

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Mus musculus) | BDBM50049168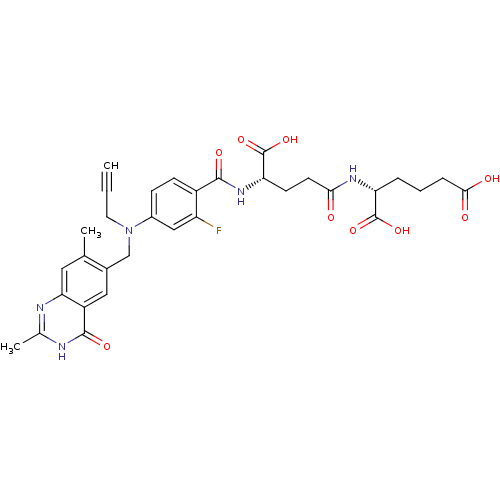 ((R)-2-((S)-4-Carboxy-4-{4-[(2,7-dimethyl-4-oxo-3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity of the compound against L1210 Thymidylate synthase. | J Med Chem 39: 73-85 (1996) Article DOI: 10.1021/jm950471+ BindingDB Entry DOI: 10.7270/Q2WH2P2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50088159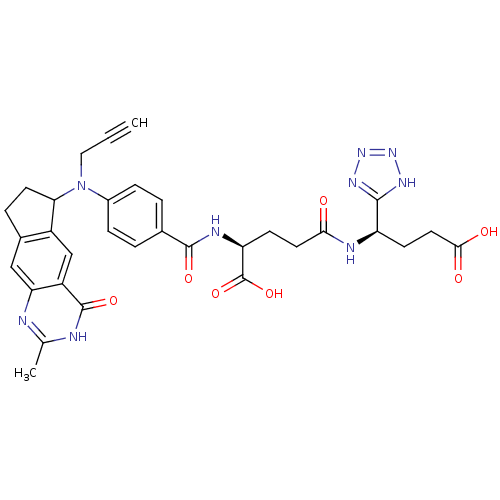 (4-[3-Carboxy-1-(1H-tetrazol-5-yl)-propylcarbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408128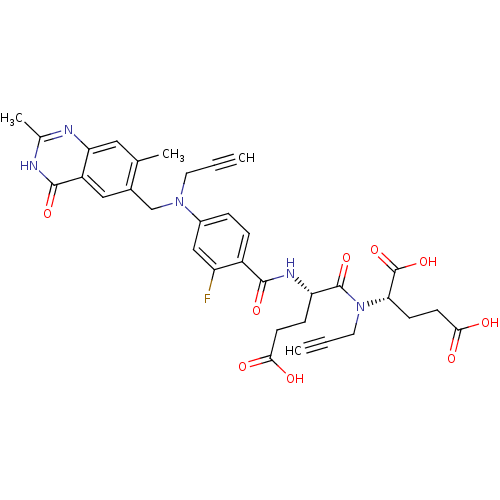 (CHEMBL30179) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50088168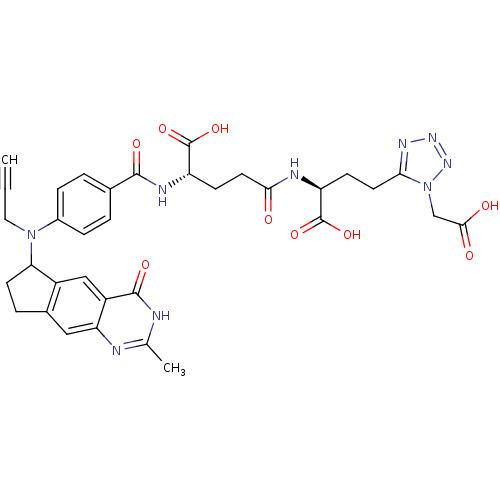 (2-(4-Carboxy-4-{4-[(2-methyl-4-oxo-4,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408130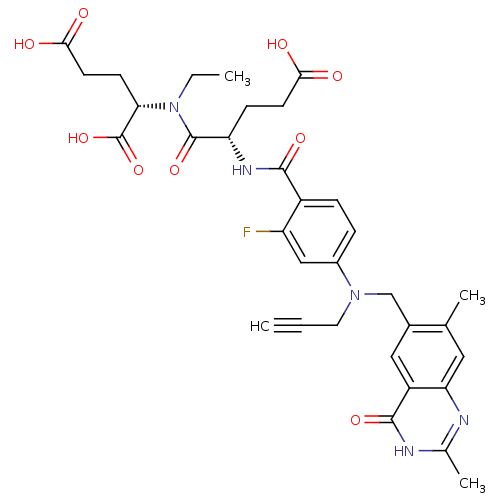 (CHEMBL35926) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50088161 (2-(4-Carboxy-4-{4-[(2-methyl-4-oxo-4,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50088164 ((S)-2-{4-[(2-Methyl-4-oxo-4,6,7,8-tetrahydro-3H-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408876 (CHEMBL434602) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50049159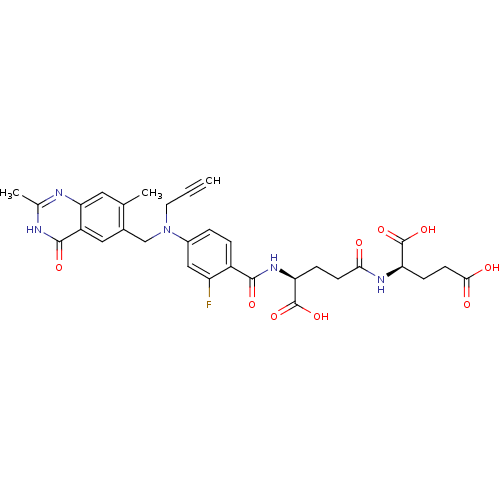 ((R)-2-((S)-4-Carboxy-4-{4-[(2,7-dimethyl-4-oxo-3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity of the compound against L1210 Thymidylate synthase. | J Med Chem 39: 73-85 (1996) Article DOI: 10.1021/jm950471+ BindingDB Entry DOI: 10.7270/Q2WH2P2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408872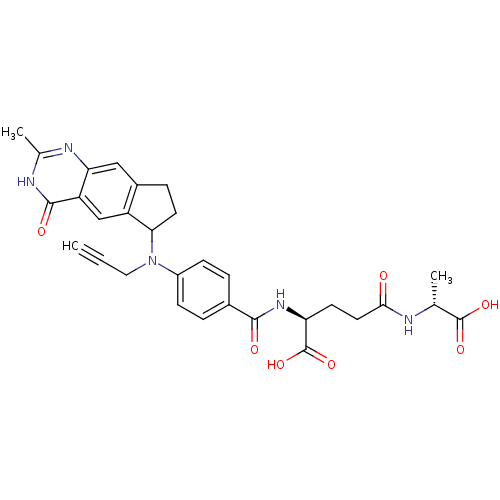 (CHEMBL58548) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408122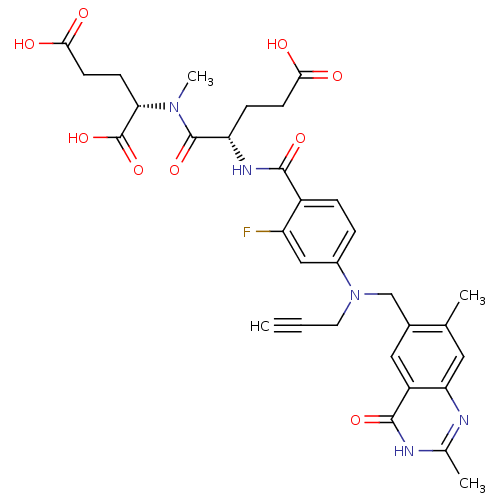 (CHEMBL37106) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408871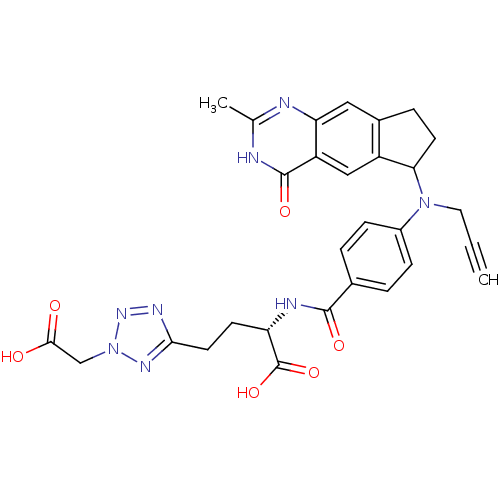 (CHEMBL60700) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50049170 ((R)-2-((S)-4-Carboxy-4-{4-[(2,7-dimethyl-4-oxo-3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408877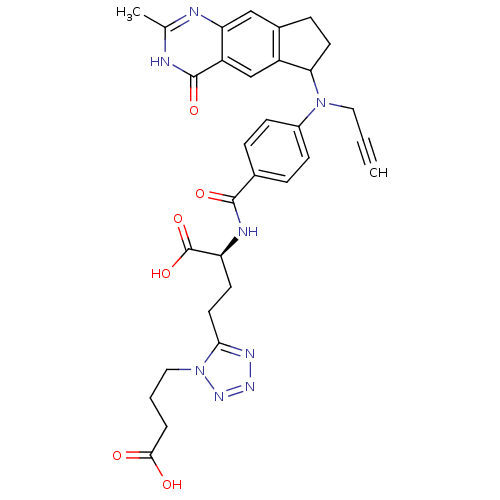 (CHEMBL412127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Compound was tested for the binding affinity against thymidylate synthase from L1210 mouse leukemia cells; value given as 0.9, 2.0 | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408135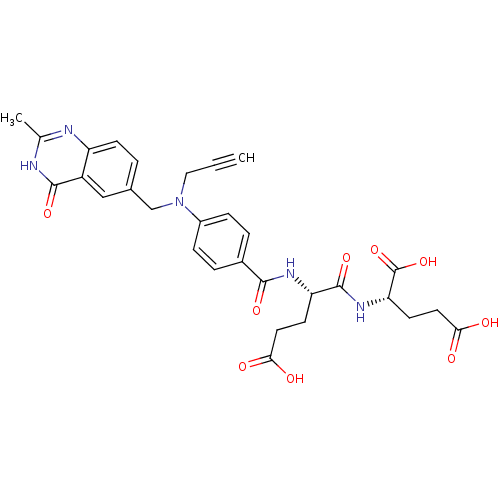 (CHEMBL288666) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408874 (CHEMBL299062) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50088157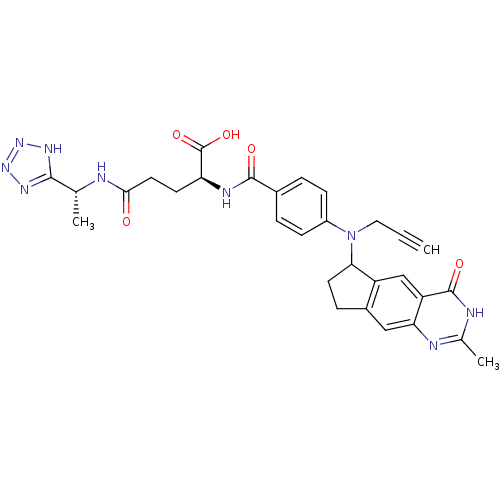 (2-{4-[(2-Methyl-4-oxo-4,6,7,8-tetrahydro-3H-cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50088169 (4-(2-Benzenesulfonylamino-1-methyl-2-oxo-ethylcarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408873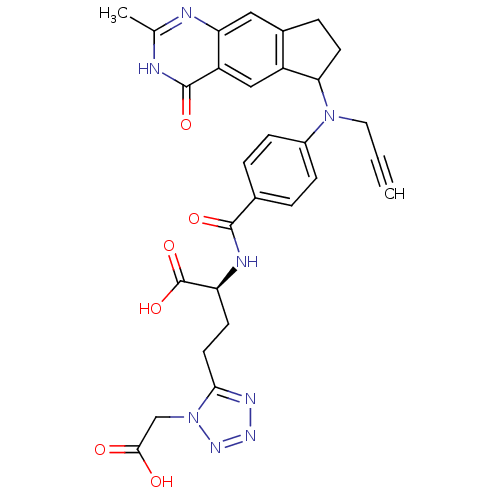 (CHEMBL292920) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Compound was tested for the binding affinity against thymidylate synthase from L1210 mouse leukemia cells; value given as 2.4,1.8 | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408136 (CHEMBL291084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408875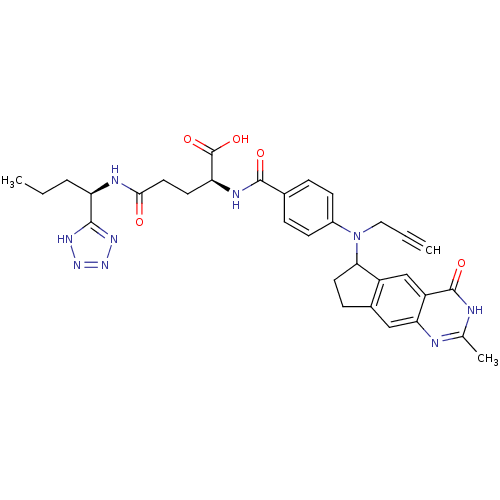 (CHEMBL57549) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408129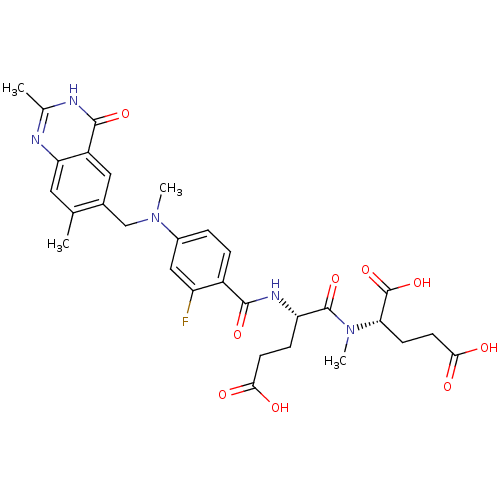 (CHEMBL289749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408127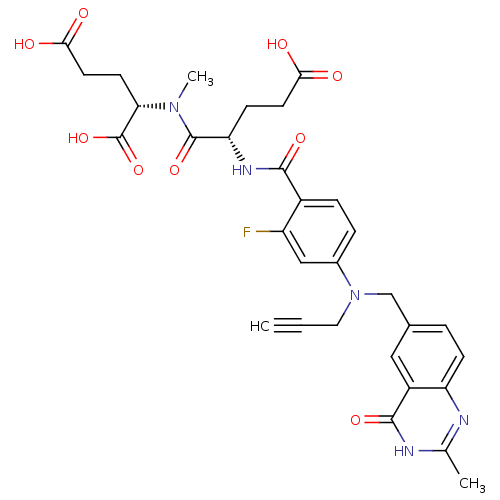 (CHEMBL264609) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408123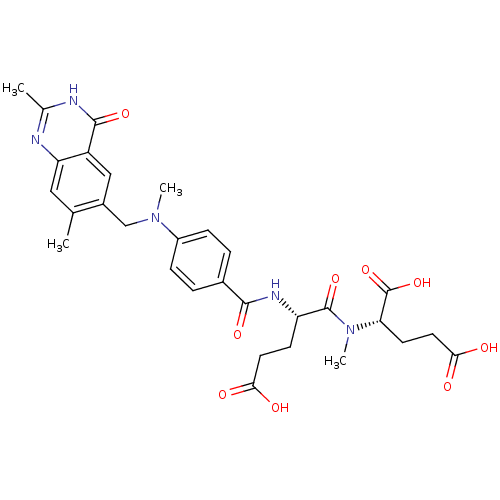 (CHEMBL36433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408126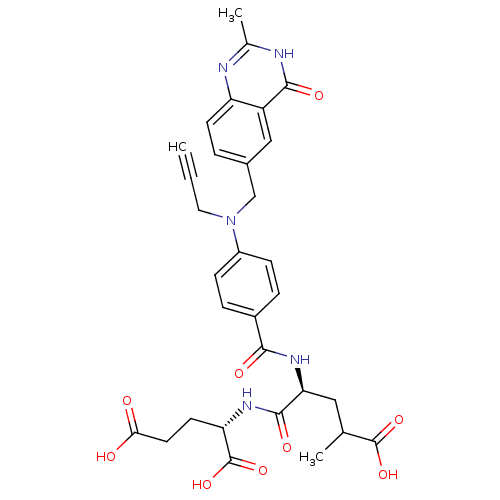 (CHEMBL36721) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408125 (CHEMBL288615) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408133 (CHEMBL287693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408131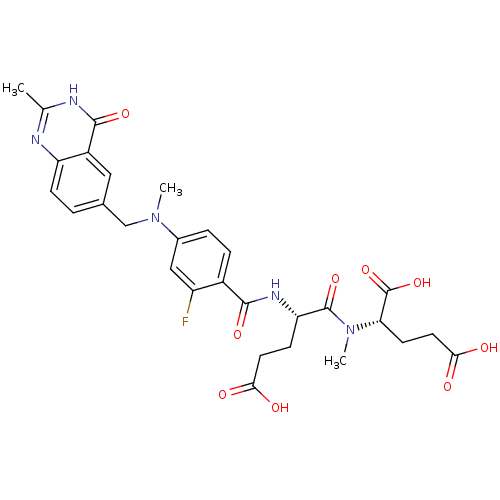 (CHEMBL36426) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408132 (CHEMBL284613) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408124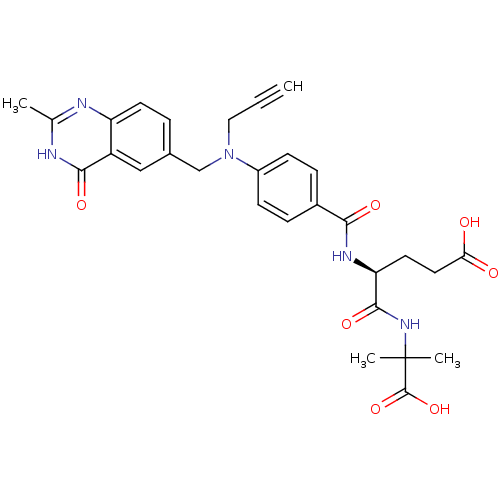 (CHEMBL34857) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408134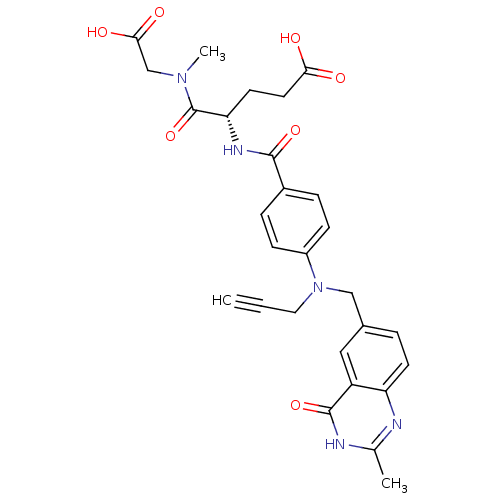 (CHEMBL35941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2 [171-432]/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM5566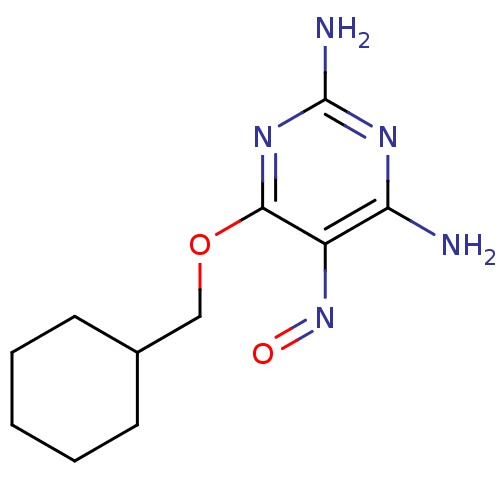 (2,6-Diamino-4-cyclohexylmethoxy-5-nitrosopyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.30E+3 | -34.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Newcastle | Assay Description The enzyme was assayed with substrate histone H1 in the presence of 12.5 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which inhibits ... | J Med Chem 43: 2797-804 (2000) Article DOI: 10.1021/jm990628o BindingDB Entry DOI: 10.7270/Q20R9MKP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 1/G2/mitotic-specific cyclin-B (Marthasterias glacialis (starfish)) | BDBM5566 (2,6-Diamino-4-cyclohexylmethoxy-5-nitrosopyrimidin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.50E+3 | -32.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Newcastle | Assay Description The enzyme was assayed with substrate histone H1 in the presence of 12.5 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which inhibits ... | J Med Chem 43: 2797-804 (2000) Article DOI: 10.1021/jm990628o BindingDB Entry DOI: 10.7270/Q20R9MKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reduced folate transporter (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Affinity towards RFC measured by the inhibition of [3H]-MTX uptake | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 1/G2/mitotic-specific cyclin-B (Marthasterias glacialis (starfish)) | BDBM5485 (6-(cyclohexylmethoxy)-9H-purin-2-amine | CHEMBL269...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.00E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Newcastle | Assay Description The enzyme was assayed with substrate histone H1 in the presence of 12.5 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which inhibits ... | J Med Chem 43: 2797-804 (2000) Article DOI: 10.1021/jm990628o BindingDB Entry DOI: 10.7270/Q20R9MKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50088164 ((S)-2-{4-[(2-Methyl-4-oxo-4,6,7,8-tetrahydro-3H-cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Affinity towards RFC (Reduced folate carrier) measured by the inhibition of [3H]-MTX uptake | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2 [171-432]/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM5485 (6-(cyclohexylmethoxy)-9H-purin-2-amine | CHEMBL269...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.20E+4 | -28.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Newcastle | Assay Description The enzyme was assayed with substrate histone H1 in the presence of 12.5 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which inhibits ... | J Med Chem 43: 2797-804 (2000) Article DOI: 10.1021/jm990628o BindingDB Entry DOI: 10.7270/Q20R9MKP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50088157 (2-{4-[(2-Methyl-4-oxo-4,6,7,8-tetrahydro-3H-cyclop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Affinity towards RFC (Reduced folate carrier) measured by the inhibition of [3H]-MTX uptake | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50088169 (4-(2-Benzenesulfonylamino-1-methyl-2-oxo-ethylcarb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Affinity towards RFC (Reduced folate carrier) measured by the inhibition of [3H]-MTX uptake | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50088168 (2-(4-Carboxy-4-{4-[(2-methyl-4-oxo-4,6,7,8-tetrahy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Affinity towards RFC (Reduced folate carrier) measured by the inhibition of [3H]-MTX uptake | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50088159 (4-[3-Carboxy-1-(1H-tetrazol-5-yl)-propylcarbamoyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Affinity towards RFC (Reduced folate carrier) measured by the inhibition of [3H]-MTX uptake | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50088161 (2-(4-Carboxy-4-{4-[(2-methyl-4-oxo-4,6,7,8-tetrahy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Affinity towards RFC (Reduced folate carrier) measured by the inhibition of [3H]-MTX uptake | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50049170 ((R)-2-((S)-4-Carboxy-4-{4-[(2,7-dimethyl-4-oxo-3,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Affinity towards RFC (Reduced folate carrier) measured by the inhibition of [3H]-MTX uptake | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50288983 ((S)-2-((S)-4-Carboxy-4-{4-[(2,7-dimethyl-4-oxo-3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Thymidylate synthase in experiment 1 | Bioorg Med Chem Lett 6: 631-636 (1996) Article DOI: 10.1016/0960-894X(96)00078-9 BindingDB Entry DOI: 10.7270/Q2XS5VC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50288983 ((S)-2-((S)-4-Carboxy-4-{4-[(2,7-dimethyl-4-oxo-3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Thymidylate synthase in experiment 1 | Bioorg Med Chem Lett 6: 631-636 (1996) Article DOI: 10.1016/0960-894X(96)00078-9 BindingDB Entry DOI: 10.7270/Q2XS5VC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50049159 ((R)-2-((S)-4-Carboxy-4-{4-[(2,7-dimethyl-4-oxo-3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate synthase activity in L1210 cells | J Med Chem 39: 73-85 (1996) Article DOI: 10.1021/jm950471+ BindingDB Entry DOI: 10.7270/Q2WH2P2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081273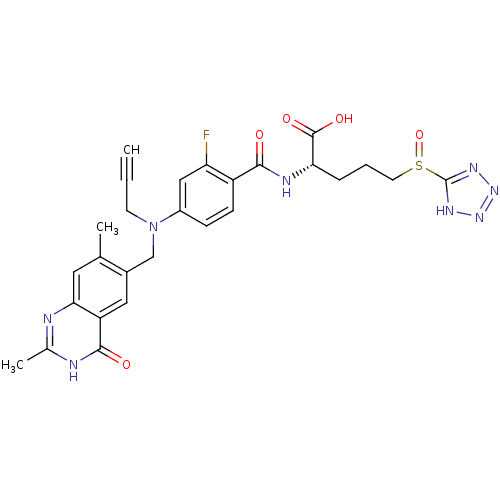 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50049168 ((R)-2-((S)-4-Carboxy-4-{4-[(2,7-dimethyl-4-oxo-3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate synthase activity in L1210 cells | J Med Chem 39: 73-85 (1996) Article DOI: 10.1021/jm950471+ BindingDB Entry DOI: 10.7270/Q2WH2P2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081256 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081259 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 961 total ) | Next | Last >> |