

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188534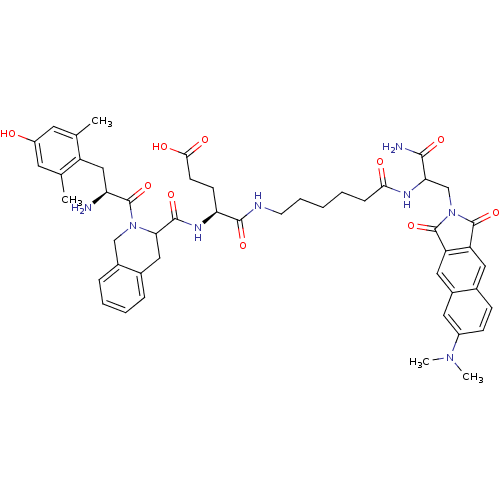 ((H-Dmt-Tic-Glu-NH-(CH(2))(5)-CO-Dap(6DMN)-NH(2) | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin C from rat delta opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188537 (CHEMBL208916 | H-Dmt-Tic-Glu-Dap(6DMN)-NH(2)) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin C from rat delta opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188534 ((H-Dmt-Tic-Glu-NH-(CH(2))(5)-CO-Dap(6DMN)-NH(2) | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188533 (CHEMBL411335 | H-Tyr-Pro-Phe-Phe-NH-(CH2)5-(C=O)-D...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188535 (CHEMBL219407 | H-Tyr-Pro-Phe-Phe-NH-(CH2)5-NH-(C=S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188535 (CHEMBL219407 | H-Tyr-Pro-Phe-Phe-NH-(CH2)5-NH-(C=S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 668 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin C from rat delta opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188536 (CHEMBL207661 | H-Tyr-Pro-Dap(6DMN)-Phe-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 839 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin C from rat delta opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445596 (CHEMBL3103447) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by LC-MS analysis | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445595 (CHEMBL3103453) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by LC-MS analysis | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188536 (CHEMBL207661 | H-Tyr-Pro-Dap(6DMN)-Phe-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188533 (CHEMBL411335 | H-Tyr-Pro-Phe-Phe-NH-(CH2)5-(C=O)-D...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin C from rat delta opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188537 (CHEMBL208916 | H-Dmt-Tic-Glu-Dap(6DMN)-NH(2)) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Helicobacter pylori) | BDBM50496975 (CHEMBL3233400) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Reversible competitive inhibition of Helicobacter pylori DHQ2 assessed as conversion of 3-dehydroquinic acid to 3-dehydroshikimic acid by Dixon plot ... | J Med Chem 57: 3494-510 (2014) Article DOI: 10.1021/jm500175z BindingDB Entry DOI: 10.7270/Q29S1V1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Helicobacter pylori) | BDBM50496972 (CHEMBL3233398) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Reversible competitive inhibition of Helicobacter pylori DHQ2 assessed as conversion of 3-dehydroquinic acid to 3-dehydroshikimic acid by Dixon plot ... | J Med Chem 57: 3494-510 (2014) Article DOI: 10.1021/jm500175z BindingDB Entry DOI: 10.7270/Q29S1V1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Helicobacter pylori) | BDBM50496970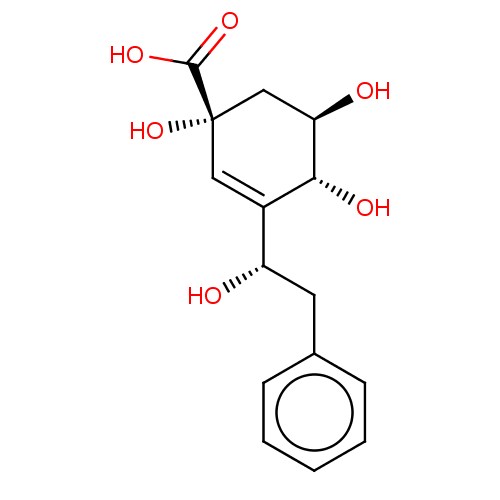 (CHEMBL3233016) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Reversible competitive inhibition of Helicobacter pylori DHQ2 assessed as conversion of 3-dehydroquinic acid to 3-dehydroshikimic acid by Dixon plot ... | J Med Chem 57: 3494-510 (2014) Article DOI: 10.1021/jm500175z BindingDB Entry DOI: 10.7270/Q29S1V1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Helicobacter pylori) | BDBM50496973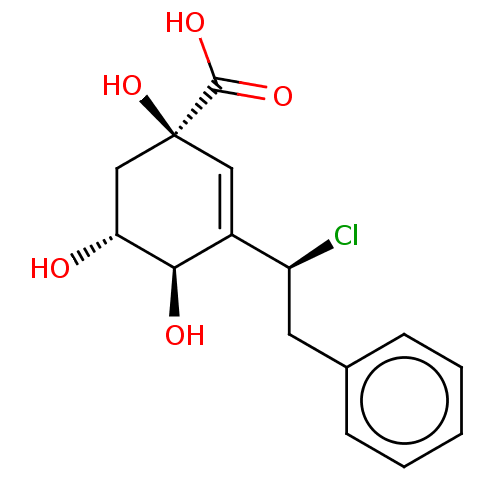 (CHEMBL3233399) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Reversible competitive inhibition of Helicobacter pylori DHQ2 assessed as conversion of 3-dehydroquinic acid to 3-dehydroshikimic acid by Dixon plot ... | J Med Chem 57: 3494-510 (2014) Article DOI: 10.1021/jm500175z BindingDB Entry DOI: 10.7270/Q29S1V1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Helicobacter pylori) | BDBM50496974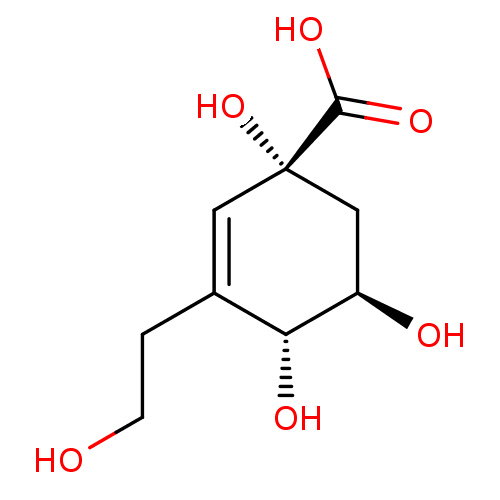 (CHEMBL3233397) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Reversible competitive inhibition of Helicobacter pylori DHQ2 assessed as conversion of 3-dehydroquinic acid to 3-dehydroshikimic acid by Dixon plot ... | J Med Chem 57: 3494-510 (2014) Article DOI: 10.1021/jm500175z BindingDB Entry DOI: 10.7270/Q29S1V1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Helicobacter pylori) | BDBM50496971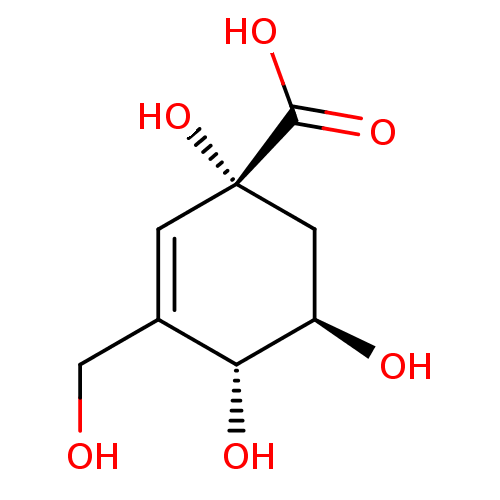 (CHEMBL3233396) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 2.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Reversible competitive inhibition of Helicobacter pylori DHQ2 assessed as conversion of 3-dehydroquinic acid to 3-dehydroshikimic acid by Dixon plot ... | J Med Chem 57: 3494-510 (2014) Article DOI: 10.1021/jm500175z BindingDB Entry DOI: 10.7270/Q29S1V1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50188537 (CHEMBL208916 | H-Dmt-Tic-Glu-Dap(6DMN)-NH(2)) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Functional bioactivity against mu opioid receptor in guinea-pig ileum | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50188535 (CHEMBL219407 | H-Tyr-Pro-Phe-Phe-NH-(CH2)5-NH-(C=S...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Functional bioactivity against mu opioid receptor in guinea-pig ileum | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50188535 (CHEMBL219407 | H-Tyr-Pro-Phe-Phe-NH-(CH2)5-NH-(C=S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Functional bioactivity against delta opioid receptor in mouse vas deferens | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50188534 ((H-Dmt-Tic-Glu-NH-(CH(2))(5)-CO-Dap(6DMN)-NH(2) | ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 244 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Functional bioactivity against mu opioid receptor in guinea-pig ileum | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50188533 (CHEMBL411335 | H-Tyr-Pro-Phe-Phe-NH-(CH2)5-(C=O)-D...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Functional bioactivity against mu opioid receptor in guinea-pig ileum | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50188533 (CHEMBL411335 | H-Tyr-Pro-Phe-Phe-NH-(CH2)5-(C=O)-D...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Functional bioactivity against delta opioid receptor in mouse vas deferens | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50188536 (CHEMBL207661 | H-Tyr-Pro-Dap(6DMN)-Phe-NH2) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 389 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Functional bioactivity against mu opioid receptor in guinea-pig ileum | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50188536 (CHEMBL207661 | H-Tyr-Pro-Dap(6DMN)-Phe-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Functional bioactivity against delta opioid receptor in mouse vas deferens | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445595 (CHEMBL3103453) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445596 (CHEMBL3103447) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445604 (CHEMBL3103448) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445602 (CHEMBL3103450) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445599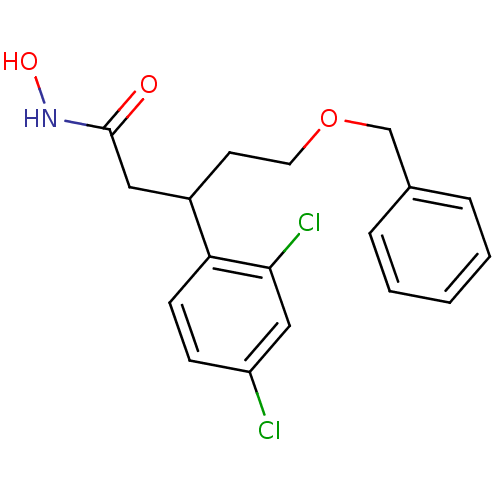 (CHEMBL3103454) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445598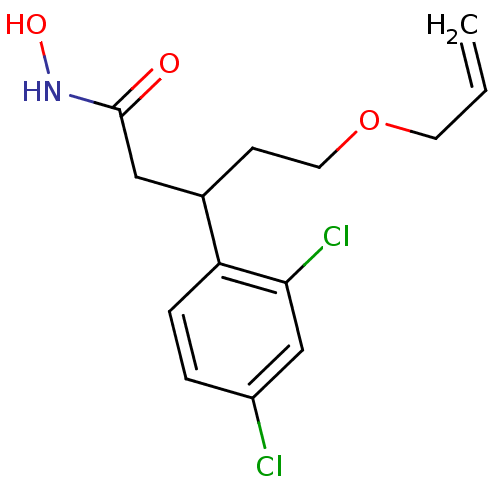 (CHEMBL3103455) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445603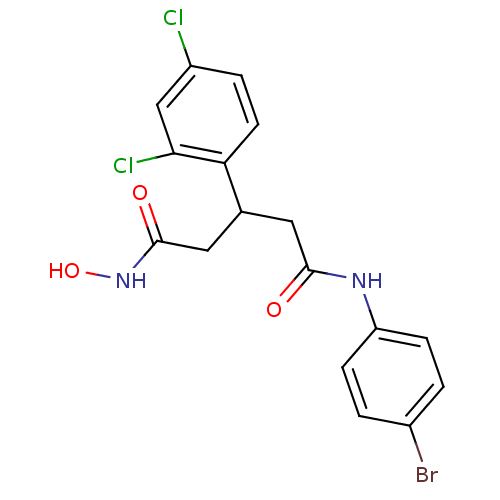 (CHEMBL3103449) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445601 (CHEMBL3103451) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445600 (CHEMBL3103452) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445597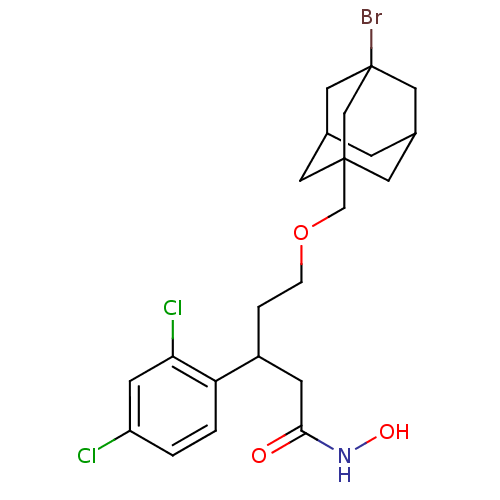 (CHEMBL3103456) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445594 (CHEMBL3103459) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall A hyper BoNT/A light chain expressed in human induced pluripotent stem cell-derived neurons after 8 hrs by W... | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445592 (CHEMBL3103461) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.98E+5 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall A hyper BoNT/A light chain expressed in human induced pluripotent stem cell-derived neurons after 8 hrs by W... | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445591 (CHEMBL3103462) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall A hyper BoNT/A light chain expressed in human induced pluripotent stem cell-derived neurons after 8 hrs by W... | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445593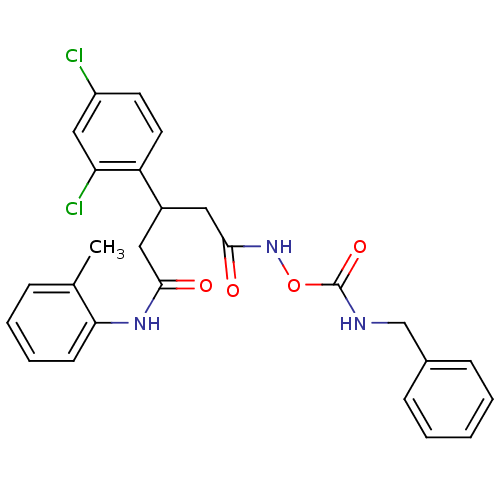 (CHEMBL3103460) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.09E+5 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall A hyper BoNT/A light chain expressed in human induced pluripotent stem cell-derived neurons after 8 hrs by W... | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445590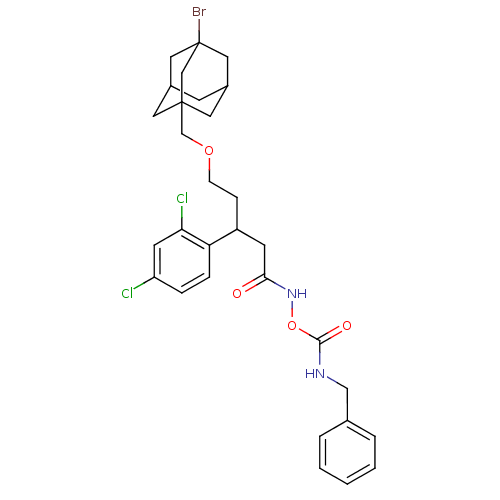 (CHEMBL3103445) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall A hyper BoNT/A light chain expressed in human induced pluripotent stem cell-derived neurons after 8 hrs by W... | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||