41.93,-40.75,;42.69,-42.09,;44.24,-42.1,;45.02,-40.76,;44.25,-39.41,;40.39,-38.08,;39.61,-39.42,;38.07,-39.41,;37.31,-38.07,;38.08,-36.74,;39.62,-36.74,;35.77,-38.06,;34.99,-39.39,;33.46,-39.39,;32.69,-38.05,;33.46,-36.72,;35,-36.73,;31.92,-39.38,;30.39,-39.38,;29.63,-38.04,;30.39,-36.71,;31.93,-36.72,;28.73,-39.28,;27.39,-38.74,;26.6,-37.56,;24.87,-37.89,;26.34,-38.44,;27.15,-39.72,;27.26,-37.14,;28.84,-36.75,;27.46,-36.35,)| Show InChI InChI=1S/C27H37NO5/c1-3-22(4-2-19(1)18-28-9-11-29-12-10-28)23-5-7-26(8-6-23)30-32-27(33-31-26)24-14-20-13-21(16-24)17-25(27)15-20/h1-4,20-21,23-25H,5-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes assessed as (S)-mephenytoin 4'-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysis |
Bioorg Med Chem 26: 2996-3005 (2018)
Article DOI: 10.1016/j.bmc.2018.05.006
BindingDB Entry DOI: 10.7270/Q2K076SB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50056190

(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C8 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50273688

(CHEMBL4127539)Show SMILES C(N1CCOCC1)c1ccc(cc1)C1CCC2(CC1)OOC1(OO2)C2CC3CC(C2)CC1C3 |TLB:32:31:27.26.25:29,THB:32:26:29:21.30.31,27:26:21:28.29.30,27:28:21:26.25.32,(41.94,-38.08,;42.71,-39.42,;41.93,-40.75,;42.69,-42.09,;44.24,-42.1,;45.02,-40.76,;44.25,-39.41,;40.39,-38.08,;39.61,-39.42,;38.07,-39.41,;37.31,-38.07,;38.08,-36.74,;39.62,-36.74,;35.77,-38.06,;34.99,-39.39,;33.46,-39.39,;32.69,-38.05,;33.46,-36.72,;35,-36.73,;31.92,-39.38,;30.39,-39.38,;29.63,-38.04,;30.39,-36.71,;31.93,-36.72,;28.73,-39.28,;27.39,-38.74,;26.6,-37.56,;24.87,-37.89,;26.34,-38.44,;27.15,-39.72,;27.26,-37.14,;28.84,-36.75,;27.46,-36.35,)| Show InChI InChI=1S/C27H37NO5/c1-3-22(4-2-19(1)18-28-9-11-29-12-10-28)23-5-7-26(8-6-23)30-32-27(33-31-26)24-14-20-13-21(16-24)17-25(27)15-20/h1-4,20-21,23-25H,5-18H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes assessed as dextromethorphan O-demethylation after 4 to 40 mins in presence of NADPH by LCMS analysis |
Bioorg Med Chem 26: 2996-3005 (2018)
Article DOI: 10.1016/j.bmc.2018.05.006
BindingDB Entry DOI: 10.7270/Q2K076SB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data