 Found 22 hits with Last Name = 'bleasdale' and Initial = 'je'
Found 22 hits with Last Name = 'bleasdale' and Initial = 'je' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Rattus norvegicus (rat)) | BDBM13614

(2-(carboxymethoxy)-5-[(2S)-2-(pentylcarbamoyl)-2-[...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)Cn1[nH]ncc1=S |r| Show InChI InChI=1S/C30H36N6O8S/c1-2-3-7-12-31-28(40)22(15-20-10-11-24(44-18-27(38)39)21(13-20)30(42)43)34-29(41)23(14-19-8-5-4-6-9-19)33-25(37)17-36-26(45)16-32-35-36/h4-6,8-11,13,16,22-23,35H,2-3,7,12,14-15,17-18H2,1H3,(H,31,40)(H,33,37)(H,34,41)(H,38,39)(H,42,43)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | -39.1 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation
| Assay Description
Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... |
Biochemistry 40: 5642-54 (2001)
Article DOI: 10.1021/bi002865v
BindingDB Entry DOI: 10.7270/Q2D50K6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13611
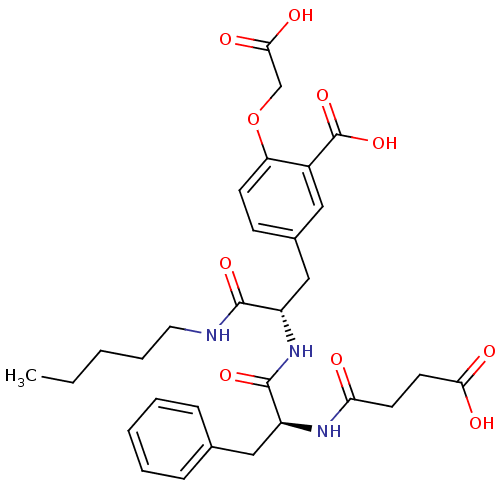
(2-(carboxymethoxy)-5-[(2S)-2-[(2S)-2-(3-formamidop...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)CCC(O)=O |r| Show InChI InChI=1S/C30H37N3O10/c1-2-3-7-14-31-28(39)22(17-20-10-11-24(43-18-27(37)38)21(15-20)30(41)42)33-29(40)23(16-19-8-5-4-6-9-19)32-25(34)12-13-26(35)36/h4-6,8-11,15,22-23H,2-3,7,12-14,16-18H2,1H3,(H,31,39)(H,32,34)(H,33,40)(H,35,36)(H,37,38)(H,41,42)/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 13: 971-5 (2003)
BindingDB Entry DOI: 10.7270/Q24M93WC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Rattus norvegicus (rat)) | BDBM13611

(2-(carboxymethoxy)-5-[(2S)-2-[(2S)-2-(3-formamidop...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)CCC(O)=O |r| Show InChI InChI=1S/C30H37N3O10/c1-2-3-7-14-31-28(39)22(17-20-10-11-24(43-18-27(37)38)21(15-20)30(41)42)33-29(40)23(16-19-8-5-4-6-9-19)32-25(34)12-13-26(35)36/h4-6,8-11,15,22-23H,2-3,7,12-14,16-18H2,1H3,(H,31,39)(H,32,34)(H,33,40)(H,35,36)(H,37,38)(H,41,42)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 250 | -37.3 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation
| Assay Description
Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... |
Biochemistry 40: 5642-54 (2001)
Article DOI: 10.1021/bi002865v
BindingDB Entry DOI: 10.7270/Q2D50K6S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50124517
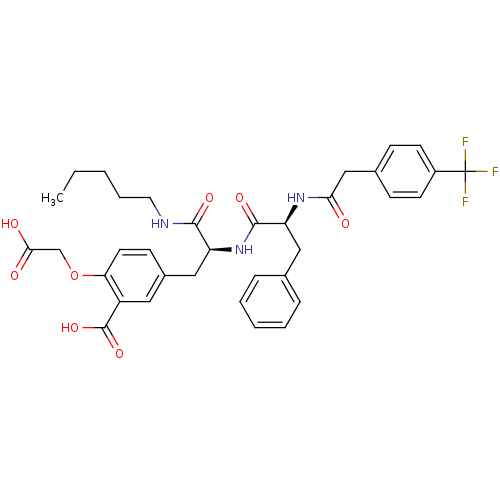
(2-Carboxymethoxy-5-(2-pentylcarbamoyl-2-{(S)-(S)-3...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C35H38F3N3O8/c1-2-3-7-16-39-32(45)27(19-24-12-15-29(49-21-31(43)44)26(17-24)34(47)48)41-33(46)28(18-22-8-5-4-6-9-22)40-30(42)20-23-10-13-25(14-11-23)35(36,37)38/h4-6,8-15,17,27-28H,2-3,7,16,18-21H2,1H3,(H,39,45)(H,40,42)(H,41,46)(H,43,44)(H,47,48)/t27-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 13: 971-5 (2003)
BindingDB Entry DOI: 10.7270/Q24M93WC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50124511

(2-Carboxymethoxy-5-(2-{(S)-(S)-2-[2-(4-methoxy-phe...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)Cc1ccc(OC)cc1 Show InChI InChI=1S/C35H41N3O9/c1-3-4-8-17-36-33(42)28(20-25-13-16-30(47-22-32(40)41)27(18-25)35(44)45)38-34(43)29(19-23-9-6-5-7-10-23)37-31(39)21-24-11-14-26(46-2)15-12-24/h5-7,9-16,18,28-29H,3-4,8,17,19-22H2,1-2H3,(H,36,42)(H,37,39)(H,38,43)(H,40,41)(H,44,45)/t28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 13: 971-5 (2003)
BindingDB Entry DOI: 10.7270/Q24M93WC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50124514
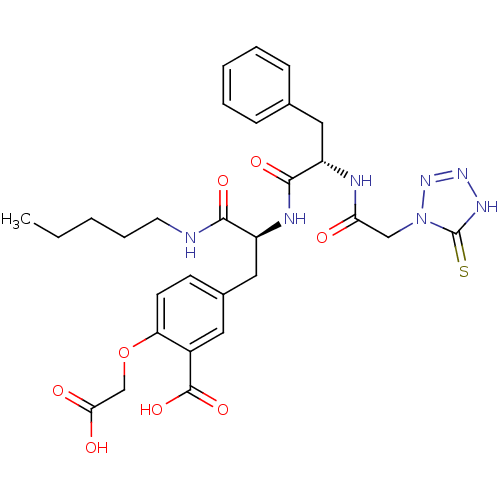
(2-Carboxymethoxy-5-[2-{(S)-2-[2-(5-mercapto-tetraz...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)Cn1nn[nH]c1=S Show InChI InChI=1S/C29H35N7O8S/c1-2-3-7-12-30-26(40)21(15-19-10-11-23(44-17-25(38)39)20(13-19)28(42)43)32-27(41)22(14-18-8-5-4-6-9-18)31-24(37)16-36-29(45)33-34-35-36/h4-6,8-11,13,21-22H,2-3,7,12,14-17H2,1H3,(H,30,40)(H,31,37)(H,32,41)(H,38,39)(H,42,43)(H,33,35,45)/t21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 13: 971-5 (2003)
BindingDB Entry DOI: 10.7270/Q24M93WC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50124513

(2-Carboxymethoxy-5-[(S)-2-pentylcarbamoyl-2-((S)-2...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)Oc1ccccc1 Show InChI InChI=1S/C33H37N3O9/c1-2-3-10-17-34-30(39)26(20-23-15-16-28(44-21-29(37)38)25(18-23)32(41)42)35-31(40)27(19-22-11-6-4-7-12-22)36-33(43)45-24-13-8-5-9-14-24/h4-9,11-16,18,26-27H,2-3,10,17,19-21H2,1H3,(H,34,39)(H,35,40)(H,36,43)(H,37,38)(H,41,42)/t26-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 13: 971-5 (2003)
BindingDB Entry DOI: 10.7270/Q24M93WC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Rattus norvegicus (rat)) | BDBM13609

(2-{4-[(2S)-2-[(2S)-2-(3-formamidopropanoic acid)-3...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OC(C(O)=O)C(O)=O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CCC(O)=O |r| Show InChI InChI=1S/C30H37N3O10/c1-2-3-7-16-31-27(37)22(18-20-10-12-21(13-11-20)43-26(29(39)40)30(41)42)33-28(38)23(17-19-8-5-4-6-9-19)32-24(34)14-15-25(35)36/h4-6,8-13,22-23,26H,2-3,7,14-18H2,1H3,(H,31,37)(H,32,34)(H,33,38)(H,35,36)(H,39,40)(H,41,42)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation
| Assay Description
Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... |
Biochemistry 40: 5642-54 (2001)
Article DOI: 10.1021/bi002865v
BindingDB Entry DOI: 10.7270/Q2D50K6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50124515
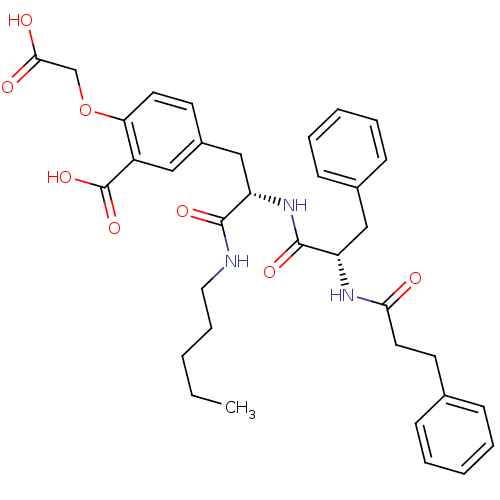
(2-Carboxymethoxy-5-{2-pentylcarbamoyl-2-[(S)-(S)-3...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)CCc1ccccc1 Show InChI InChI=1S/C35H41N3O8/c1-2-3-10-19-36-33(42)28(22-26-15-17-30(46-23-32(40)41)27(20-26)35(44)45)38-34(43)29(21-25-13-8-5-9-14-25)37-31(39)18-16-24-11-6-4-7-12-24/h4-9,11-15,17,20,28-29H,2-3,10,16,18-19,21-23H2,1H3,(H,36,42)(H,37,39)(H,38,43)(H,40,41)(H,44,45)/t28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 13: 971-5 (2003)
BindingDB Entry DOI: 10.7270/Q24M93WC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50112100
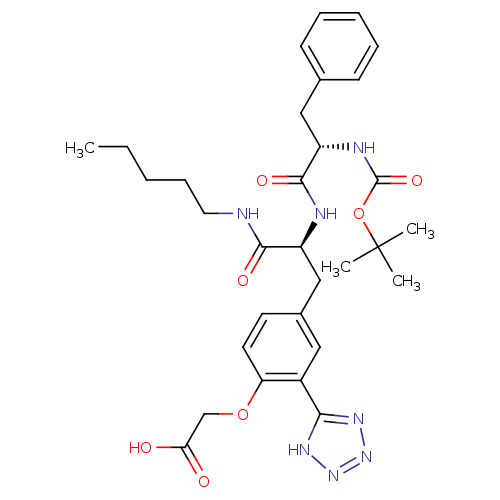
(CHEMBL51202 | [4-[2-(2-tert-Butoxycarbonylamino-3-...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)-c1nnn[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C31H41N7O7/c1-5-6-10-15-32-28(41)23(18-21-13-14-25(44-19-26(39)40)22(16-21)27-35-37-38-36-27)33-29(42)24(17-20-11-8-7-9-12-20)34-30(43)45-31(2,3)4/h7-9,11-14,16,23-24H,5-6,10,15,17-19H2,1-4H3,(H,32,41)(H,33,42)(H,34,43)(H,39,40)(H,35,36,37,38)/t23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB
Curated by ChEMBL
| Assay Description
Inhibitory constant of the compound against Protein-tyrosine phosphatase 1B |
J Med Chem 45: 1785-98 (2002)
BindingDB Entry DOI: 10.7270/Q27D2TFF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50112101

(5-[2-(2-tert-Butoxycarbonylamino-3-phenyl-propiony...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C31H41N3O9/c1-5-6-10-15-32-27(37)23(18-21-13-14-25(42-19-26(35)36)22(16-21)29(39)40)33-28(38)24(17-20-11-8-7-9-12-20)34-30(41)43-31(2,3)4/h7-9,11-14,16,23-24H,5-6,10,15,17-19H2,1-4H3,(H,32,37)(H,33,38)(H,34,41)(H,35,36)(H,39,40)/t23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 13: 971-5 (2003)
BindingDB Entry DOI: 10.7270/Q24M93WC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50112101

(5-[2-(2-tert-Butoxycarbonylamino-3-phenyl-propiony...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C31H41N3O9/c1-5-6-10-15-32-27(37)23(18-21-13-14-25(42-19-26(35)36)22(16-21)29(39)40)33-28(38)24(17-20-11-8-7-9-12-20)34-30(41)43-31(2,3)4/h7-9,11-14,16,23-24H,5-6,10,15,17-19H2,1-4H3,(H,32,37)(H,33,38)(H,34,41)(H,35,36)(H,39,40)/t23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB
Curated by ChEMBL
| Assay Description
Inhibitory constant of the compound against Protein-tyrosine phosphatase 1B |
J Med Chem 45: 1785-98 (2002)
BindingDB Entry DOI: 10.7270/Q27D2TFF |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Rattus norvegicus (rat)) | BDBM13610

(2-(carboxymethoxy)-5-[(2S)-2-(3-formamidopropanoic...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)CCC(O)=O |r| Show InChI InChI=1S/C21H28N2O9/c1-2-3-4-9-22-20(29)15(23-17(24)7-8-18(25)26)11-13-5-6-16(32-12-19(27)28)14(10-13)21(30)31/h5-6,10,15H,2-4,7-9,11-12H2,1H3,(H,22,29)(H,23,24)(H,25,26)(H,27,28)(H,30,31)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | -31.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation
| Assay Description
Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... |
Biochemistry 40: 5642-54 (2001)
Article DOI: 10.1021/bi002865v
BindingDB Entry DOI: 10.7270/Q2D50K6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Rattus norvegicus (rat)) | BDBM13613

(2-{4-[(2S)-2-({[(1S)-1-carboxy-2-phenylethyl]carba...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OC(C(O)=O)C(O)=O)cc1)NC(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C27H33N3O9/c1-2-3-7-14-28-23(31)20(15-18-10-12-19(13-11-18)39-22(25(34)35)26(36)37)29-27(38)30-21(24(32)33)16-17-8-5-4-6-9-17/h4-6,8-13,20-22H,2-3,7,14-16H2,1H3,(H,28,31)(H,32,33)(H,34,35)(H,36,37)(H2,29,30,38)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 3.40E+3 | -30.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation
| Assay Description
Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... |
Biochemistry 40: 5642-54 (2001)
Article DOI: 10.1021/bi002865v
BindingDB Entry DOI: 10.7270/Q2D50K6S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Rattus norvegicus (rat)) | BDBM13606
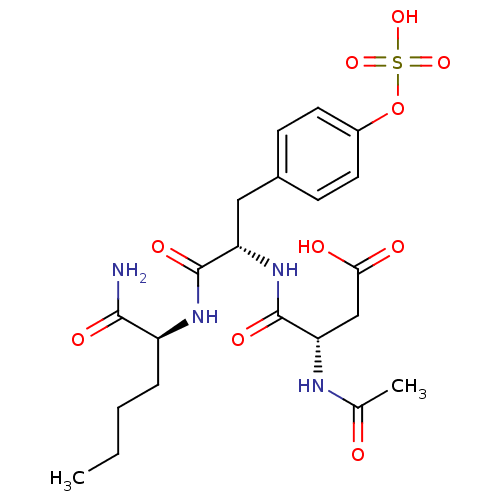
((3S)-3-{[(1S)-1-{[(1S)-1-carbamoylpentyl]carbamoyl...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(N)=O |r| Show InChI InChI=1S/C21H30N4O10S/c1-3-4-5-15(19(22)29)24-20(30)16(25-21(31)17(11-18(27)28)23-12(2)26)10-13-6-8-14(9-7-13)35-36(32,33)34/h6-9,15-17H,3-5,10-11H2,1-2H3,(H2,22,29)(H,23,26)(H,24,30)(H,25,31)(H,27,28)(H,32,33,34)/t15-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+3 | -30.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation
| Assay Description
Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... |
Biochemistry 40: 5642-54 (2001)
Article DOI: 10.1021/bi002865v
BindingDB Entry DOI: 10.7270/Q2D50K6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13606

((3S)-3-{[(1S)-1-{[(1S)-1-carbamoylpentyl]carbamoyl...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(N)=O |r| Show InChI InChI=1S/C21H30N4O10S/c1-3-4-5-15(19(22)29)24-20(30)16(25-21(31)17(11-18(27)28)23-12(2)26)10-13-6-8-14(9-7-13)35-36(32,33)34/h6-9,15-17H,3-5,10-11H2,1-2H3,(H2,22,29)(H,23,26)(H,24,30)(H,25,31)(H,27,28)(H,32,33,34)/t15-,16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 13: 971-5 (2003)
BindingDB Entry DOI: 10.7270/Q24M93WC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50112099
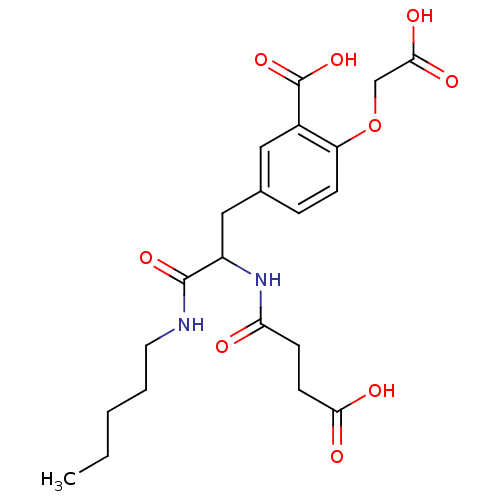
(2-Carboxymethoxy-5-[2-(3-carboxy-propionylamino)-2...)Show SMILES CCCCCNC(=O)C(Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)CCC(O)=O Show InChI InChI=1S/C21H28N2O9/c1-2-3-4-9-22-20(29)15(23-17(24)7-8-18(25)26)11-13-5-6-16(32-12-19(27)28)14(10-13)21(30)31/h5-6,10,15H,2-4,7-9,11-12H2,1H3,(H,22,29)(H,23,24)(H,25,26)(H,27,28)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB
Curated by ChEMBL
| Assay Description
Percent inhibition of compound against Protein-tyrosine phosphatase 1B at a 65 microM concentration |
J Med Chem 45: 1785-98 (2002)
BindingDB Entry DOI: 10.7270/Q27D2TFF |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50124512

(2-{4-[(S)-2-((S)-2-tert-Butoxycarbonylamino-3-phen...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OC(C(O)=O)C(O)=O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C31H41N3O9/c1-5-6-10-17-32-26(35)23(19-21-13-15-22(16-14-21)42-25(28(37)38)29(39)40)33-27(36)24(18-20-11-8-7-9-12-20)34-30(41)43-31(2,3)4/h7-9,11-16,23-25H,5-6,10,17-19H2,1-4H3,(H,32,35)(H,33,36)(H,34,41)(H,37,38)(H,39,40)/t23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 13: 971-5 (2003)
BindingDB Entry DOI: 10.7270/Q24M93WC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Rattus norvegicus (rat)) | BDBM13608
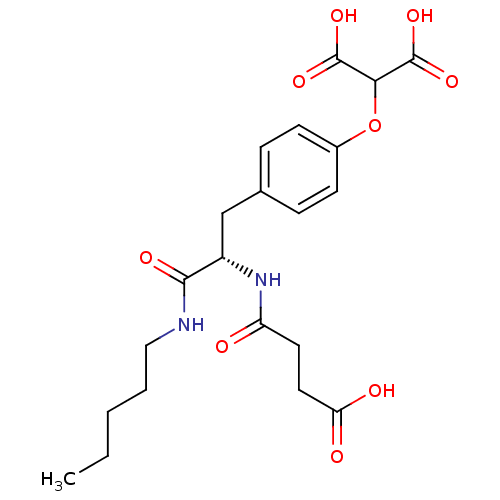
(2-{4-[(2S)-2-(3-formamidopropanoic acid)-2-(pentyl...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OC(C(O)=O)C(O)=O)cc1)NC(=O)CCC(O)=O |r| Show InChI InChI=1S/C21H28N2O9/c1-2-3-4-11-22-19(27)15(23-16(24)9-10-17(25)26)12-13-5-7-14(8-6-13)32-18(20(28)29)21(30)31/h5-8,15,18H,2-4,9-12H2,1H3,(H,22,27)(H,23,24)(H,25,26)(H,28,29)(H,30,31)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | -27.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation
| Assay Description
Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... |
Biochemistry 40: 5642-54 (2001)
Article DOI: 10.1021/bi002865v
BindingDB Entry DOI: 10.7270/Q2D50K6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50124516
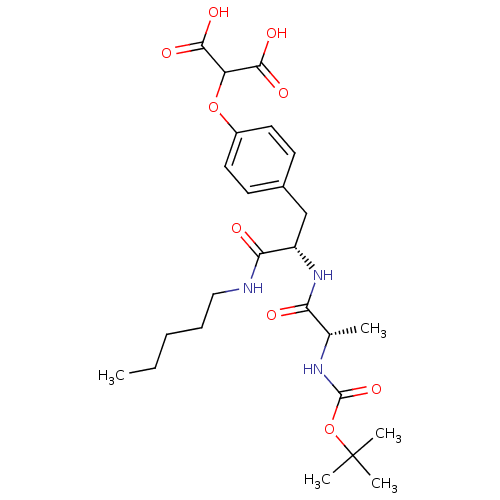
(2-{4-[(S)-2-((S)-2-tert-Butoxycarbonylamino-propio...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OC(C(O)=O)C(O)=O)cc1)NC(=O)[C@H](C)NC(=O)OC(C)(C)C Show InChI InChI=1S/C25H37N3O9/c1-6-7-8-13-26-21(30)18(28-20(29)15(2)27-24(35)37-25(3,4)5)14-16-9-11-17(12-10-16)36-19(22(31)32)23(33)34/h9-12,15,18-19H,6-8,13-14H2,1-5H3,(H,26,30)(H,27,35)(H,28,29)(H,31,32)(H,33,34)/t15-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 13: 971-5 (2003)
BindingDB Entry DOI: 10.7270/Q24M93WC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Rattus norvegicus (rat)) | BDBM13607
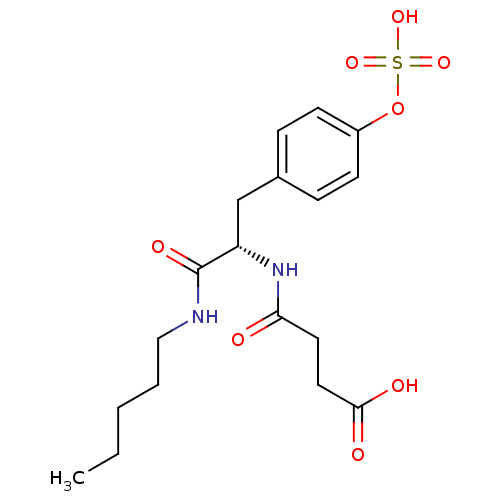
(3-{[(1S)-1-(pentylcarbamoyl)-2-[4-(sulfooxy)phenyl...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)CCC(O)=O |r| Show InChI InChI=1S/C18H26N2O8S/c1-2-3-4-11-19-18(24)15(20-16(21)9-10-17(22)23)12-13-5-7-14(8-6-13)28-29(25,26)27/h5-8,15H,2-4,9-12H2,1H3,(H,19,24)(H,20,21)(H,22,23)(H,25,26,27)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+4 | -25.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation
| Assay Description
Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... |
Biochemistry 40: 5642-54 (2001)
Article DOI: 10.1021/bi002865v
BindingDB Entry DOI: 10.7270/Q2D50K6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Rattus norvegicus (rat)) | BDBM13612
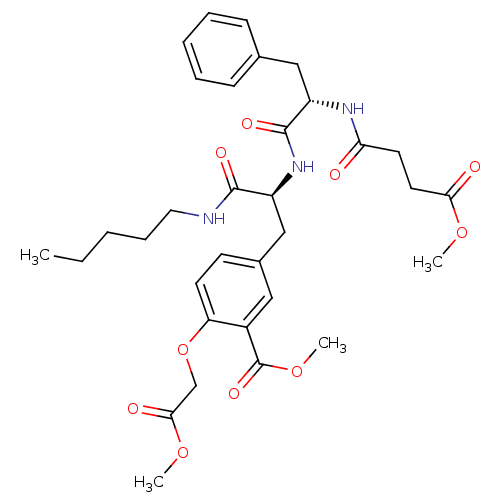
(Compound VII | methyl 2-(2-methoxy-2-oxoethoxy)-5-...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(=O)OC)c(c1)C(=O)OC)NC(=O)[C@H](Cc1ccccc1)NC(=O)CCC(=O)OC |r| Show InChI InChI=1S/C33H43N3O10/c1-5-6-10-17-34-31(40)25(20-23-13-14-27(46-21-30(39)44-3)24(18-23)33(42)45-4)36-32(41)26(19-22-11-8-7-9-12-22)35-28(37)15-16-29(38)43-2/h7-9,11-14,18,25-26H,5-6,10,15-17,19-21H2,1-4H3,(H,34,40)(H,35,37)(H,36,41)/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-22.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation
| Assay Description
Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... |
Biochemistry 40: 5642-54 (2001)
Article DOI: 10.1021/bi002865v
BindingDB Entry DOI: 10.7270/Q2D50K6S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data