 Found 140 hits with Last Name = 'boucher' and Initial = 'd'
Found 140 hits with Last Name = 'boucher' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FLT3 by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557859
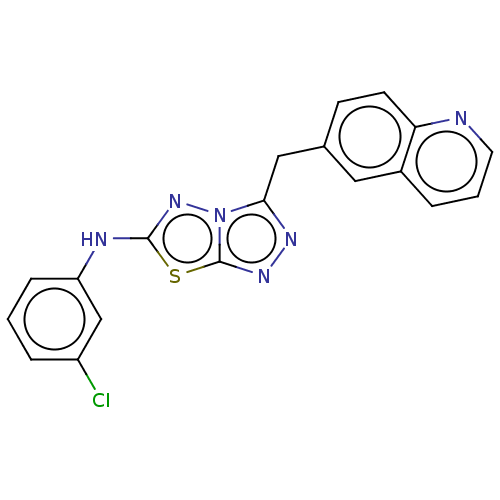
(CHEMBL4784332) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557853

(CHEMBL4753090) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557856
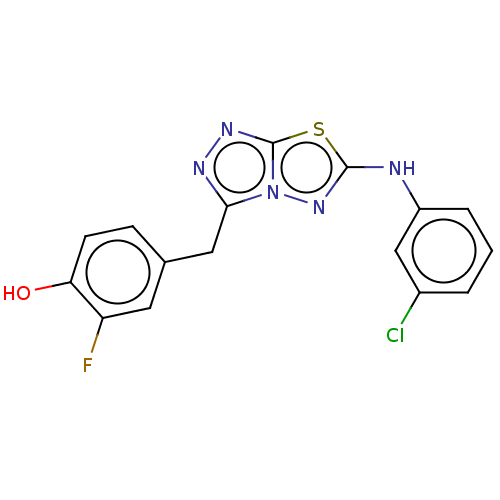
(CHEMBL4790331) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557867

(CHEMBL4783289)Show SMILES C[C@H](c1nnc2sc(nn12)-c1cn(C)nc1C)c1ccc2ncccc2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557864
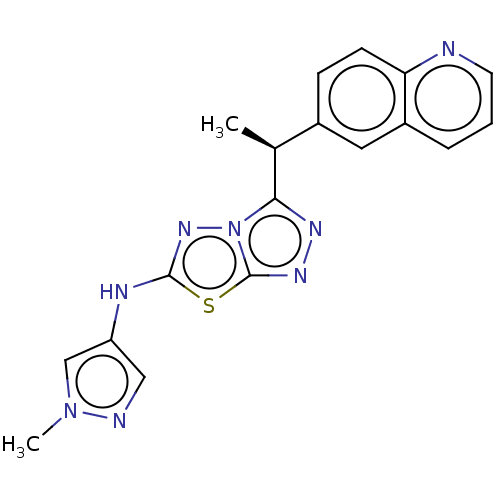
(CHEMBL4787386)Show SMILES C[C@H](c1nnc2sc(Nc3cnn(C)c3)nn12)c1ccc2ncccc2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557863
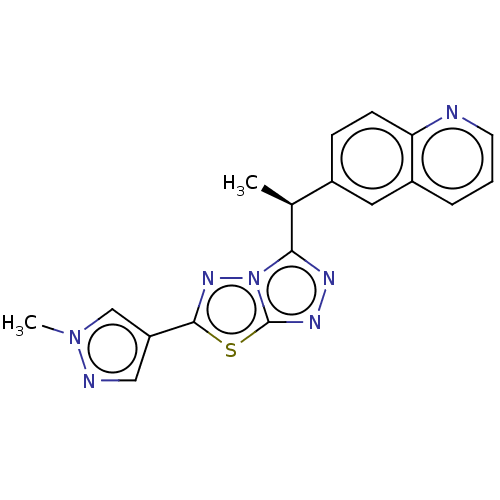
(CHEMBL4791034)Show SMILES C[C@H](c1nnc2sc(nn12)-c1cnn(C)c1)c1ccc2ncccc2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557865
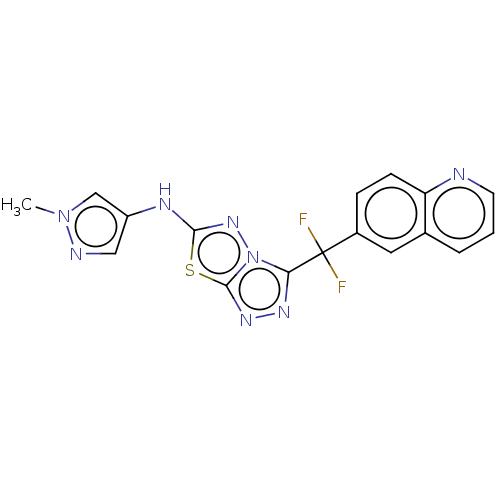
(CHEMBL4778083)Show SMILES Cn1cc(Nc2nn3c(nnc3s2)C(F)(F)c2ccc3ncccc3c2)cn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557866
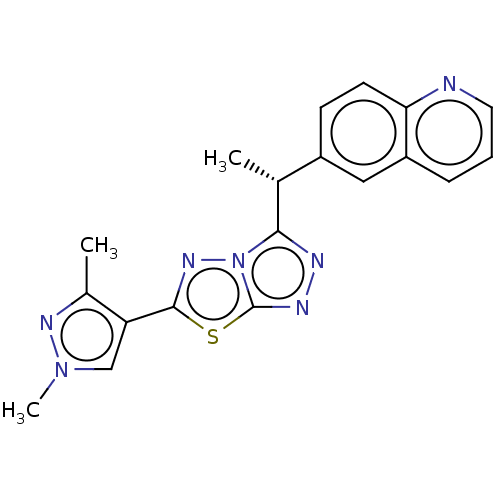
(CHEMBL4750387)Show SMILES C[C@@H](c1nnc2sc(nn12)-c1cn(C)nc1C)c1ccc2ncccc2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557852

(CHEMBL4793526) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of AXL |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557860
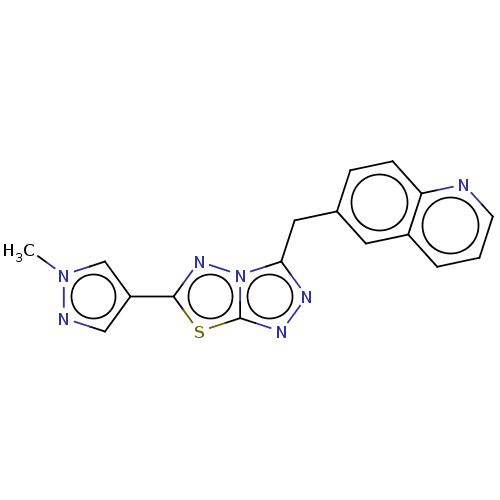
(CHEMBL4760101) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557858
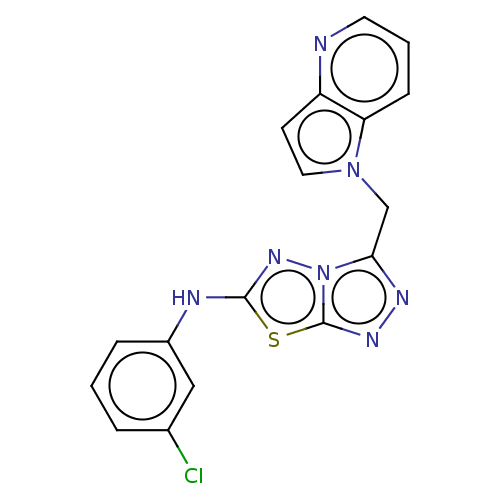
(CHEMBL4786582) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557861
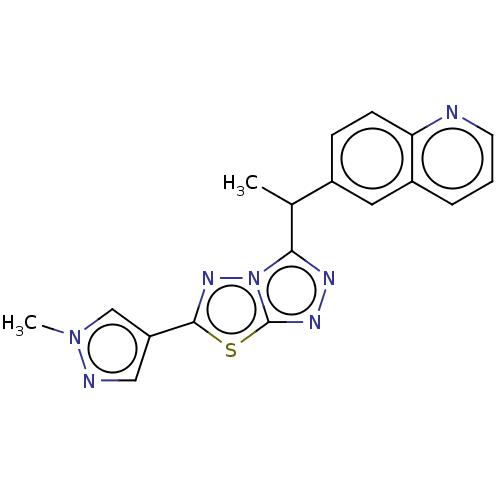
(CHEMBL4762193)Show SMILES CC(c1nnc2sc(nn12)-c1cnn(C)c1)c1ccc2ncccc2c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557857
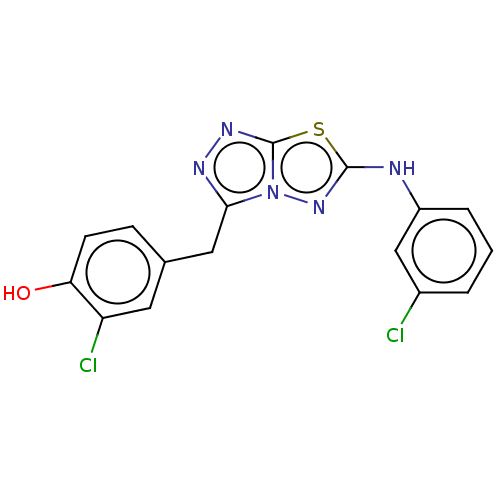
(CHEMBL4743756) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396960

(CHEMBL2170957)Show InChI InChI=1S/C17H12FN5O/c18-13-5-3-12(4-6-13)15-10-19-17-21-20-16(23(17)22-15)9-11-1-7-14(24)8-2-11/h1-8,10,24H,9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557850
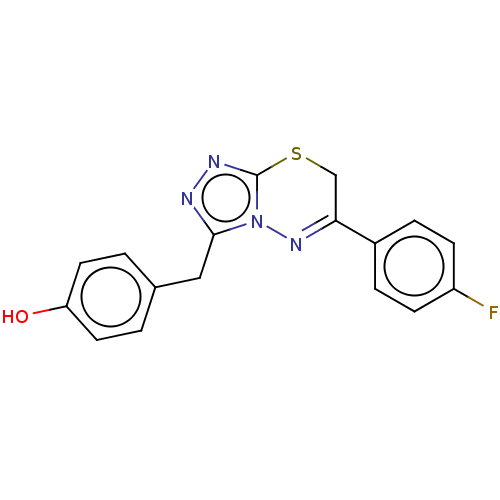
(CHEMBL4748465)Show SMILES Oc1ccc(Cc2nnc3SCC(=Nn23)c2ccc(F)cc2)cc1 |c:12| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557855

(CHEMBL4740644) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of cMET |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557854
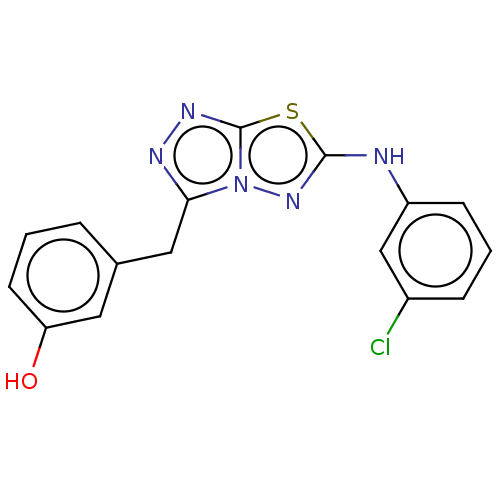
(CHEMBL4740776) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440886
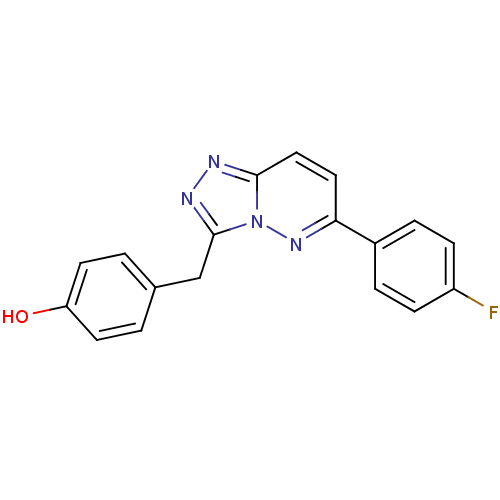
(CHEMBL2431818)Show InChI InChI=1S/C18H13FN4O/c19-14-5-3-13(4-6-14)16-9-10-17-20-21-18(23(17)22-16)11-12-1-7-15(24)8-2-12/h1-10,24H,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of PDGFR-beta |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of Tie2 |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of SYK |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557862
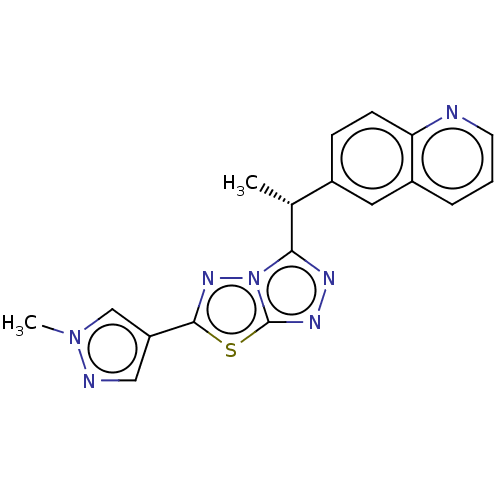
(CHEMBL4763399)Show SMILES C[C@@H](c1nnc2sc(nn12)-c1cnn(C)c1)c1ccc2ncccc2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of Jak3 |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557851

(CHEMBL4764695) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50351673
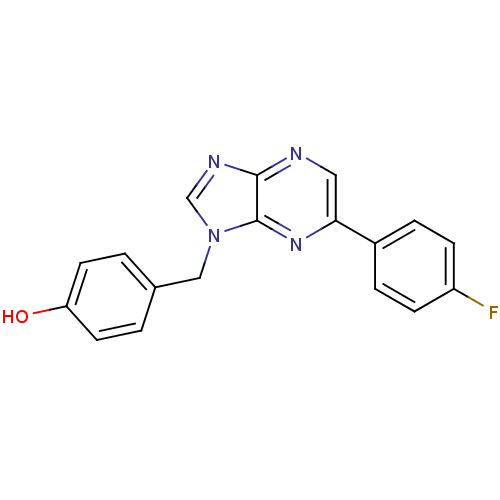
(CHEMBL1822377)Show InChI InChI=1S/C18H13FN4O/c19-14-5-3-13(4-6-14)16-9-20-17-18(22-16)23(11-21-17)10-12-1-7-15(24)8-2-12/h1-9,11,24H,10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of Lyn |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of FGFR3 |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of ABL |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of Src |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50557863

(CHEMBL4791034)Show SMILES C[C@H](c1nnc2sc(nn12)-c1cnn(C)c1)c1ccc2ncccc2c1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FLT3 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50557863

(CHEMBL4791034)Show SMILES C[C@H](c1nnc2sc(nn12)-c1cnn(C)c1)c1ccc2ncccc2c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JAK2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50557863

(CHEMBL4791034)Show SMILES C[C@H](c1nnc2sc(nn12)-c1cnn(C)c1)c1ccc2ncccc2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of KDR (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50557863

(CHEMBL4791034)Show SMILES C[C@H](c1nnc2sc(nn12)-c1cnn(C)c1)c1ccc2ncccc2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SRC (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50557863

(CHEMBL4791034)Show SMILES C[C@H](c1nnc2sc(nn12)-c1cnn(C)c1)c1ccc2ncccc2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SKY (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557867

(CHEMBL4783289)Show SMILES C[C@H](c1nnc2sc(nn12)-c1cn(C)nc1C)c1ccc2ncccc2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-MET in human SNU-5 cells measured after 6 hrs by steady-glo luciferase reporter gene assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355489
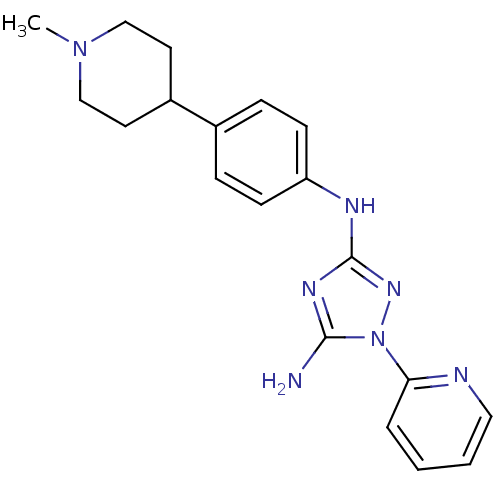
(CHEMBL1835867)Show SMILES CN1CCC(CC1)c1ccc(Nc2nc(N)n(n2)-c2ccccn2)cc1 Show InChI InChI=1S/C19H23N7/c1-25-12-9-15(10-13-25)14-5-7-16(8-6-14)22-19-23-18(20)26(24-19)17-4-2-3-11-21-17/h2-8,11,15H,9-10,12-13H2,1H3,(H3,20,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FLT3 by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557866

(CHEMBL4750387)Show SMILES C[C@@H](c1nnc2sc(nn12)-c1cn(C)nc1C)c1ccc2ncccc2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-MET in human SNU-5 cells measured after 6 hrs by steady-glo luciferase reporter gene assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50355489

(CHEMBL1835867)Show SMILES CN1CCC(CC1)c1ccc(Nc2nc(N)n(n2)-c2ccccn2)cc1 Show InChI InChI=1S/C19H23N7/c1-25-12-9-15(10-13-25)14-5-7-16(8-6-14)22-19-23-18(20)26(24-19)17-4-2-3-11-21-17/h2-8,11,15H,9-10,12-13H2,1H3,(H3,20,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant c-Kit by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of c-KIT-mediated ERK 1/2 phosphorylation in human TF1 cells at 4 to 12 nM after 1 hr by immunoblot analysis |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of SCF-induced phosphorylation of c-KIT in human TF1 cells after 1 hr by immunoblot analysis |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557865

(CHEMBL4778083)Show SMILES Cn1cc(Nc2nn3c(nnc3s2)C(F)(F)c2ccc3ncccc3c2)cn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-MET in human SNU-5 cells measured after 6 hrs by steady-glo luciferase reporter gene assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50557864

(CHEMBL4787386)Show SMILES C[C@H](c1nnc2sc(Nc3cnn(C)c3)nn12)c1ccc2ncccc2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-MET in human SNU-5 cells measured after 6 hrs by steady-glo luciferase reporter gene assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00094
BindingDB Entry DOI: 10.7270/Q28919JG |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant c-Kit by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data