

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180524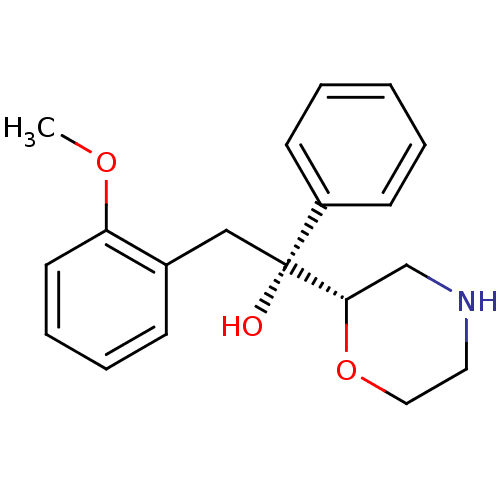 ((R)-2-(2-methoxyphenyl)-1-((S)-morpholin-2-yl)-1-p...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180521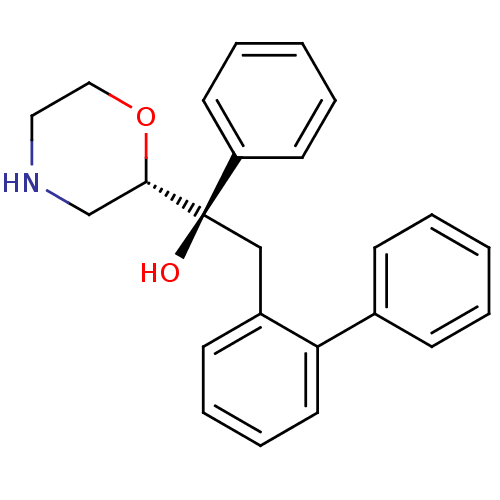 ((R)-2-biphenyl-2-yl-1-(S)-morpholin-2-yl-1-phenyl-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180531 ((R)-2-(2-ethoxyphenyl)-1-((S)-morpholin-2-yl)-1-ph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180526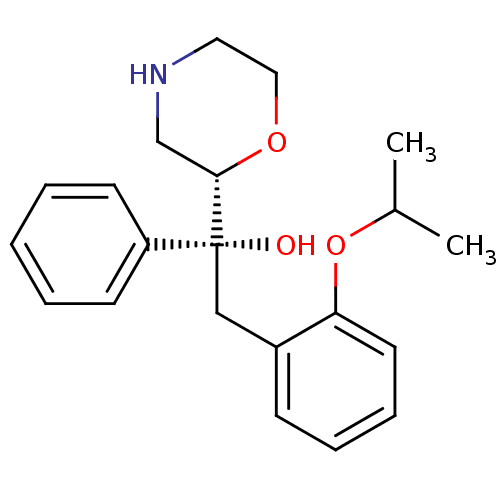 ((R)-2-(2-isopropoxyphenyl)-1-((S)-morpholin-2-yl)-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180526 ((R)-2-(2-isopropoxyphenyl)-1-((S)-morpholin-2-yl)-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180523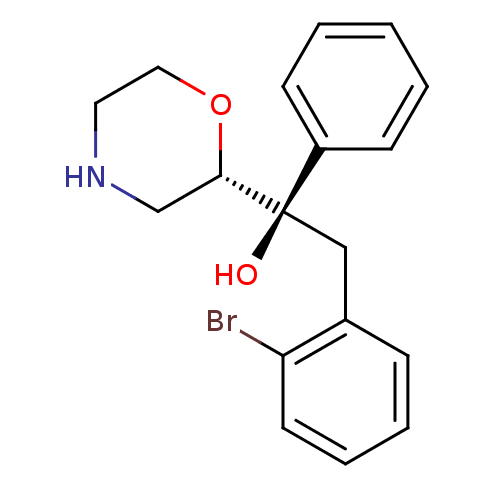 ((R)-2-(2-bromophenyl)-1-((S)-morpholin-2-yl)-1-phe...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180525 ((R)-2-(2-chlorophenyl)-1-((S)-morpholin-2-yl)-1-ph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180522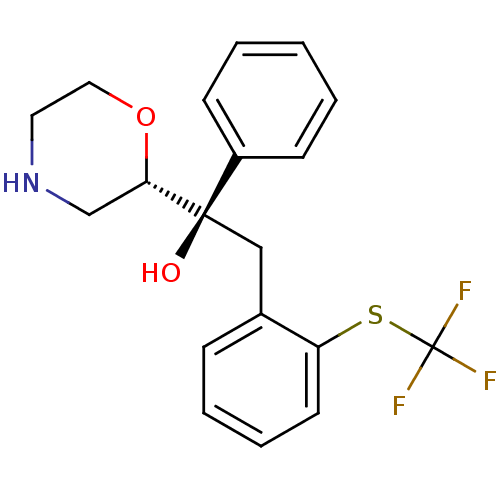 ((R)-1-((S)-morpholin-2-yl)-1-phenyl-2-(2-(trifluor...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 54.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180524 ((R)-2-(2-methoxyphenyl)-1-((S)-morpholin-2-yl)-1-p...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180527 ((R)-1-((S)-morpholin-2-yl)-1,2-diphenylethanol | C...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180524 ((R)-2-(2-methoxyphenyl)-1-((S)-morpholin-2-yl)-1-p...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180524 ((R)-2-(2-methoxyphenyl)-1-((S)-morpholin-2-yl)-1-p...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against hCyp-18 PPIase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50369811 (CHEMBL1790316) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50068939 ((E)-(9S,12S,13R,14S,17R,21S,23S,24R,25S,27S)-17-Et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against hCyp-18 PPIase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50087860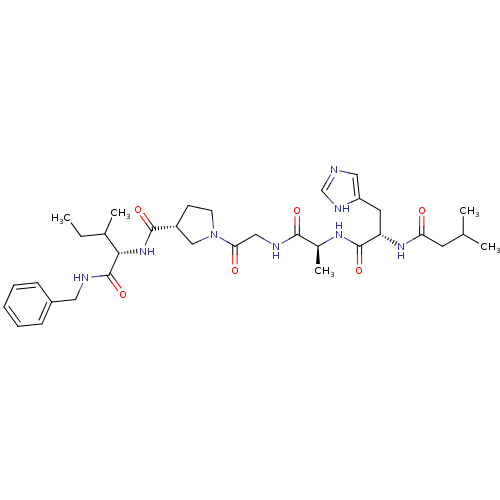 (1-(2-{2-[3-(3H-Imidazol-4-yl)-2-(3-methyl-butyryla...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against hCyp-18 PPIase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50369808 (CHEMBL1793898) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50369806 (CHEMBL1790326) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50369804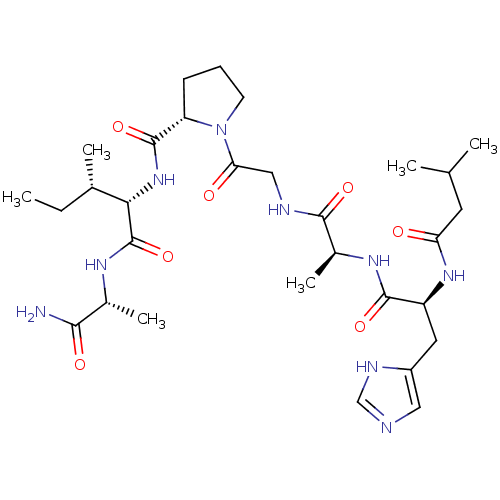 (CHEMBL1790324) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50369803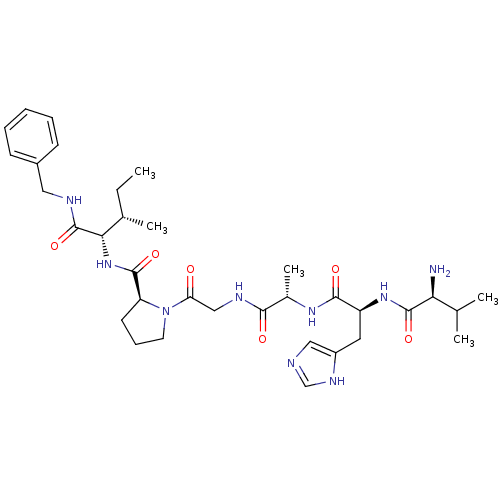 (CHEMBL1790327) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50369816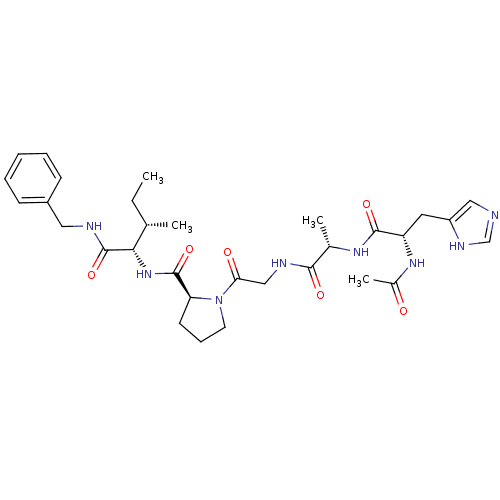 (CHEMBL1790319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50369809 (CHEMBL1790321) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against hCyp-18 PPIase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50369810 (CHEMBL1790320) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against hCyp-18 PPIase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50369805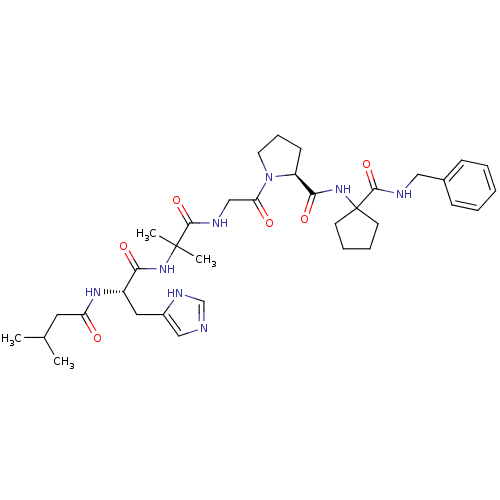 (CHEMBL1793899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50369817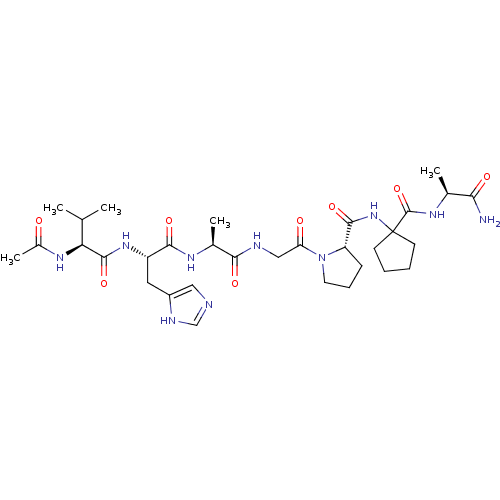 (CHEMBL1793897) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50087868 (1-(2-{2-[2-(2-Acetylamino-3-methyl-butyrylamino)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50087875 (1-(2-{2-[2-(2-Acetylamino-3-methyl-butyrylamino)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50087877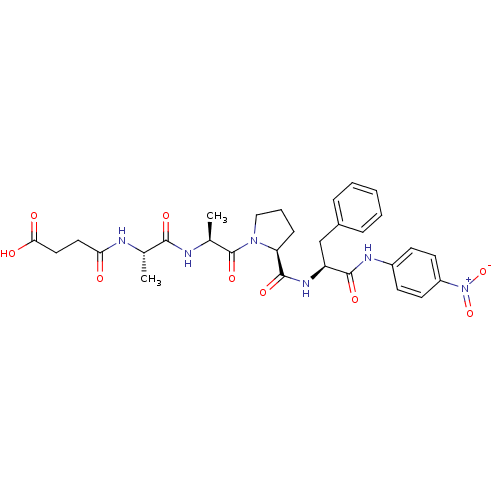 (CHEMBL298712 | N-[1-(1-Methyl-2-{2-[1-(4-nitro-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against hCyp-18 PPIase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50087882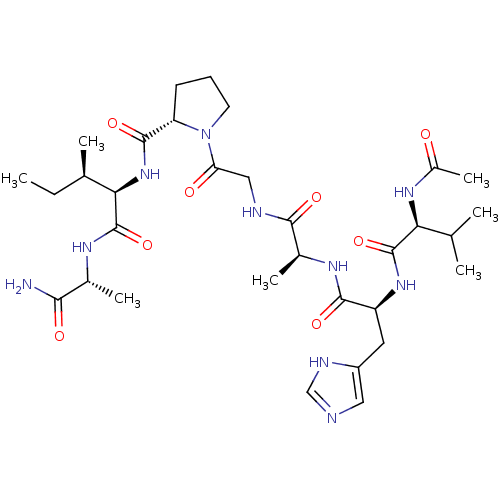 (1-(2-{2-[2-(2-Acetylamino-3-methyl-butyrylamino)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50087855 (1-(2-{2-[2-(2-Acetylamino-3-methyl-butyrylamino)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50369812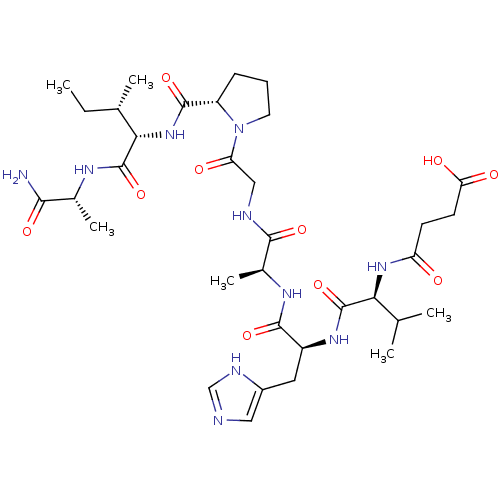 (CHEMBL1790308) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50087854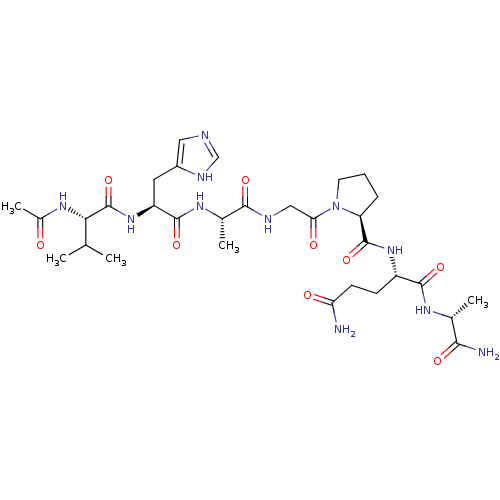 (2-{[1-(2-{2-[2-(2-Acetylamino-3-methyl-butyrylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50369807 (CHEMBL1790325) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50087874 (1-(2-{2-[2-(2-Acetylamino-3-methyl-butyrylamino)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50369814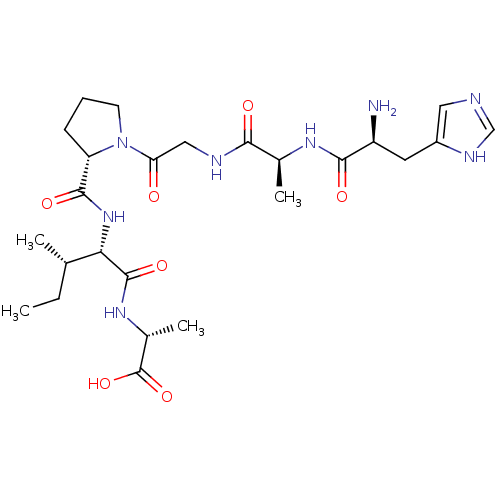 (CHEMBL1790322) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50369813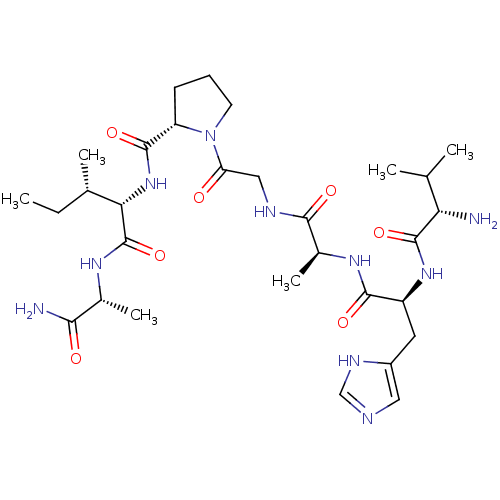 (CHEMBL1790309) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50087872 (2-(2-Acetylamino-3-methyl-butyrylamino)-pentanedio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50369815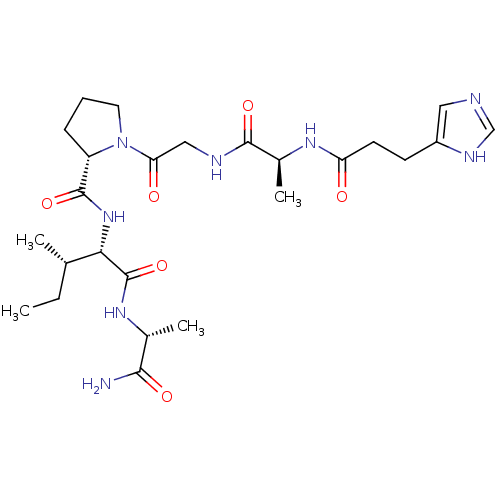 (CHEMBL1790323) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50087866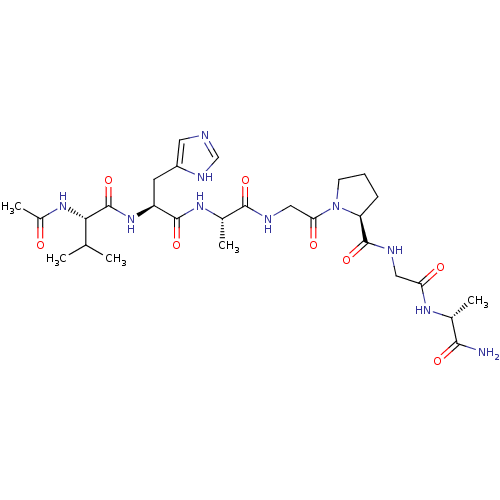 (1-(2-{2-[2-(2-Acetylamino-3-methyl-butyrylamino)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50369818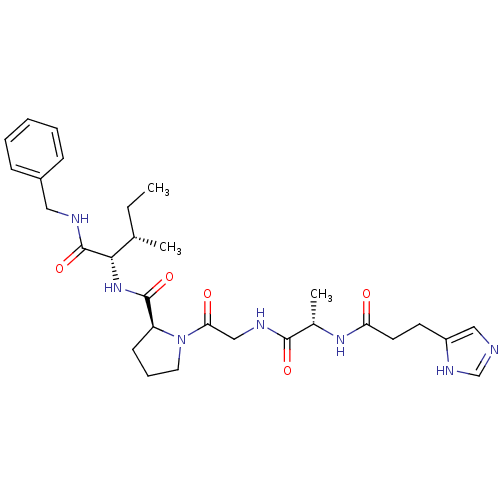 (CHEMBL1790318) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50087873 (1-(2-{2-[2-(2-Acetylamino-3-methyl-butyrylamino)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50087861 (1-(2-{2-[2-(2-Acetylamino-3-methyl-butyrylamino)-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against hCyp-18 PPIase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50087857 (1-(2-{2-[3-(3H-Imidazol-4-yl)-2-(3-methyl-butyryla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50087870 (4-{[1-(2-{2-[2-(2-Acetylamino-3-methyl-butyrylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement D'Ing�nierie et D'Etudes des Prot�ines Curated by ChEMBL | Assay Description 50% inhibitory concentration of competitive binding against human Peptidyl-prolyl isomerase activity using uncoupled assay | J Med Chem 43: 1770-9 (2000) BindingDB Entry DOI: 10.7270/Q2SQ9135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||