

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR Biosciences Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase assessed as residual enzyme activity at 50 uM after 1 hr by ELISA | Bioorg Med Chem 19: 4227-37 (2011) Article DOI: 10.1016/j.bmc.2011.05.062 BindingDB Entry DOI: 10.7270/Q29K4F22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396489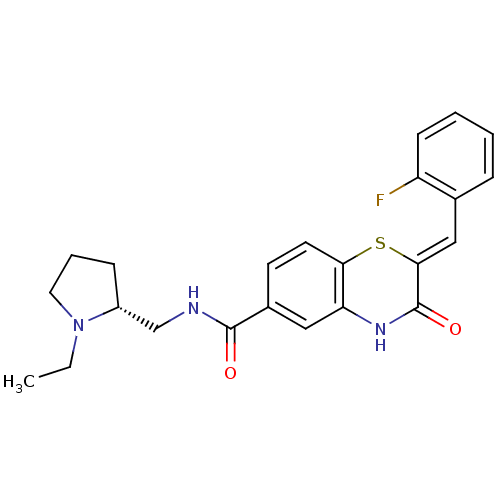 (CHEMBL2170943) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396486 (CHEMBL2171113) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396521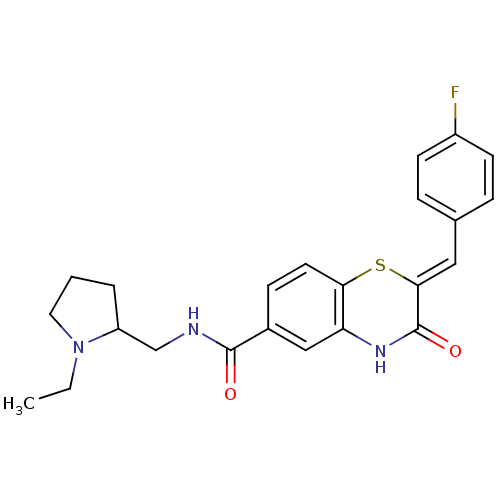 (CHEMBL2170936) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM2195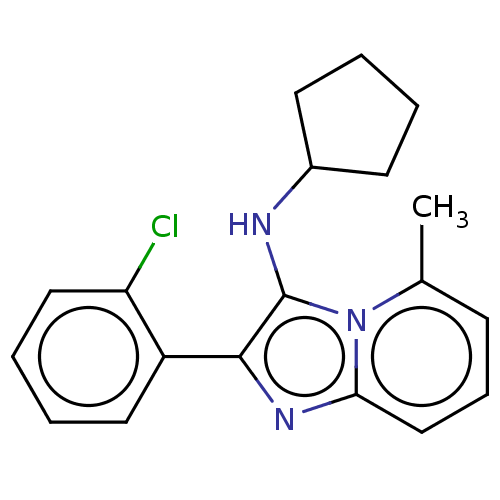 (SM36 | US8501787, 171) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR US Patent | Assay Description The assay is based on a sandwich-ELISA protocol employing the ROCHE colorimetric reverse transcriptase kit (cat # 1468120910). | US Patent US8501767 (2013) BindingDB Entry DOI: 10.7270/Q2Z60MPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM2184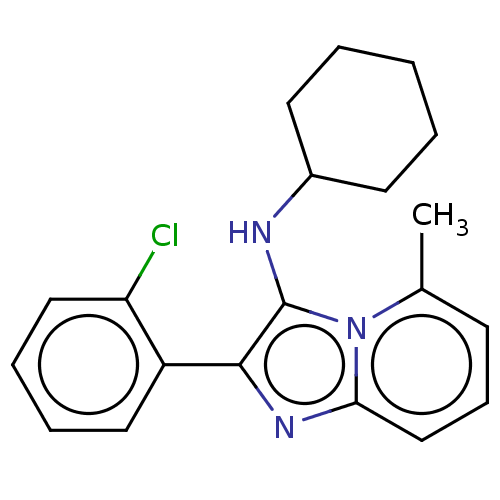 (SM30 | US8501787, 166) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR US Patent | Assay Description The assay is based on a sandwich-ELISA protocol employing the ROCHE colorimetric reverse transcriptase kit (cat # 1468120910). | US Patent US8501767 (2013) BindingDB Entry DOI: 10.7270/Q2Z60MPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2184 (SM30 | US8501787, 166) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR Biosciences Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase assessed as residual enzyme activity at 50 uM after 1 hr by ELISA | Bioorg Med Chem 19: 4227-37 (2011) Article DOI: 10.1016/j.bmc.2011.05.062 BindingDB Entry DOI: 10.7270/Q29K4F22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396519 (CHEMBL2170938) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396486 (CHEMBL2171113) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396489 (CHEMBL2170943) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396521 (CHEMBL2170936) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396522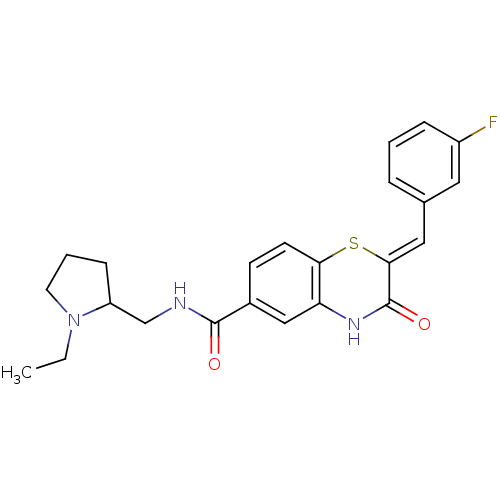 (CHEMBL2170935) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396517 (CHEMBL2170940) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396488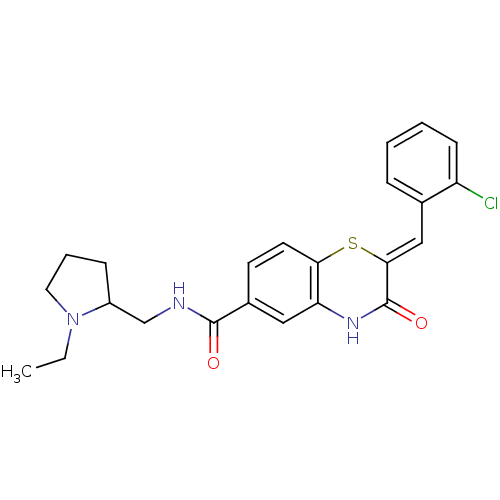 (CHEMBL2170929) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396519 (CHEMBL2170938) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396517 (CHEMBL2170940) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396515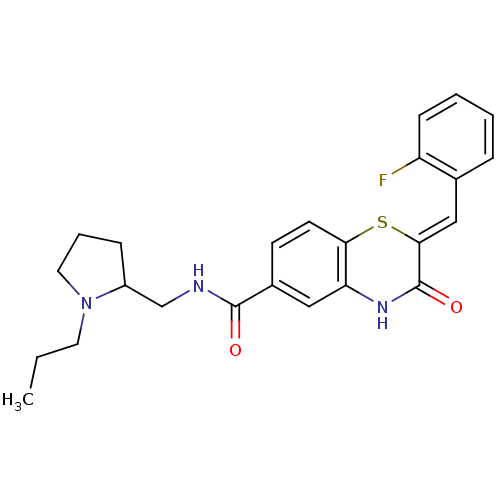 (CHEMBL2170945) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396488 (CHEMBL2170929) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2173 (CW23836 | US8501787, 116) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR Biosciences Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase assessed as residual enzyme activity at 50 uM after 1 hr by ELISA | Bioorg Med Chem 19: 4227-37 (2011) Article DOI: 10.1016/j.bmc.2011.05.062 BindingDB Entry DOI: 10.7270/Q29K4F22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM2173 (CW23836 | US8501787, 116) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR US Patent | Assay Description The assay is based on a sandwich-ELISA protocol employing the ROCHE colorimetric reverse transcriptase kit (cat # 1468120910). | US Patent US8501767 (2013) BindingDB Entry DOI: 10.7270/Q2Z60MPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM2172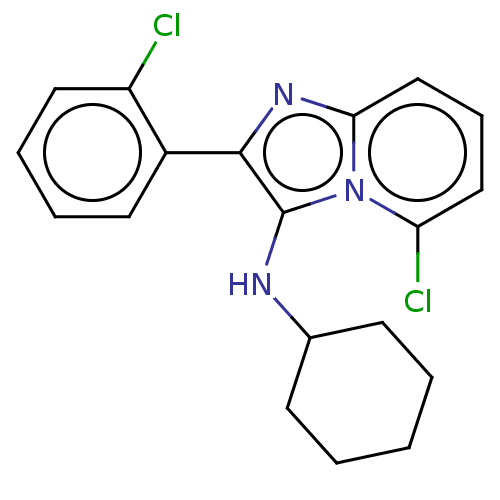 (DG 402-49748 | US8501787, 104) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR US Patent | Assay Description The assay is based on a sandwich-ELISA protocol employing the ROCHE colorimetric reverse transcriptase kit (cat # 1468120910). | US Patent US8501767 (2013) BindingDB Entry DOI: 10.7270/Q2Z60MPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2172 (DG 402-49748 | US8501787, 104) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR Biosciences Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase assessed as residual enzyme activity at 50 uM after 1 hr by ELISA | Bioorg Med Chem 19: 4227-37 (2011) Article DOI: 10.1016/j.bmc.2011.05.062 BindingDB Entry DOI: 10.7270/Q29K4F22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396520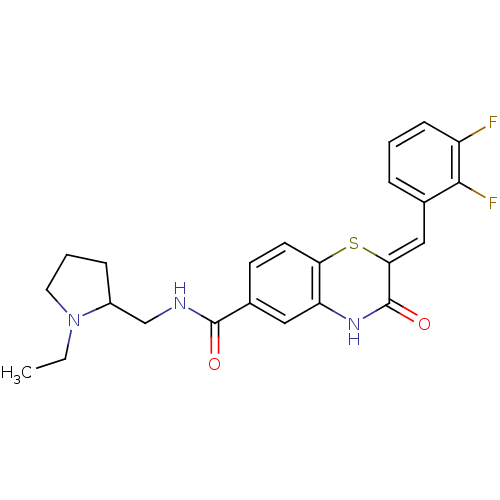 (CHEMBL2170937) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396515 (CHEMBL2170945) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396522 (CHEMBL2170935) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396518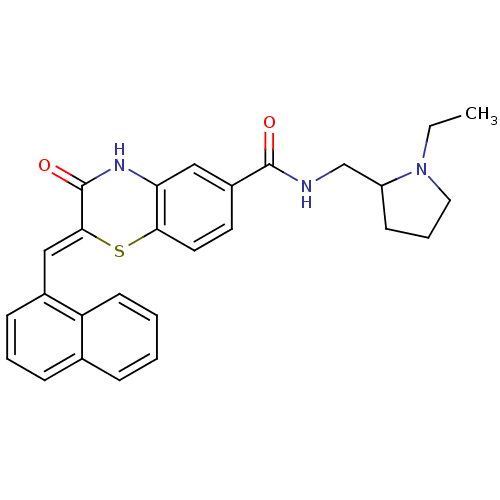 (CHEMBL2170939) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396518 (CHEMBL2170939) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM2115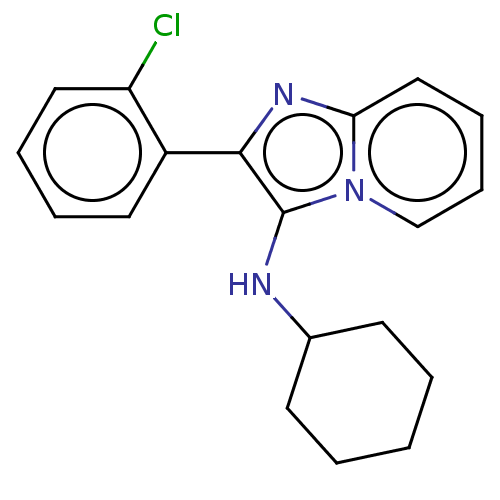 (MLB133-641A | US8501787, 8) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR US Patent | Assay Description The assay is based on a sandwich-ELISA protocol employing the ROCHE colorimetric reverse transcriptase kit (cat # 1468120910). | US Patent US8501767 (2013) BindingDB Entry DOI: 10.7270/Q2Z60MPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2115 (MLB133-641A | US8501787, 8) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR Biosciences Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase assessed as residual enzyme activity at 50 uM after 1 hr by ELISA | Bioorg Med Chem 19: 4227-37 (2011) Article DOI: 10.1016/j.bmc.2011.05.062 BindingDB Entry DOI: 10.7270/Q29K4F22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396487 (CHEMBL2170930) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM2116 (DG 402-49706 | US8501787, 42) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR US Patent | Assay Description The assay is based on a sandwich-ELISA protocol employing the ROCHE colorimetric reverse transcriptase kit (cat # 1468120910). | US Patent US8501767 (2013) BindingDB Entry DOI: 10.7270/Q2Z60MPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2116 (DG 402-49706 | US8501787, 42) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR Biosciences Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase assessed as residual enzyme activity at 50 uM after 1 hr by ELISA | Bioorg Med Chem 19: 4227-37 (2011) Article DOI: 10.1016/j.bmc.2011.05.062 BindingDB Entry DOI: 10.7270/Q29K4F22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396516 (CHEMBL2170944) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396508 (CHEMBL2170923) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396508 (CHEMBL2170923) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396513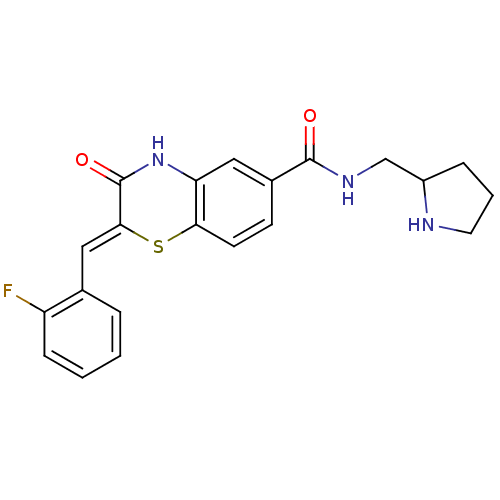 (CHEMBL2171111) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396524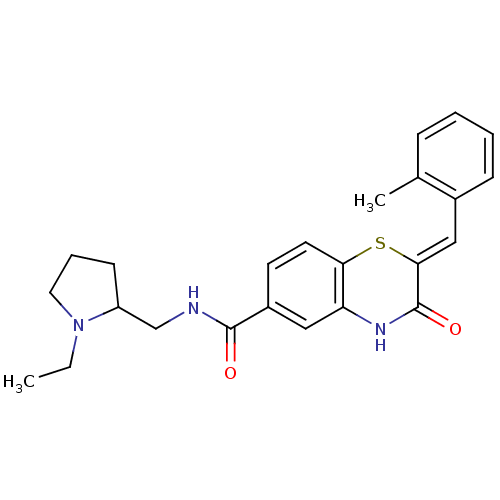 (CHEMBL2170933) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM2126 (DG 402-49760 | US8501787, 48) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR US Patent | Assay Description The assay is based on a sandwich-ELISA protocol employing the ROCHE colorimetric reverse transcriptase kit (cat # 1468120910). | US Patent US8501767 (2013) BindingDB Entry DOI: 10.7270/Q2Z60MPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2177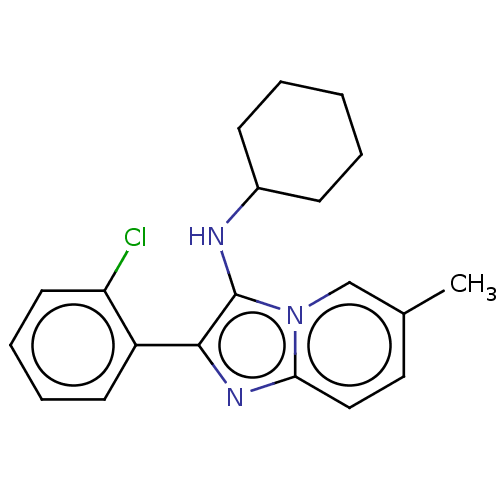 (SM27 | US8501787, 163) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR Biosciences Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase assessed as residual enzyme activity at 50 uM after 1 hr by ELISA | Bioorg Med Chem 19: 4227-37 (2011) Article DOI: 10.1016/j.bmc.2011.05.062 BindingDB Entry DOI: 10.7270/Q29K4F22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM2177 (SM27 | US8501787, 163) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR US Patent | Assay Description The assay is based on a sandwich-ELISA protocol employing the ROCHE colorimetric reverse transcriptase kit (cat # 1468120910). | US Patent US8501767 (2013) BindingDB Entry DOI: 10.7270/Q2Z60MPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396520 (CHEMBL2170937) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396513 (CHEMBL2171111) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396524 (CHEMBL2170933) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396516 (CHEMBL2170944) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM99893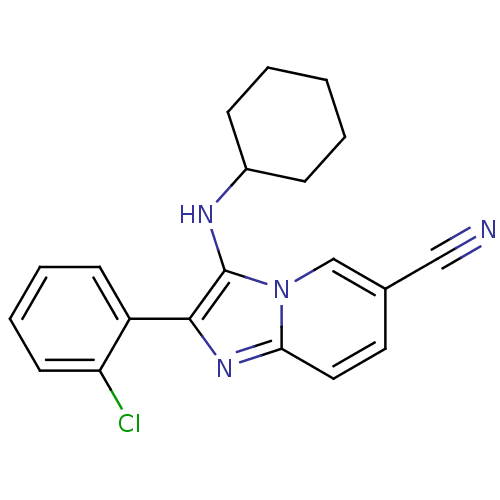 (US8501767, 96) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR Biosciences Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase assessed as residual enzyme activity at 50 uM after 1 hr by ELISA | Bioorg Med Chem 19: 4227-37 (2011) Article DOI: 10.1016/j.bmc.2011.05.062 BindingDB Entry DOI: 10.7270/Q29K4F22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM99893 (US8501767, 96) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR US Patent | Assay Description The assay is based on a sandwich-ELISA protocol employing the ROCHE colorimetric reverse transcriptase kit (cat # 1468120910). | US Patent US8501767 (2013) BindingDB Entry DOI: 10.7270/Q2Z60MPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396508 (CHEMBL2170923) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50538823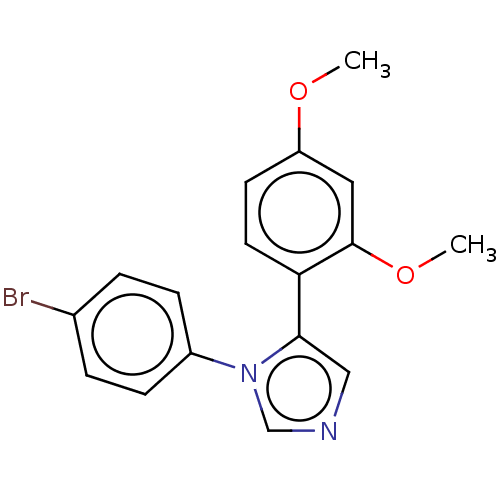 (CHEMBL4649334) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Witwatersrand Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase assessed as inhibition of interaction of HIV-1 IN with LEDGF/p75 (unknown origin) preincubated with enzyme for 30 mins ... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115210 BindingDB Entry DOI: 10.7270/Q2348PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM2182 (SM29 | US8501787, 165) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR US Patent | Assay Description The assay is based on a sandwich-ELISA protocol employing the ROCHE colorimetric reverse transcriptase kit (cat # 1468120910). | US Patent US8501767 (2013) BindingDB Entry DOI: 10.7270/Q2Z60MPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2182 (SM29 | US8501787, 165) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR Biosciences Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase assessed as residual enzyme activity at 50 uM after 1 hr by ELISA | Bioorg Med Chem 19: 4227-37 (2011) Article DOI: 10.1016/j.bmc.2011.05.062 BindingDB Entry DOI: 10.7270/Q29K4F22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 179 total ) | Next | Last >> |