

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511450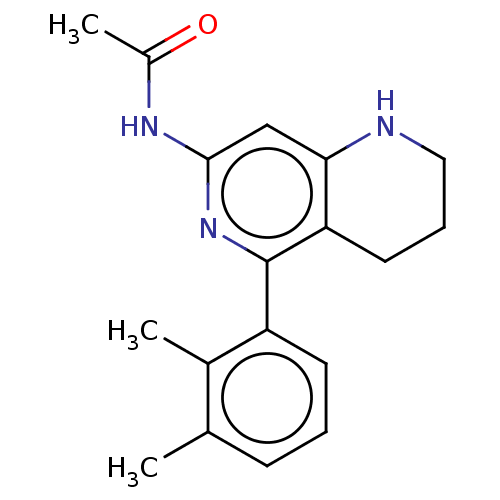 (CHEMBL4436749) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511450 (CHEMBL4436749) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111939 (US8618107, 105) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length His-tagged BTK expressed in baculovirus expression system by Z-LYTE assay | ACS Med Chem Lett 8: 608-613 (2017) Article DOI: 10.1021/acsmedchemlett.7b00103 BindingDB Entry DOI: 10.7270/Q24M96ZH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511450 (CHEMBL4436749) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511431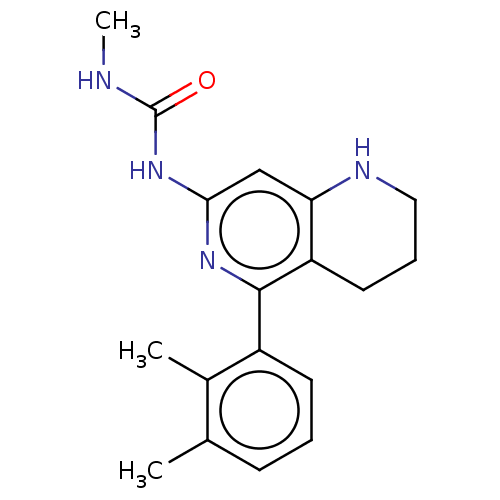 (CHEMBL4468099) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511430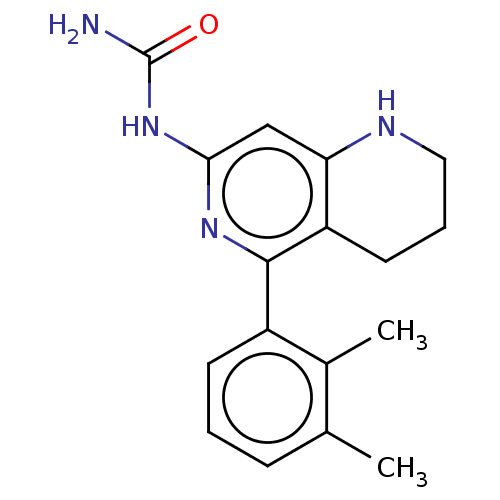 (CHEMBL4455353) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511453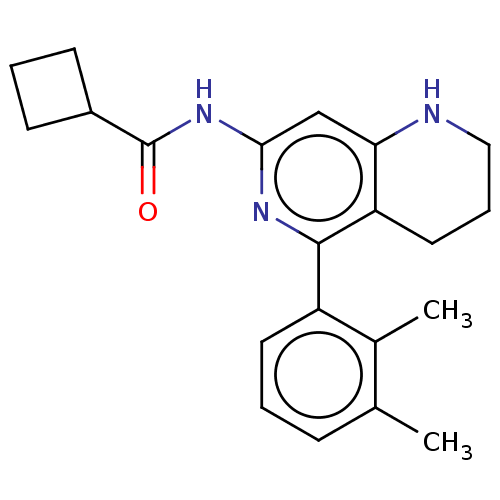 (CHEMBL4451394) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511452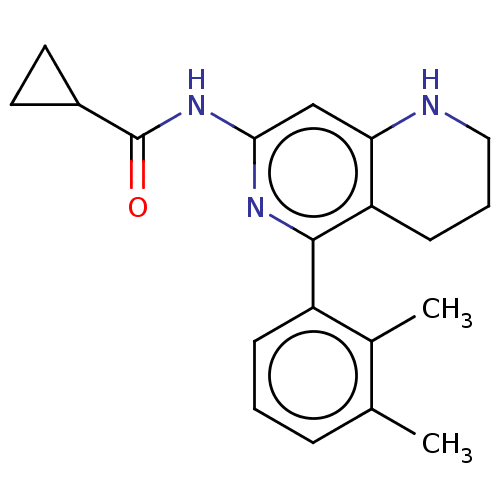 (CHEMBL4570266) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511454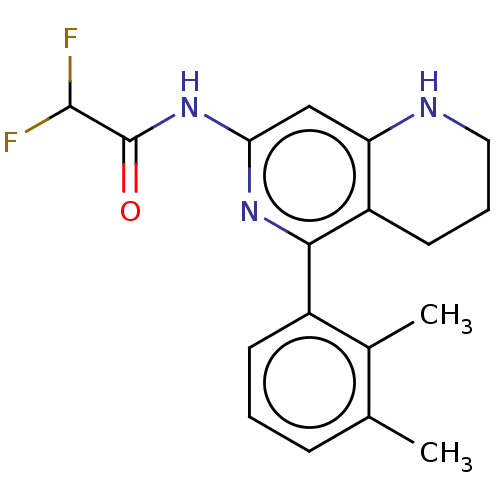 (CHEMBL4460228) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511432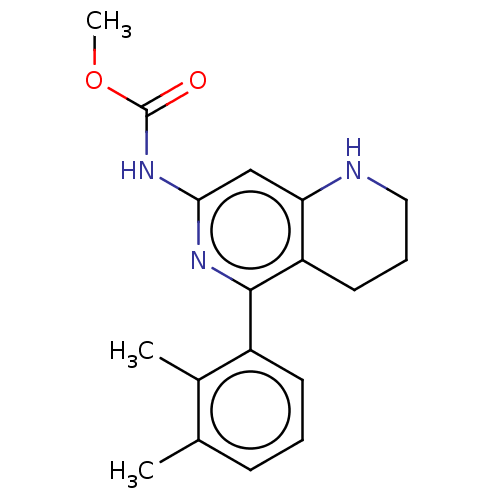 (CHEMBL4435945) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511466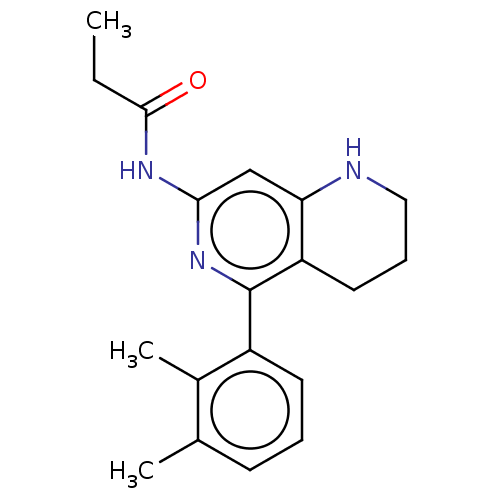 (CHEMBL4529335) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50152144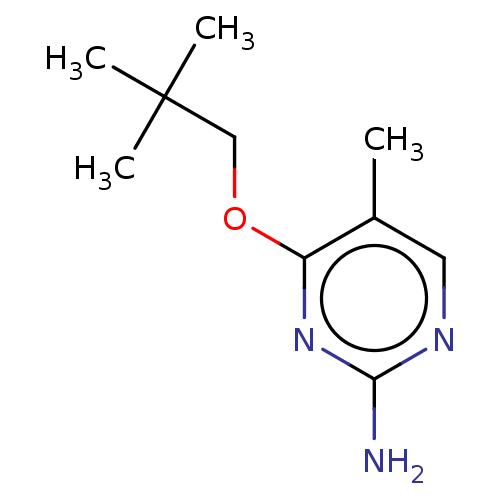 (CHEMBL3781661) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511448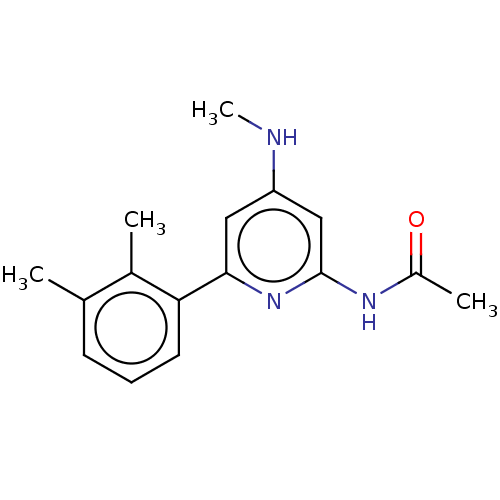 (CHEMBL4460446) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015453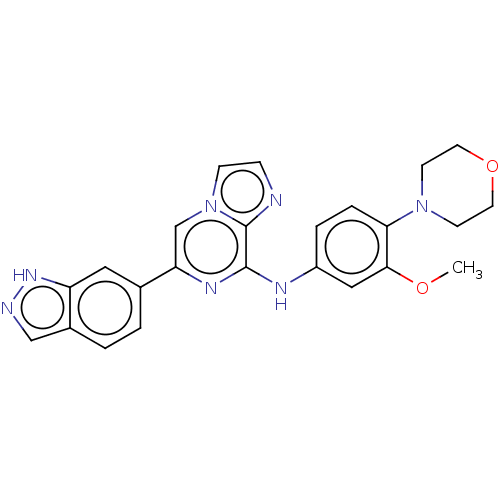 (CHEMBL3265037) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511428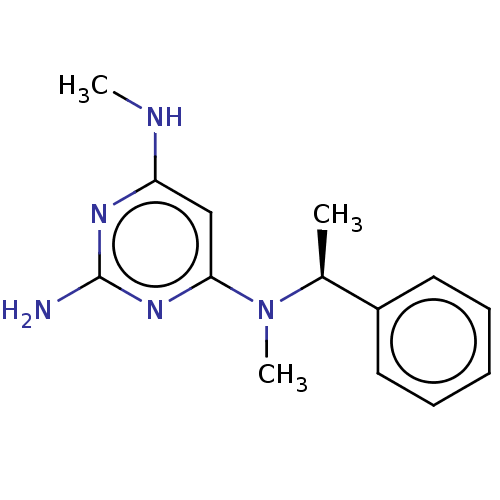 (CHEMBL4435983) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511463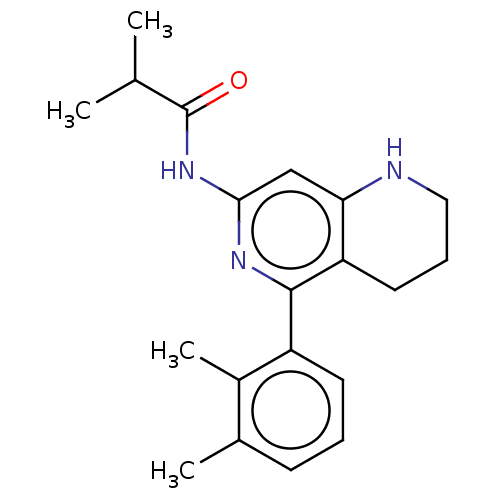 (CHEMBL4577002) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511457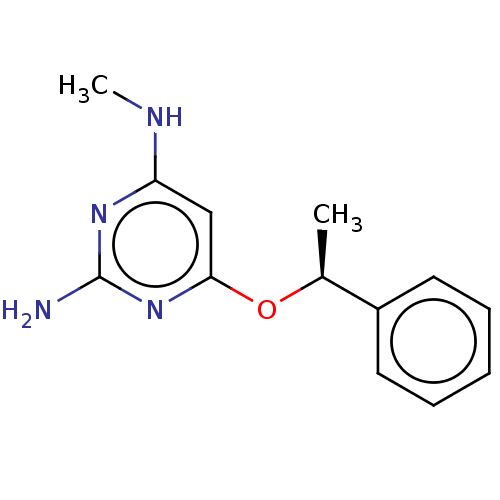 (CHEMBL4537820) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50152125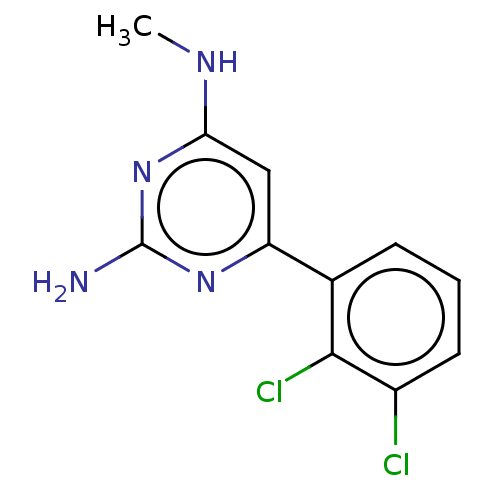 (CHEMBL3781316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using dGTP as substrate measured after 30 mins by mala... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511439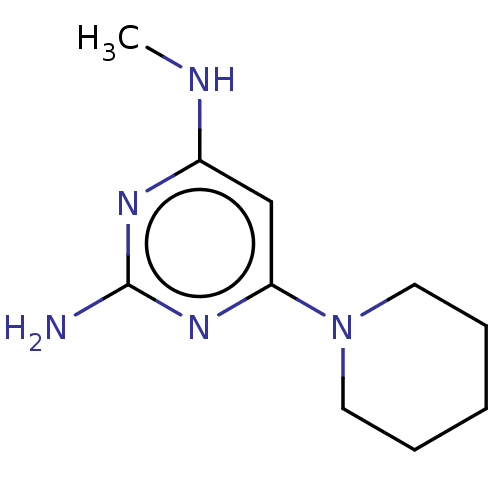 (CHEMBL4565830) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538456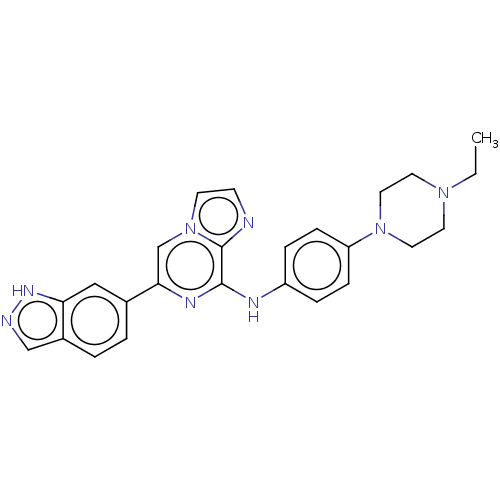 (CHEMBL4649800) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511458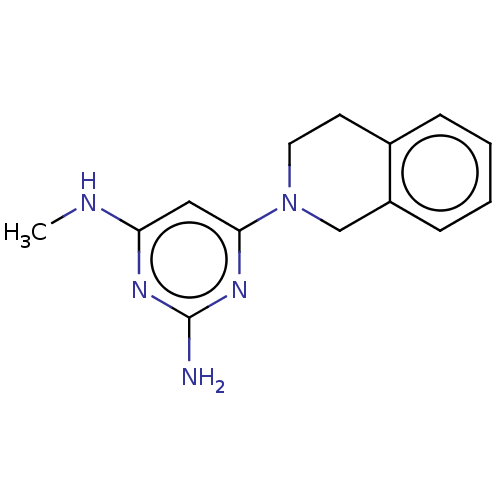 (CHEMBL4534555) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015454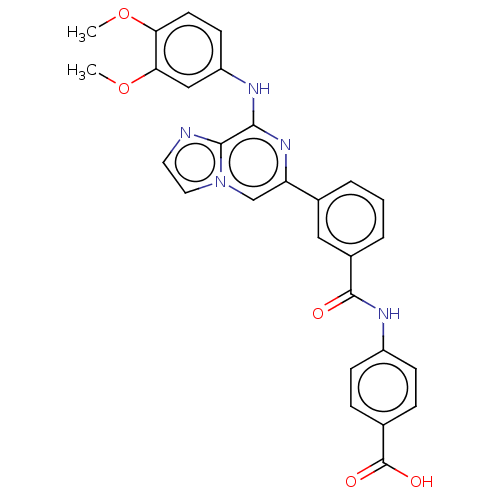 (CHEMBL3264995) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111938 (US8618107, 104) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538455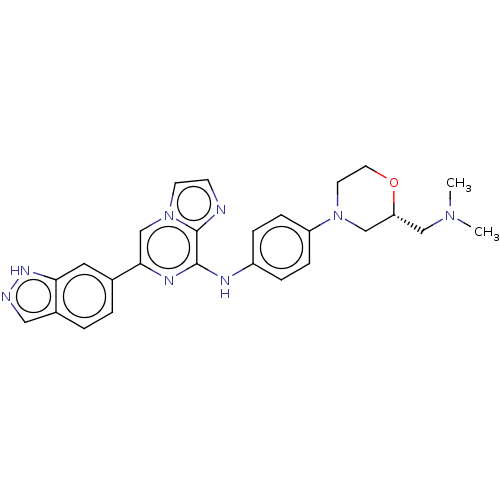 (CHEMBL4637943) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538457 (CHEMBL4638089) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50134365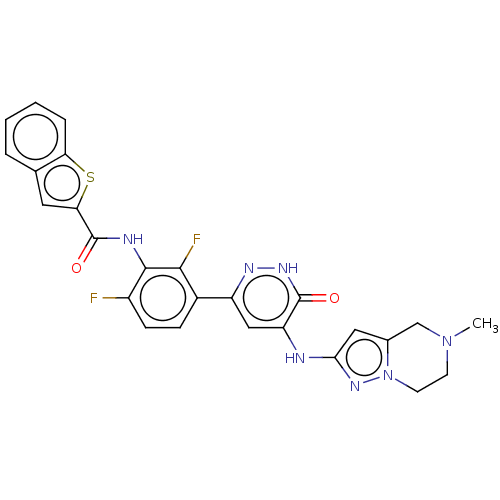 (CHEMBL3745935) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of BTK (unknown origin) by Lanthascreen assay | Bioorg Med Chem Lett 26: 575-9 (2016) Article DOI: 10.1016/j.bmcl.2015.11.076 BindingDB Entry DOI: 10.7270/Q2222WMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111938 (US8618107, 104) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... | ACS Med Chem Lett 8: 608-613 (2017) Article DOI: 10.1021/acsmedchemlett.7b00103 BindingDB Entry DOI: 10.7270/Q24M96ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111940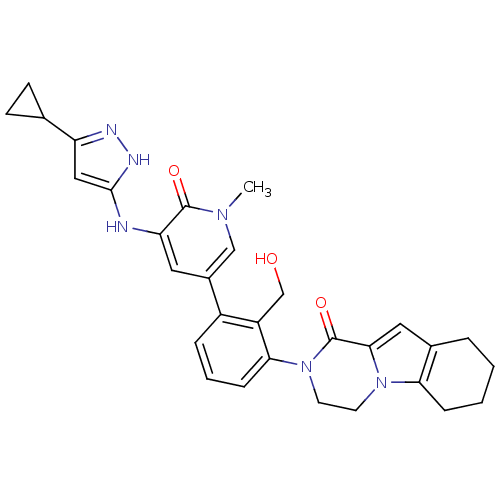 (US8618107, 106) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111953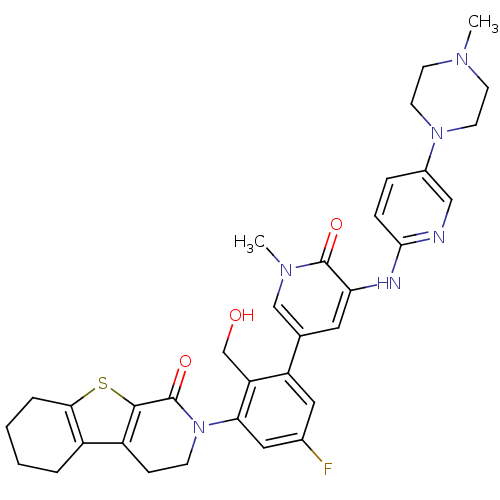 (US8618107, 212) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511441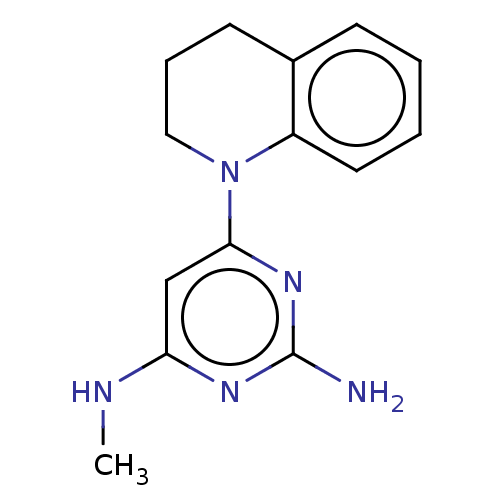 (CHEMBL4464305) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538454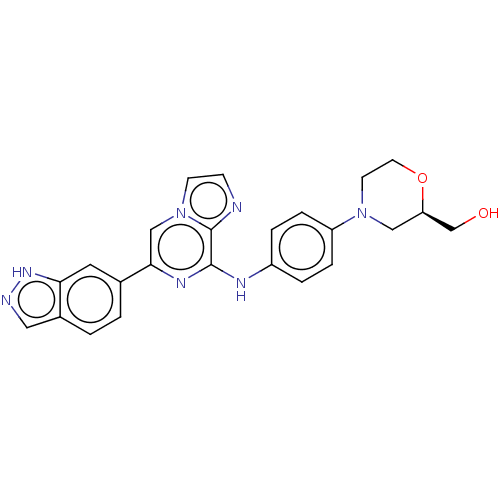 (CHEMBL4643354) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538453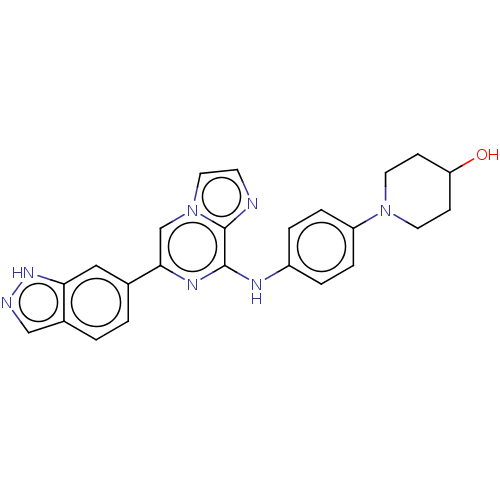 (CHEMBL4647927) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111975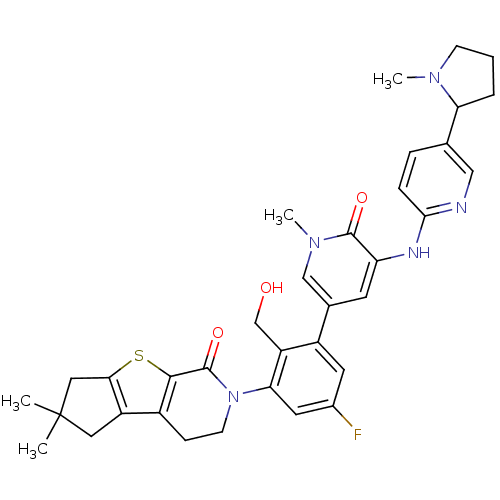 (US8618107, 287) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015447 (CHEMBL3265031) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015460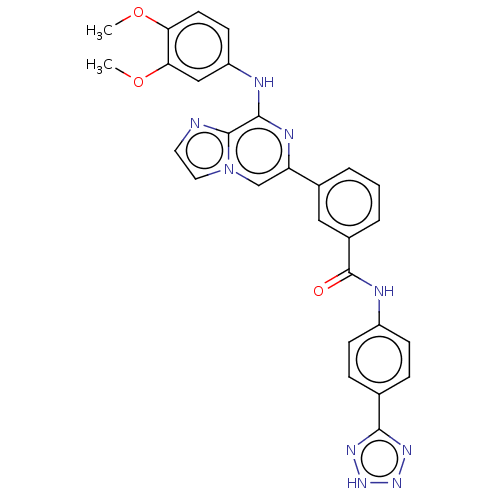 (CHEMBL3265001) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538469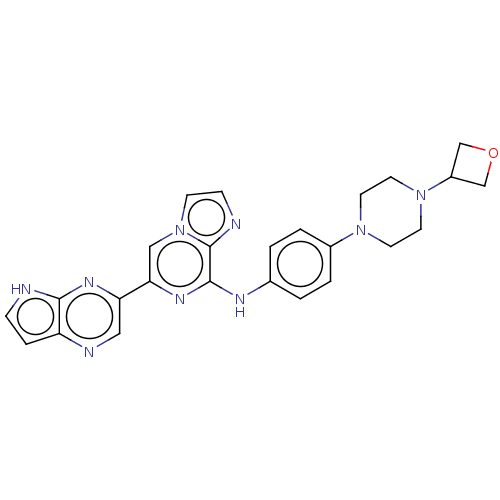 (CHEMBL4643695) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111978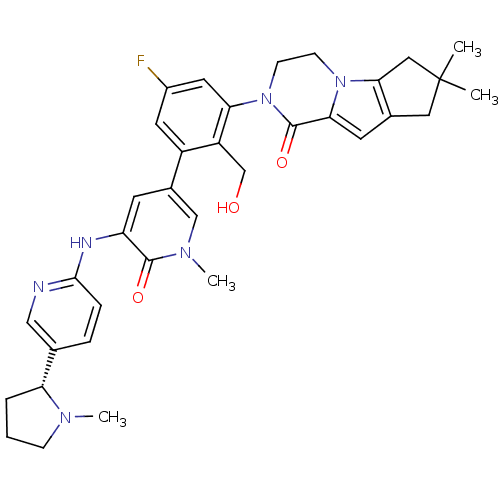 (US8618107, 291) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.74 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111956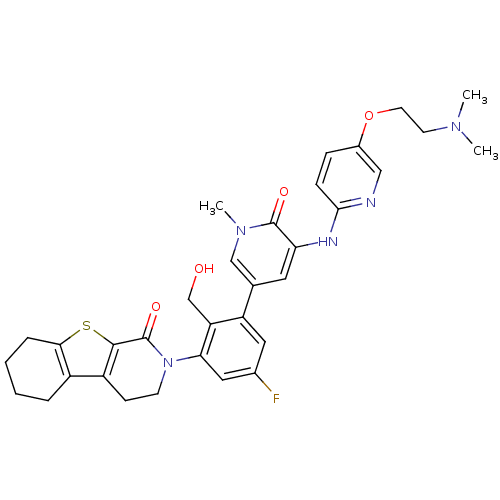 (US8618107, 268) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111949 (US8618107, 131) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111939 (US8618107, 105) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... | ACS Med Chem Lett 8: 608-613 (2017) Article DOI: 10.1021/acsmedchemlett.7b00103 BindingDB Entry DOI: 10.7270/Q24M96ZH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111935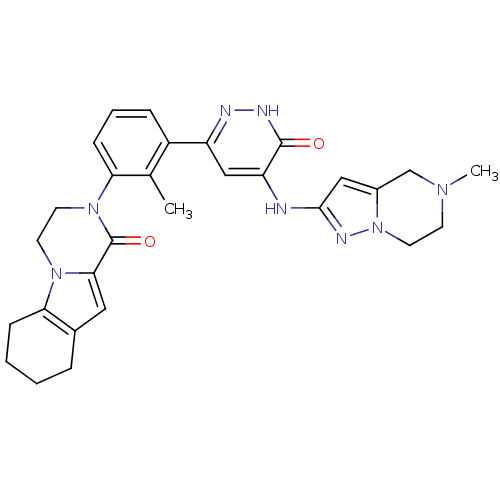 (US8618107, 101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111951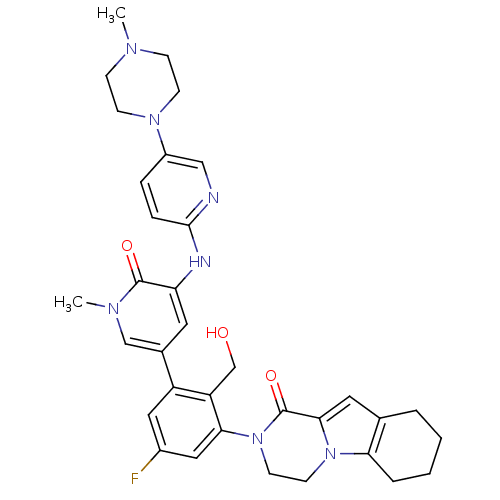 (US8618107, 197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... | ACS Med Chem Lett 8: 608-613 (2017) Article DOI: 10.1021/acsmedchemlett.7b00103 BindingDB Entry DOI: 10.7270/Q24M96ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50254236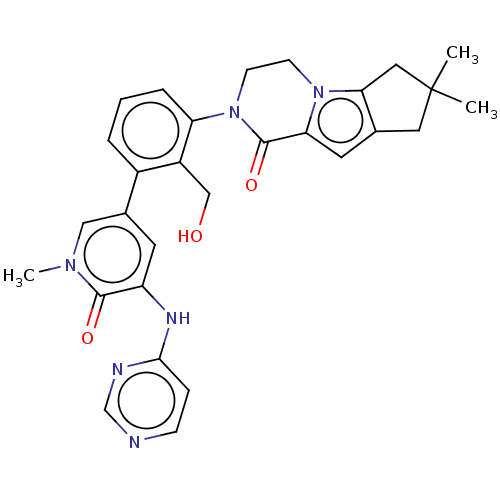 (CHEMBL4090189) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... | ACS Med Chem Lett 8: 608-613 (2017) Article DOI: 10.1021/acsmedchemlett.7b00103 BindingDB Entry DOI: 10.7270/Q24M96ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111951 (US8618107, 197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111976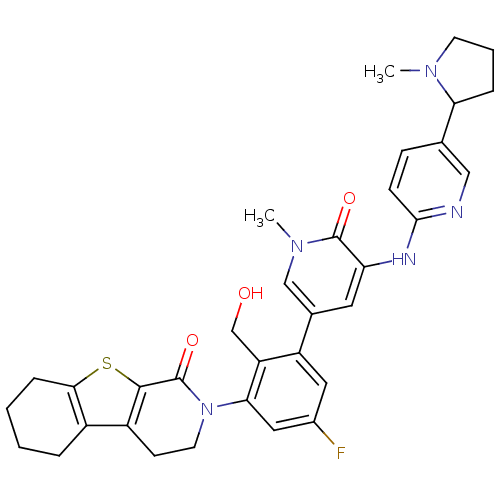 (US8618107, 288) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015449 (CHEMBL3265033) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111964 (US8618107, 276) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015459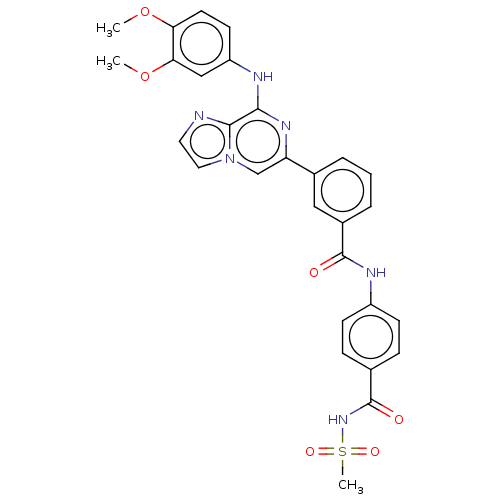 (CHEMBL3265000) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111982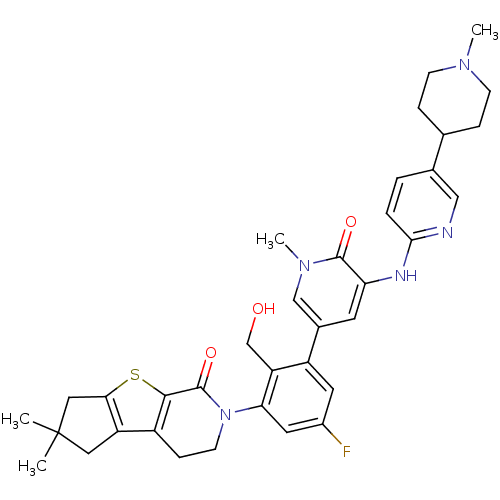 (US8618107, 295) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255583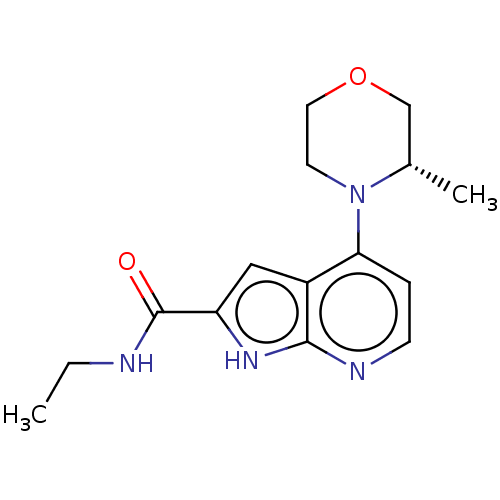 (CHEMBL4096813) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 827 total ) | Next | Last >> |