

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248793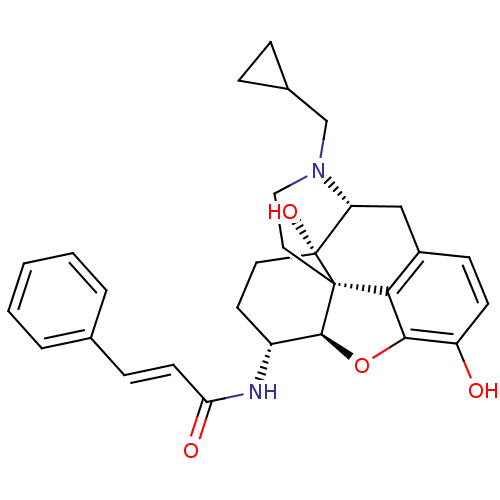 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248825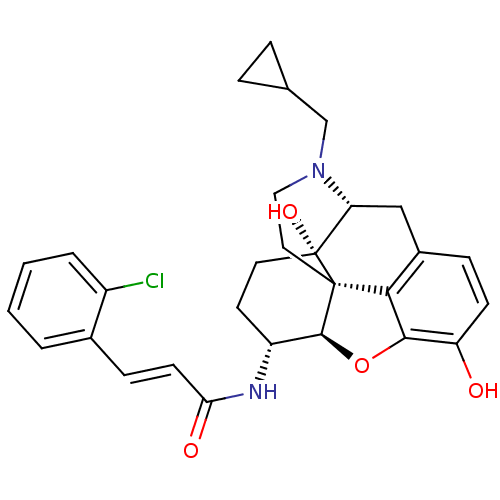 ((2E)-3-(2-chlorophenyl)-N-[(1S,5R,13R,14R,17S)-4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248826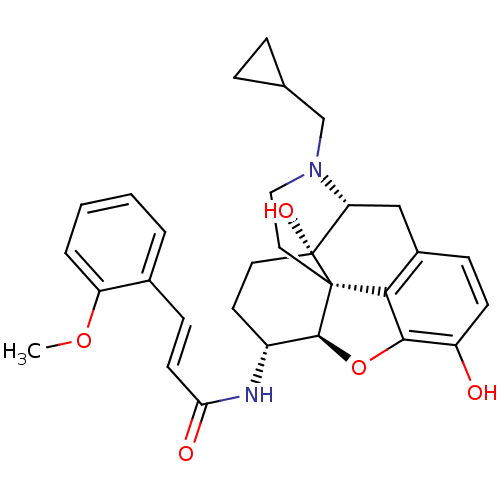 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248795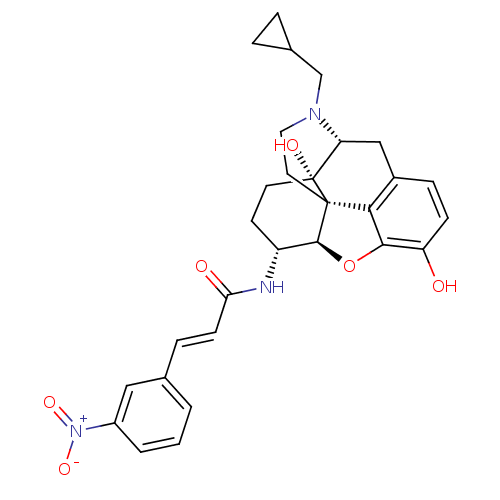 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248828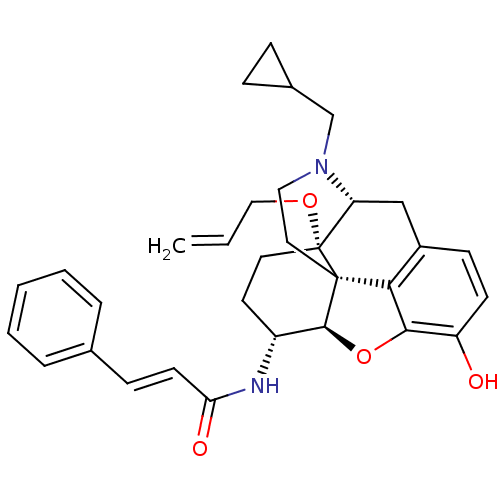 (14beta-Allyloxy-17-cyclopropylmethyl-4,5alpha-epox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248827 ((2E)-N-[(1S,5R,13R,14R,17S)-17-(benzyloxy)-4-(cycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248793 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248855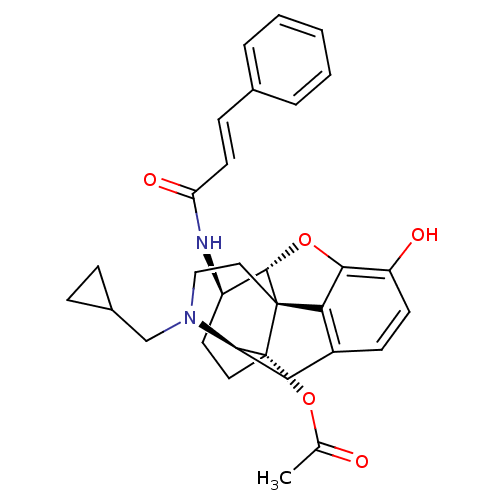 (14beta-Acetoyloxy-17-cyclopropylmethyl-4,5alpha-ep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248828 (14beta-Allyloxy-17-cyclopropylmethyl-4,5alpha-epox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248857 ((1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-10-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248825 ((2E)-3-(2-chlorophenyl)-N-[(1S,5R,13R,14R,17S)-4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248857 ((1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-10-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248794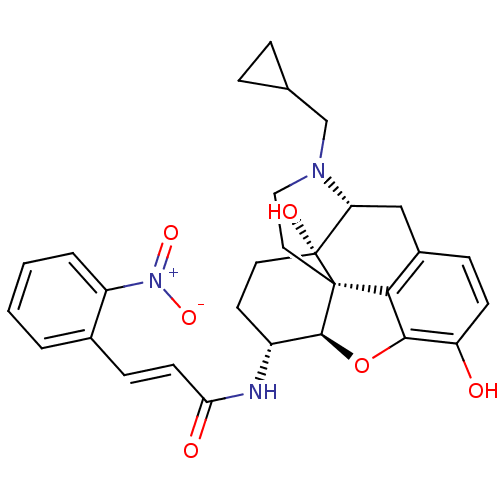 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248826 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248794 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248795 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248856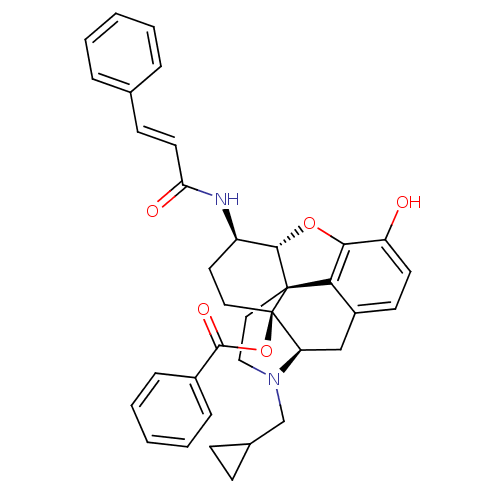 ((1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-10-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248855 (14beta-Acetoyloxy-17-cyclopropylmethyl-4,5alpha-ep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248827 ((2E)-N-[(1S,5R,13R,14R,17S)-17-(benzyloxy)-4-(cycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248856 ((1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-10-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membrane by liquid scintillation and luminescence counter | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248828 (14beta-Allyloxy-17-cyclopropylmethyl-4,5alpha-epox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248855 (14beta-Acetoyloxy-17-cyclopropylmethyl-4,5alpha-ep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248857 ((1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-10-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248793 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248794 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248795 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248825 ((2E)-3-(2-chlorophenyl)-N-[(1S,5R,13R,14R,17S)-4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248826 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248827 ((2E)-N-[(1S,5R,13R,14R,17S)-17-(benzyloxy)-4-(cycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248828 (14beta-Allyloxy-17-cyclopropylmethyl-4,5alpha-epox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248855 (14beta-Acetoyloxy-17-cyclopropylmethyl-4,5alpha-ep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248856 ((1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-10-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50248857 ((1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-10-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248827 ((2E)-N-[(1S,5R,13R,14R,17S)-17-(benzyloxy)-4-(cycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248793 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248794 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248826 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248825 ((2E)-3-(2-chlorophenyl)-N-[(1S,5R,13R,14R,17S)-4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248795 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||