 Found 50 hits with Last Name = 'bortolato' and Initial = 'm'
Found 50 hits with Last Name = 'bortolato' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
AP-2 complex subunit sigma
(RAT) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1E
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Rattus norvegicus) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 102: 18620-5 (2005)
Article DOI: 10.1073/pnas.0509591102
BindingDB Entry DOI: 10.7270/Q2F47MQJ |
More data for this
Ligand-Target Pair | |
GABA A receptor alpha-4/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50580362
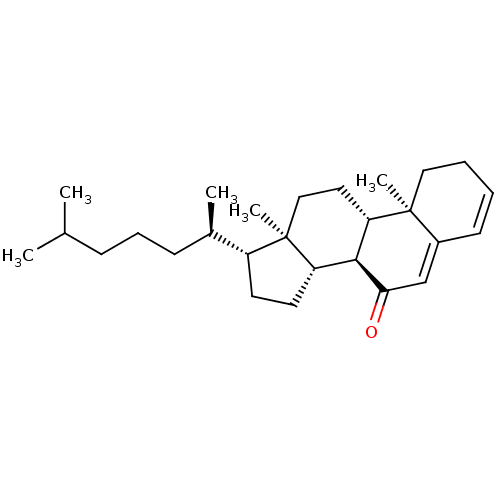
(CHEMBL5086854)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])C(=O)C=C4C=CCC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |r,c:12,t:10| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Negative allosteric modulation activity at human GABAA alpha4beta3gamma2 stably expressed in HEK cells by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00562
BindingDB Entry DOI: 10.7270/Q2TX3K7P |
More data for this
Ligand-Target Pair | |
GABA A receptor alpha-1/beta-1/gamma-2
(Homo sapiens (Human)) | BDBM50580362

(CHEMBL5086854)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])C(=O)C=C4C=CCC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |r,c:12,t:10| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Negative allosteric modulation activity at human GABAA alpha1beta1gamma2 stably expressed in HEK cells by whole cell patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00562
BindingDB Entry DOI: 10.7270/Q2TX3K7P |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50580362

(CHEMBL5086854)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])C(=O)C=C4C=CCC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |r,c:12,t:10| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Negative allosteric modulation activity at human GABAA alpha3beta3gamma2 stably expressed in HEK cells by whole cell patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00562
BindingDB Entry DOI: 10.7270/Q2TX3K7P |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-2/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50580362

(CHEMBL5086854)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])C(=O)C=C4C=CCC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |r,c:12,t:10| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Negative allosteric modulation activity at human GABAA alpha2beta3gamma2 stably expressed in HEK cells by whole cell patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00562
BindingDB Entry DOI: 10.7270/Q2TX3K7P |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50580362

(CHEMBL5086854)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])C(=O)C=C4C=CCC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |r,c:12,t:10| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Negative allosteric modulation activity at human GABAA alpha5beta3gamma2 stably expressed in HEK cells by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00562
BindingDB Entry DOI: 10.7270/Q2TX3K7P |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50580362

(CHEMBL5086854)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])C(=O)C=C4C=CCC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |r,c:12,t:10| | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Negative allosteric modulation activity at human GABAA alpha1beta3gamma2 stably expressed in HEK cells by whole cell patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00562
BindingDB Entry DOI: 10.7270/Q2TX3K7P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data