 Found 142 hits with Last Name = 'biggers' and Initial = 'ms'
Found 142 hits with Last Name = 'biggers' and Initial = 'ms' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215854

(CHEMBL301829)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(ccc1C(O)=O)C(F)(F)F Show InChI InChI=1S/C24H19F3O4/c25-24(26,27)17-7-9-18(23(29)30)20(12-17)15-6-8-19-21(11-15)31-13-16(22(19)28)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,28H,10,13H2,(H,29,30)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215854

(CHEMBL301829)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(ccc1C(O)=O)C(F)(F)F Show InChI InChI=1S/C24H19F3O4/c25-24(26,27)17-7-9-18(23(29)30)20(12-17)15-6-8-19-21(11-15)31-13-16(22(19)28)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,28H,10,13H2,(H,29,30)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215854

(CHEMBL301829)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(ccc1C(O)=O)C(F)(F)F Show InChI InChI=1S/C24H19F3O4/c25-24(26,27)17-7-9-18(23(29)30)20(12-17)15-6-8-19-21(11-15)31-13-16(22(19)28)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,28H,10,13H2,(H,29,30)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215738
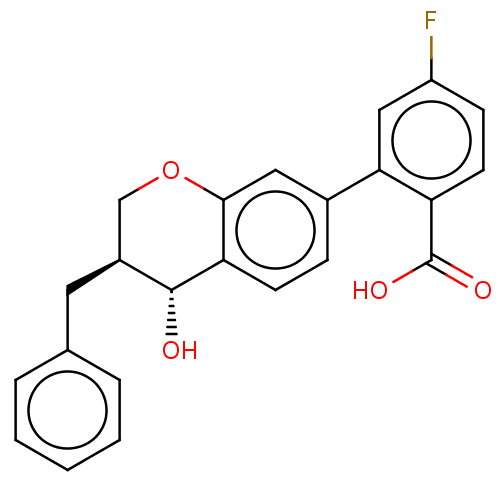
(CHEMBL52675)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-17-7-9-18(23(26)27)20(12-17)15-6-8-19-21(11-15)28-13-16(22(19)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Mus musculus) | BDBM50470784
(CHEMBL282934)Show SMILES COc1ccc(cc1O[C@H]1CC2CCC1C2)-c1ccc(cc1)C(O)=O |TLB:8:9:15:13.12| Show InChI InChI=1S/C21H22O4/c1-24-18-9-8-16(14-4-6-15(7-5-14)21(22)23)12-20(18)25-19-11-13-2-3-17(19)10-13/h4-9,12-13,17,19H,2-3,10-11H2,1H3,(H,22,23)/t13?,17?,19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]rolipram from mouse brain homogenates |
J Med Chem 39: 120-5 (1996)
Article DOI: 10.1021/jm9505066
BindingDB Entry DOI: 10.7270/Q20P12Q4 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Mus musculus) | BDBM50470788
(CHEMBL278386)Show SMILES COc1ccc(cc1O[C@@H]1CC2CCC1C2)-c1ccc(cc1)C(O)=O |TLB:8:9:15:13.12| Show InChI InChI=1S/C21H22O4/c1-24-18-9-8-16(14-4-6-15(7-5-14)21(22)23)12-20(18)25-19-11-13-2-3-17(19)10-13/h4-9,12-13,17,19H,2-3,10-11H2,1H3,(H,22,23)/t13?,17?,19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]rolipram from mouse brain homogenates |
J Med Chem 39: 120-5 (1996)
Article DOI: 10.1021/jm9505066
BindingDB Entry DOI: 10.7270/Q20P12Q4 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215740

(CHEMBL48906)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1C(O)=O Show InChI InChI=1S/C29H23FO4/c30-23-11-13-24(29(32)33)26(16-23)21-10-12-25-27(15-21)34-17-22(28(25)31)14-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h1-13,15-16,22,28,31H,14,17H2,(H,32,33)/t22-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Mus musculus) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]rolipram from mouse brain homogenates |
J Med Chem 39: 120-5 (1996)
Article DOI: 10.1021/jm9505066
BindingDB Entry DOI: 10.7270/Q20P12Q4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215740

(CHEMBL48906)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1C(O)=O Show InChI InChI=1S/C29H23FO4/c30-23-11-13-24(29(32)33)26(16-23)21-10-12-25-27(15-21)34-17-22(28(25)31)14-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h1-13,15-16,22,28,31H,14,17H2,(H,32,33)/t22-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215738

(CHEMBL52675)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-17-7-9-18(23(26)27)20(12-17)15-6-8-19-21(11-15)28-13-16(22(19)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Mus musculus) | BDBM50470782
(CHEMBL280185)Show SMILES COc1ccc(cc1OC1Cc2ccccc2C1)-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C23H21NO3/c1-26-21-11-10-19(15-6-8-16(9-7-15)23(24)25)14-22(21)27-20-12-17-4-2-3-5-18(17)13-20/h2-11,14,20H,12-13H2,1H3,(H2,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]rolipram from mouse brain homogenates |
J Med Chem 39: 120-5 (1996)
Article DOI: 10.1021/jm9505066
BindingDB Entry DOI: 10.7270/Q20P12Q4 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215738

(CHEMBL52675)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-17-7-9-18(23(26)27)20(12-17)15-6-8-19-21(11-15)28-13-16(22(19)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215855
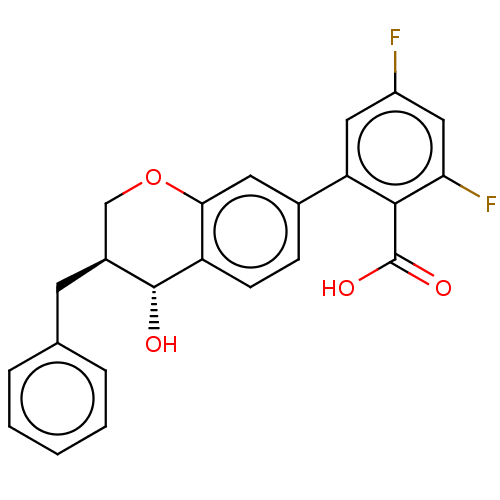
(CHEMBL51467)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(F)cc(F)c1C(O)=O Show InChI InChI=1S/C23H18F2O4/c24-16-10-18(21(23(27)28)19(25)11-16)14-6-7-17-20(9-14)29-12-15(22(17)26)8-13-4-2-1-3-5-13/h1-7,9-11,15,22,26H,8,12H2,(H,27,28)/t15-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50037218
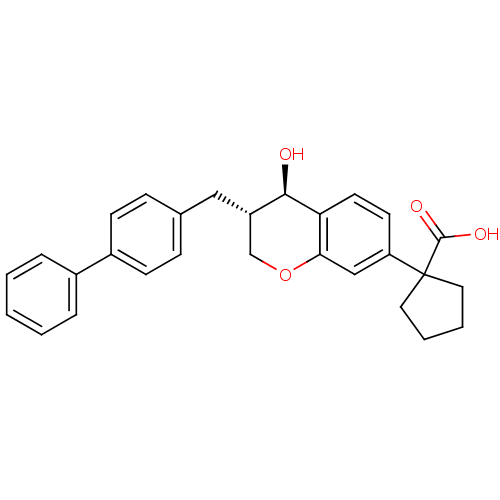
(1-((3S,4R)-3-Biphenyl-4-ylmethyl-4-hydroxy-chroman...)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)C1(CCCC1)C(O)=O Show InChI InChI=1S/C28H28O4/c29-26-22(16-19-8-10-21(11-9-19)20-6-2-1-3-7-20)18-32-25-17-23(12-13-24(25)26)28(27(30)31)14-4-5-15-28/h1-3,6-13,17,22,26,29H,4-5,14-16,18H2,(H,30,31)/t22-,26+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced CD11b up-regulation on isolated human neutrophils in whole blood |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50037218

(1-((3S,4R)-3-Biphenyl-4-ylmethyl-4-hydroxy-chroman...)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)C1(CCCC1)C(O)=O Show InChI InChI=1S/C28H28O4/c29-26-22(16-19-8-10-21(11-9-19)20-6-2-1-3-7-20)18-32-25-17-23(12-13-24(25)26)28(27(30)31)14-4-5-15-28/h1-3,6-13,17,22,26,29H,4-5,14-16,18H2,(H,30,31)/t22-,26+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Tested for binding activity against [3H]-LTB4 to whole human neutrophil with halh maximal inhibition |
J Med Chem 37: 3197-9 (1994)
BindingDB Entry DOI: 10.7270/Q298862K |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215853

(CHEMBL417364)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)-c1cc(ccc1C(O)=O)C(F)(F)F Show InChI InChI=1S/C30H23F3O4/c31-30(32,33)23-11-13-24(29(35)36)26(16-23)21-10-12-25-27(15-21)37-17-22(28(25)34)14-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h1-13,15-16,22,28,34H,14,17H2,(H,35,36)/t22-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215853

(CHEMBL417364)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)-c1cc(ccc1C(O)=O)C(F)(F)F Show InChI InChI=1S/C30H23F3O4/c31-30(32,33)23-11-13-24(29(35)36)26(16-23)21-10-12-25-27(15-21)37-17-22(28(25)34)14-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h1-13,15-16,22,28,34H,14,17H2,(H,35,36)/t22-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50037218

(1-((3S,4R)-3-Biphenyl-4-ylmethyl-4-hydroxy-chroman...)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)C1(CCCC1)C(O)=O Show InChI InChI=1S/C28H28O4/c29-26-22(16-19-8-10-21(11-9-19)20-6-2-1-3-7-20)18-32-25-17-23(12-13-24(25)26)28(27(30)31)14-4-5-15-28/h1-3,6-13,17,22,26,29H,4-5,14-16,18H2,(H,30,31)/t22-,26+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to inhibit [3H]LTB4 binding to LTB4 receptors on guinea pig spleen membranes |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215854

(CHEMBL301829)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(ccc1C(O)=O)C(F)(F)F Show InChI InChI=1S/C24H19F3O4/c25-24(26,27)17-7-9-18(23(29)30)20(12-17)15-6-8-19-21(11-15)31-13-16(22(19)28)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,28H,10,13H2,(H,29,30)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215737

(CHEMBL50425)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)-c1cc(Cl)ccc1C(O)=O Show InChI InChI=1S/C29H23ClO4/c30-23-11-13-24(29(32)33)26(16-23)21-10-12-25-27(15-21)34-17-22(28(25)31)14-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h1-13,15-16,22,28,31H,14,17H2,(H,32,33)/t22-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215737

(CHEMBL50425)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)-c1cc(Cl)ccc1C(O)=O Show InChI InChI=1S/C29H23ClO4/c30-23-11-13-24(29(32)33)26(16-23)21-10-12-25-27(15-21)34-17-22(28(25)31)14-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h1-13,15-16,22,28,31H,14,17H2,(H,32,33)/t22-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50037218

(1-((3S,4R)-3-Biphenyl-4-ylmethyl-4-hydroxy-chroman...)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)C1(CCCC1)C(O)=O Show InChI InChI=1S/C28H28O4/c29-26-22(16-19-8-10-21(11-9-19)20-6-2-1-3-7-20)18-32-25-17-23(12-13-24(25)26)28(27(30)31)14-4-5-15-28/h1-3,6-13,17,22,26,29H,4-5,14-16,18H2,(H,30,31)/t22-,26+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Mus musculus) | BDBM50470786
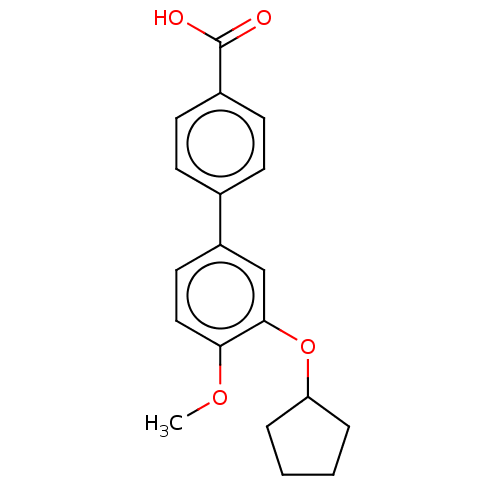
(CHEMBL21964)Show InChI InChI=1S/C19H20O4/c1-22-17-11-10-15(12-18(17)23-16-4-2-3-5-16)13-6-8-14(9-7-13)19(20)21/h6-12,16H,2-5H2,1H3,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]rolipram from mouse brain homogenates |
J Med Chem 39: 120-5 (1996)
Article DOI: 10.1021/jm9505066
BindingDB Entry DOI: 10.7270/Q20P12Q4 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215739

(CHEMBL52374)Show SMILES OCc1ccc(cc1-c1ccc2[C@H](O)[C@@H](Cc3ccccc3)COc2c1)C(F)(F)F Show InChI InChI=1S/C24H21F3O3/c25-24(26,27)19-8-6-17(13-28)21(12-19)16-7-9-20-22(11-16)30-14-18(23(20)29)10-15-4-2-1-3-5-15/h1-9,11-12,18,23,28-29H,10,13-14H2/t18-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50037218

(1-((3S,4R)-3-Biphenyl-4-ylmethyl-4-hydroxy-chroman...)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)C1(CCCC1)C(O)=O Show InChI InChI=1S/C28H28O4/c29-26-22(16-19-8-10-21(11-9-19)20-6-2-1-3-7-20)18-32-25-17-23(12-13-24(25)26)28(27(30)31)14-4-5-15-28/h1-3,6-13,17,22,26,29H,4-5,14-16,18H2,(H,30,31)/t22-,26+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Tested for antagonistic activity against LTB4 receptor in guinea pig spleen membrane |
J Med Chem 37: 3197-9 (1994)
BindingDB Entry DOI: 10.7270/Q298862K |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215861

(CHEMBL298724)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cccc(F)c1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-19-8-4-7-17(21(19)23(26)27)15-9-10-18-20(12-15)28-13-16(22(18)25)11-14-5-2-1-3-6-14/h1-10,12,16,22,25H,11,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215861

(CHEMBL298724)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cccc(F)c1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-19-8-4-7-17(21(19)23(26)27)15-9-10-18-20(12-15)28-13-16(22(18)25)11-14-5-2-1-3-6-14/h1-10,12,16,22,25H,11,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215861

(CHEMBL298724)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cccc(F)c1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-19-8-4-7-17(21(19)23(26)27)15-9-10-18-20(12-15)28-13-16(22(18)25)11-14-5-2-1-3-6-14/h1-10,12,16,22,25H,11,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215738

(CHEMBL52675)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-17-7-9-18(23(26)27)20(12-17)15-6-8-19-21(11-15)28-13-16(22(19)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215738

(CHEMBL52675)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-17-7-9-18(23(26)27)20(12-17)15-6-8-19-21(11-15)28-13-16(22(19)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215741
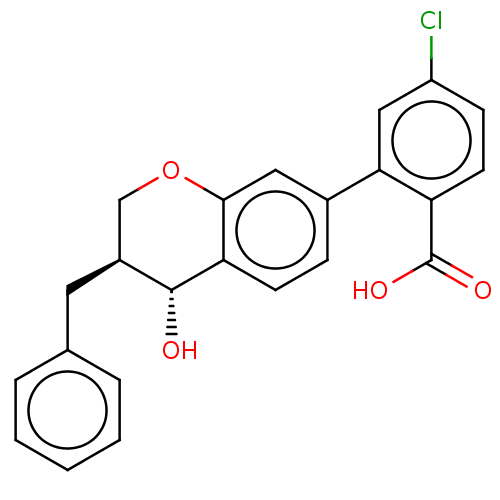
(CHEMBL418264)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(Cl)ccc1C(O)=O Show InChI InChI=1S/C23H19ClO4/c24-17-7-9-18(23(26)27)20(12-17)15-6-8-19-21(11-15)28-13-16(22(19)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Mus musculus) | BDBM50470781
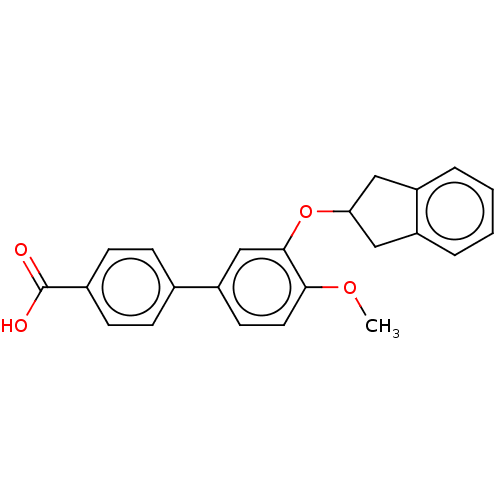
(CHEMBL281781)Show SMILES COc1ccc(cc1OC1Cc2ccccc2C1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H20O4/c1-26-21-11-10-19(15-6-8-16(9-7-15)23(24)25)14-22(21)27-20-12-17-4-2-3-5-18(17)13-20/h2-11,14,20H,12-13H2,1H3,(H,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]rolipram from mouse brain homogenates |
J Med Chem 39: 120-5 (1996)
Article DOI: 10.1021/jm9505066
BindingDB Entry DOI: 10.7270/Q20P12Q4 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Mus musculus) | BDBM50470779
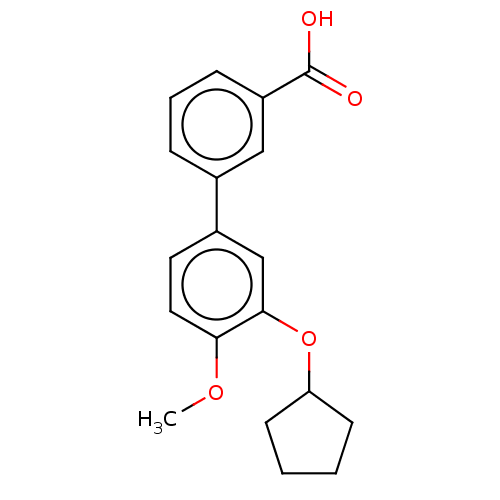
(CHEMBL21463)Show InChI InChI=1S/C19H20O4/c1-22-17-10-9-14(12-18(17)23-16-7-2-3-8-16)13-5-4-6-15(11-13)19(20)21/h4-6,9-12,16H,2-3,7-8H2,1H3,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]rolipram from mouse brain homogenates |
J Med Chem 39: 120-5 (1996)
Article DOI: 10.1021/jm9505066
BindingDB Entry DOI: 10.7270/Q20P12Q4 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215868

(CHEMBL51535)Show SMILES NS(=O)(=O)c1ccc(F)cc1-c1ccc2[C@H](O)[C@@H](Cc3ccccc3)COc2c1 Show InChI InChI=1S/C22H20FNO4S/c23-17-7-9-21(29(24,26)27)19(12-17)15-6-8-18-20(11-15)28-13-16(22(18)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H2,24,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215859
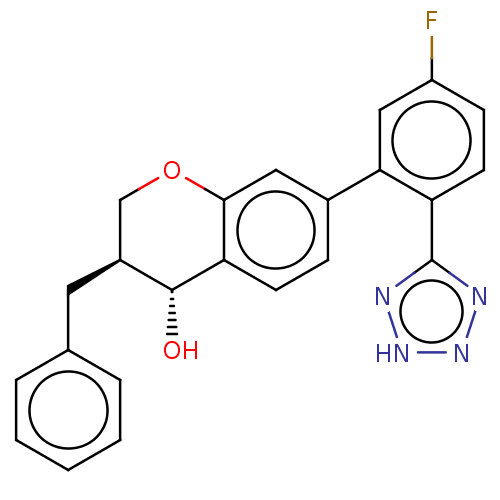
(CHEMBL51775)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1-c1nn[nH]n1 Show InChI InChI=1S/C23H19FN4O2/c24-17-7-9-18(23-25-27-28-26-23)20(12-17)15-6-8-19-21(11-15)30-13-16(22(19)29)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,29H,10,13H2,(H,25,26,27,28)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215861

(CHEMBL298724)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cccc(F)c1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-19-8-4-7-17(21(19)23(26)27)15-9-10-18-20(12-15)28-13-16(22(18)25)11-14-5-2-1-3-6-14/h1-10,12,16,22,25H,11,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215861

(CHEMBL298724)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cccc(F)c1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-19-8-4-7-17(21(19)23(26)27)15-9-10-18-20(12-15)28-13-16(22(18)25)11-14-5-2-1-3-6-14/h1-10,12,16,22,25H,11,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215743
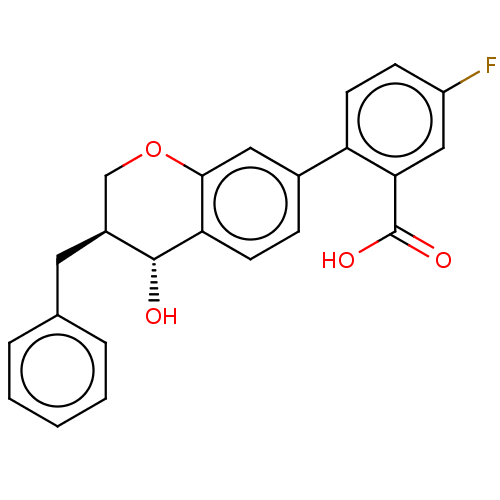
(CHEMBL51219)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1ccc(F)cc1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-17-7-9-18(20(12-17)23(26)27)15-6-8-19-21(11-15)28-13-16(22(19)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215744
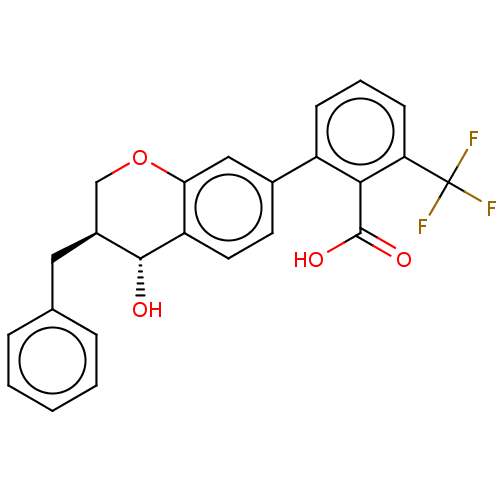
(CHEMBL299150)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cccc(c1C(O)=O)C(F)(F)F Show InChI InChI=1S/C24H19F3O4/c25-24(26,27)19-8-4-7-17(21(19)23(29)30)15-9-10-18-20(12-15)31-13-16(22(18)28)11-14-5-2-1-3-6-14/h1-10,12,16,22,28H,11,13H2,(H,29,30)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215737

(CHEMBL50425)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)-c1cc(Cl)ccc1C(O)=O Show InChI InChI=1S/C29H23ClO4/c30-23-11-13-24(29(32)33)26(16-23)21-10-12-25-27(15-21)34-17-22(28(25)31)14-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h1-13,15-16,22,28,31H,14,17H2,(H,32,33)/t22-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215737

(CHEMBL50425)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)-c1cc(Cl)ccc1C(O)=O Show InChI InChI=1S/C29H23ClO4/c30-23-11-13-24(29(32)33)26(16-23)21-10-12-25-27(15-21)34-17-22(28(25)31)14-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h1-13,15-16,22,28,31H,14,17H2,(H,32,33)/t22-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215741

(CHEMBL418264)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(Cl)ccc1C(O)=O Show InChI InChI=1S/C23H19ClO4/c24-17-7-9-18(23(26)27)20(12-17)15-6-8-19-21(11-15)28-13-16(22(19)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Mus musculus) | BDBM50037218

(1-((3S,4R)-3-Biphenyl-4-ylmethyl-4-hydroxy-chroman...)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)C1(CCCC1)C(O)=O Show InChI InChI=1S/C28H28O4/c29-26-22(16-19-8-10-21(11-9-19)20-6-2-1-3-7-20)18-32-25-17-23(12-13-24(25)26)28(27(30)31)14-4-5-15-28/h1-3,6-13,17,22,26,29H,4-5,14-16,18H2,(H,30,31)/t22-,26+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Tested for antagonistic activity in mouse spleen membrane against LTB4 receptor |
J Med Chem 37: 3197-9 (1994)
BindingDB Entry DOI: 10.7270/Q298862K |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215742

(CHEMBL51492)Show SMILES O[C@@H]1[C@@H](CCc2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1C(O)=O Show InChI InChI=1S/C24H21FO4/c25-18-9-11-19(24(27)28)21(13-18)16-8-10-20-22(12-16)29-14-17(23(20)26)7-6-15-4-2-1-3-5-15/h1-5,8-13,17,23,26H,6-7,14H2,(H,27,28)/t17-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215742

(CHEMBL51492)Show SMILES O[C@@H]1[C@@H](CCc2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1C(O)=O Show InChI InChI=1S/C24H21FO4/c25-18-9-11-19(24(27)28)21(13-18)16-8-10-20-22(12-16)29-14-17(23(20)26)7-6-15-4-2-1-3-5-15/h1-5,8-13,17,23,26H,6-7,14H2,(H,27,28)/t17-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215868

(CHEMBL51535)Show SMILES NS(=O)(=O)c1ccc(F)cc1-c1ccc2[C@H](O)[C@@H](Cc3ccccc3)COc2c1 Show InChI InChI=1S/C22H20FNO4S/c23-17-7-9-21(29(24,26)27)19(12-17)15-6-8-18-20(11-15)28-13-16(22(18)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H2,24,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215853

(CHEMBL417364)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)-c1cc(ccc1C(O)=O)C(F)(F)F Show InChI InChI=1S/C30H23F3O4/c31-30(32,33)23-11-13-24(29(35)36)26(16-23)21-10-12-25-27(15-21)37-17-22(28(25)34)14-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h1-13,15-16,22,28,34H,14,17H2,(H,35,36)/t22-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215853

(CHEMBL417364)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)-c1cc(ccc1C(O)=O)C(F)(F)F Show InChI InChI=1S/C30H23F3O4/c31-30(32,33)23-11-13-24(29(35)36)26(16-23)21-10-12-25-27(15-21)37-17-22(28(25)34)14-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h1-13,15-16,22,28,34H,14,17H2,(H,35,36)/t22-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215855

(CHEMBL51467)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(F)cc(F)c1C(O)=O Show InChI InChI=1S/C23H18F2O4/c24-16-10-18(21(23(27)28)19(25)11-16)14-6-7-17-20(9-14)29-12-15(22(17)26)8-13-4-2-1-3-5-13/h1-7,9-11,15,22,26H,8,12H2,(H,27,28)/t15-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215861

(CHEMBL298724)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cccc(F)c1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-19-8-4-7-17(21(19)23(26)27)15-9-10-18-20(12-15)28-13-16(22(18)25)11-14-5-2-1-3-6-14/h1-10,12,16,22,25H,11,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data