 Found 482 hits with Last Name = 'brock' and Initial = 'a'
Found 482 hits with Last Name = 'brock' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537072

(CHEMBL440072)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N Show InChI InChI=1S/C63H88N16O16S2/c1-34(66)53(84)69-30-51(83)70-48-32-96-97-33-49(63(94)95)78-60(91)47(31-80)77-62(93)52(35(2)81)79-55(86)42(22-12-14-24-65)71-58(89)45(27-38-29-68-40-20-10-9-19-39(38)40)75-57(88)44(26-37-17-7-4-8-18-37)73-56(87)43(25-36-15-5-3-6-16-36)74-59(90)46(28-50(67)82)76-54(85)41(72-61(48)92)21-11-13-23-64/h3-10,15-20,29,34-35,41-49,52,68,80-81H,11-14,21-28,30-33,64-66H2,1-2H3,(H2,67,82)(H,69,84)(H,70,83)(H,71,89)(H,72,92)(H,73,87)(H,74,90)(H,75,88)(H,76,85)(H,77,93)(H,78,91)(H,79,86)(H,94,95)/t34-,35+,41+,42-,43+,44-,45+,46-,47-,48+,49-,52+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537063

(CHEMBL4590517)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(O)=O |r,t:17,19| Show InChI InChI=1S/C83H108ClN13O21S4/c1-44-18-17-24-65(115-9)83(113)39-64(116-81(112)95-83)45(2)71-82(5,118-71)66(38-68(101)97(7)62-35-50(32-44)36-63(114-8)69(62)84)117-80(111)46(3)96(6)67(100)29-31-119-120-43-61(79(109)110)93-77(107)60-42-122-121-41-59(91-72(102)54(86)33-48-19-11-10-12-20-48)76(106)89-57(34-49-25-27-52(99)28-26-49)74(104)90-58(37-51-40-87-55-22-14-13-21-53(51)55)75(105)88-56(23-15-16-30-85)73(103)94-70(47(4)98)78(108)92-60/h10-14,17-22,24-28,35-36,40,45-47,54,56-61,64-66,70-71,87,98-99,113H,15-16,23,29-34,37-39,41-43,85-86H2,1-9H3,(H,88,105)(H,89,106)(H,90,104)(H,91,102)(H,92,108)(H,93,107)(H,94,103)(H,95,112)(H,109,110)/b24-17+,44-18+/t45-,46+,47-,54-,56+,57+,58-,59+,60+,61+,64+,65-,66+,70+,71+,82-,83+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537077

(CHEMBL4550617)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCOc1ccc(C[C@@H](N)C(=O)N[C@H]2CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](Cc3ccc(O)cc3)NC2=O)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)cc1 |r,t:17,19| Show InChI InChI=1S/C86H114ClN13O23S4/c1-45-16-15-20-67(119-10)86(117)41-66(121-84(116)98-86)46(2)74-85(6,123-74)68(40-70(105)100(8)64-37-52(34-45)38-65(118-9)71(64)87)122-83(115)47(3)99(7)69(104)29-32-124-125-33-31-120-55-27-23-50(24-28-55)35-57(89)75(106)94-62-43-126-127-44-63(80(111)97-73(49(5)102)82(113)114)95-81(112)72(48(4)101)96-76(107)59(19-13-14-30-88)91-78(109)61(39-53-42-90-58-18-12-11-17-56(53)58)93-77(108)60(92-79(62)110)36-51-21-25-54(103)26-22-51/h11-12,15-18,20-28,37-38,42,46-49,57,59-63,66-68,72-74,90,101-103,117H,13-14,19,29-36,39-41,43-44,88-89H2,1-10H3,(H,91,109)(H,92,110)(H,93,108)(H,94,106)(H,95,112)(H,96,107)(H,97,111)(H,98,116)(H,113,114)/b20-15+,45-16+/t46-,47+,48-,49-,57-,59+,60+,61-,62+,63+,66+,67-,68+,72+,73+,74+,85-,86+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537069
(CHEMBL4584764)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC(C)(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O |r,t:17,19| Show InChI InChI=1S/C85H113ClN14O20S4/c1-45-20-19-26-65(117-11)85(115)41-64(118-82(114)98-85)46(2)72-84(7,120-72)66(40-68(104)100(9)62-37-51(34-45)38-63(116-10)69(62)86)119-81(113)47(3)99(8)67(103)31-33-121-124-83(5,6)71(73(89)105)97-79(111)61-44-123-122-43-60(94-74(106)55(88)35-49-21-13-12-14-22-49)78(110)92-58(36-50-27-29-53(102)30-28-50)76(108)93-59(39-52-42-90-56-24-16-15-23-54(52)56)77(109)91-57(25-17-18-32-87)75(107)96-70(48(4)101)80(112)95-61/h12-16,19-24,26-30,37-38,42,46-48,55,57-61,64-66,70-72,90,101-102,115H,17-18,25,31-36,39-41,43-44,87-88H2,1-11H3,(H2,89,105)(H,91,109)(H,92,110)(H,93,108)(H,94,106)(H,95,112)(H,96,107)(H,97,111)(H,98,114)/b26-19+,45-20+/t46-,47+,48-,55-,57+,58+,59-,60+,61+,64+,65-,66+,70+,71-,72+,84-,85+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537066

(CHEMBL4541310)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCCN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O |r,t:17,19| Show InChI InChI=1S/C87H116ClN13O22S4/c1-47-20-18-26-68(120-10)87(118)43-67(121-85(117)99-87)48(2)75-86(6,123-75)69(42-71(106)101(8)65-39-54(36-47)40-66(119-9)72(65)88)122-84(116)49(3)100(7)70(105)31-35-125-124-34-19-33-90-60(37-52-21-12-11-13-22-52)77(108)95-63-45-126-127-46-64(81(112)98-74(51(5)103)83(114)115)96-82(113)73(50(4)102)97-76(107)59(25-16-17-32-89)92-79(110)62(41-55-44-91-58-24-15-14-23-57(55)58)94-78(109)61(93-80(63)111)38-53-27-29-56(104)30-28-53/h11-15,18,20-24,26-30,39-40,44,48-51,59-64,67-69,73-75,90-91,102-104,118H,16-17,19,25,31-38,41-43,45-46,89H2,1-10H3,(H,92,110)(H,93,111)(H,94,109)(H,95,108)(H,96,113)(H,97,107)(H,98,112)(H,99,117)(H,114,115)/b26-18+,47-20+/t48-,49+,50-,51-,59+,60-,61+,62-,63+,64+,67+,68-,69+,73+,74+,75+,86-,87+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537076

(CHEMBL4564727)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC[C@@H](CO)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1 |r,t:17,19| Show InChI InChI=1S/C83H110ClN13O20S4/c1-45-18-17-24-66(114-9)83(112)39-65(115-81(111)95-83)46(2)72-82(5,117-72)67(38-69(102)97(7)63-35-51(32-45)36-64(113-8)70(63)84)116-80(110)47(3)96(6)68(101)29-31-118-119-42-53(41-98)88-77(107)61-43-120-121-44-62(92-73(103)56(86)33-49-19-11-10-12-20-49)78(108)90-59(34-50-25-27-54(100)28-26-50)75(105)91-60(37-52-40-87-57-22-14-13-21-55(52)57)76(106)89-58(23-15-16-30-85)74(104)94-71(48(4)99)79(109)93-61/h10-14,17-22,24-28,35-36,40,46-48,53,56,58-62,65-67,71-72,87,98-100,112H,15-16,23,29-34,37-39,41-44,85-86H2,1-9H3,(H,88,107)(H,89,106)(H,90,108)(H,91,105)(H,92,103)(H,93,109)(H,94,104)(H,95,111)/b24-17+,45-18+/t46-,47+,48-,53-,56-,58+,59+,60-,61+,62+,65+,66-,67+,71+,72+,82-,83+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537061
(CHEMBL4527856)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O |r,t:17,19| Show InChI InChI=1S/C83H109ClN14O20S4/c1-44-18-17-24-65(115-9)83(113)39-64(116-81(112)96-83)45(2)71-82(5,118-71)66(38-68(102)98(7)62-35-50(32-44)36-63(114-8)69(62)84)117-80(111)46(3)97(6)67(101)29-31-119-120-41-59(72(87)103)92-78(109)61-43-122-121-42-60(93-73(104)54(86)33-48-19-11-10-12-20-48)77(108)90-57(34-49-25-27-52(100)28-26-49)75(106)91-58(37-51-40-88-55-22-14-13-21-53(51)55)76(107)89-56(23-15-16-30-85)74(105)95-70(47(4)99)79(110)94-61/h10-14,17-22,24-28,35-36,40,45-47,54,56-61,64-66,70-71,88,99-100,113H,15-16,23,29-34,37-39,41-43,85-86H2,1-9H3,(H2,87,103)(H,89,107)(H,90,108)(H,91,106)(H,92,109)(H,93,104)(H,94,110)(H,95,105)(H,96,112)/b24-17+,44-18+/t45-,46+,47-,54-,56+,57+,58-,59+,60+,61+,64+,65-,66+,70+,71+,82-,83+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50051007
((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...)Show SMILES CCCCN(CCCC)C(=O)CN1C[C@@H]([C@H]([C@@H]1c1ccc(OC)cc1)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O6/c1-4-6-14-30(15-7-5-2)26(32)18-31-17-23(21-10-13-24-25(16-21)37-19-36-24)27(29(33)34)28(31)20-8-11-22(35-3)12-9-20/h8-13,16,23,27-28H,4-7,14-15,17-19H2,1-3H3,(H,33,34)/t23-,27-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards Endothelin A receptor |
J Med Chem 47: 1969-86 (2004)
Article DOI: 10.1021/jm030528p
BindingDB Entry DOI: 10.7270/Q2HD7WD6 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50143784
((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-butyl-7-[2-((...)Show SMILES CCCCc1ccc2[C@@H]([C@H]([C@@H](c2n1)c1ccc(OC)cc1C[C@H](C)C(O)=O)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C31H33NO7/c1-4-5-6-20-8-10-23-26(18-7-12-24-25(15-18)39-16-38-24)28(31(35)36)27(29(23)32-20)22-11-9-21(37-3)14-19(22)13-17(2)30(33)34/h7-12,14-15,17,26-28H,4-6,13,16H2,1-3H3,(H,33,34)(H,35,36)/t17-,26-,27-,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards Endothelin A receptor |
J Med Chem 47: 1969-86 (2004)
Article DOI: 10.1021/jm030528p
BindingDB Entry DOI: 10.7270/Q2HD7WD6 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537068
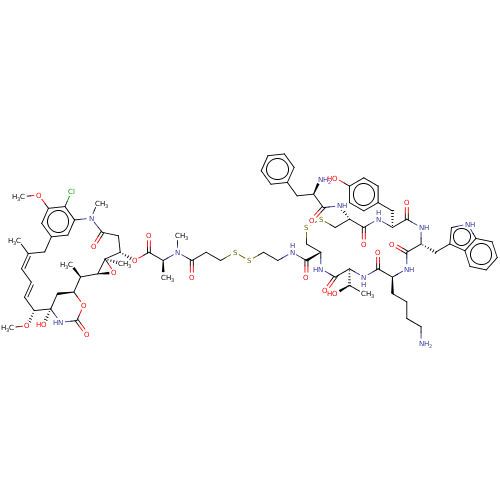
(CHEMBL4592483)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCNC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1 |r,t:17,19| Show InChI InChI=1S/C82H108ClN13O19S4/c1-45-18-17-24-65(112-9)82(110)41-64(113-80(109)94-82)46(2)71-81(5,115-71)66(40-68(100)96(7)62-37-51(34-45)38-63(111-8)69(62)83)114-79(108)47(3)95(6)67(99)29-32-116-117-33-31-86-73(102)60-43-118-119-44-61(91-72(101)55(85)35-49-19-11-10-12-20-49)77(106)89-58(36-50-25-27-53(98)28-26-50)75(104)90-59(39-52-42-87-56-22-14-13-21-54(52)56)76(105)88-57(23-15-16-30-84)74(103)93-70(48(4)97)78(107)92-60/h10-14,17-22,24-28,37-38,42,46-48,55,57-61,64-66,70-71,87,97-98,110H,15-16,23,29-36,39-41,43-44,84-85H2,1-9H3,(H,86,102)(H,88,105)(H,89,106)(H,90,104)(H,91,101)(H,92,107)(H,93,103)(H,94,109)/b24-17+,45-18+/t46-,47+,48-,55-,57+,58+,59-,60+,61+,64+,65-,66+,70+,71+,81-,82+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537070

(CHEMBL4581874)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCC(C)(C)SSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O |r,t:17,19| Show InChI InChI=1S/C86H115ClN14O20S4/c1-46-20-19-26-67(118-11)86(116)41-66(119-83(115)99-86)47(2)73-85(7,121-73)68(40-70(105)101(9)64-37-52(34-46)38-65(117-10)71(64)87)120-82(114)48(3)100(8)69(104)31-32-84(5,6)125-124-43-61(74(90)106)95-80(112)63-45-123-122-44-62(96-75(107)56(89)35-50-21-13-12-14-22-50)79(111)93-59(36-51-27-29-54(103)30-28-51)77(109)94-60(39-53-42-91-57-24-16-15-23-55(53)57)78(110)92-58(25-17-18-33-88)76(108)98-72(49(4)102)81(113)97-63/h12-16,19-24,26-30,37-38,42,47-49,56,58-63,66-68,72-73,91,102-103,116H,17-18,25,31-36,39-41,43-45,88-89H2,1-11H3,(H2,90,106)(H,92,110)(H,93,111)(H,94,109)(H,95,112)(H,96,107)(H,97,113)(H,98,108)(H,99,115)/b26-19+,46-20+/t47-,48+,49-,56-,58+,59+,60-,61+,62+,63+,66+,67-,68+,72+,73+,85-,86+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537074

(CHEMBL4556000)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCC(=O)NCCCC[C@@H]1N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)[C@@H](C)O |r,t:17,19| Show InChI InChI=1S/C84H111ClN12O19S2/c1-48-21-20-28-67(113-10)84(111)46-66(114-82(110)94-84)49(2)74-83(5,116-74)68(45-71(102)96(7)64-42-54(39-48)43-65(112-9)72(64)85)115-81(109)50(3)95(6)70(101)34-38-118-117-37-33-69(100)87-36-19-17-27-63-78(106)91-60(40-53-29-31-56(99)32-30-53)76(104)90-61(44-55-47-88-58-25-15-14-24-57(55)58)77(105)89-59(26-16-18-35-86)75(103)93-73(51(4)98)79(107)92-62(80(108)97(63)8)41-52-22-12-11-13-23-52/h11-15,20-25,28-32,42-43,47,49-51,59-63,66-68,73-74,88,98-99,111H,16-19,26-27,33-41,44-46,86H2,1-10H3,(H,87,100)(H,89,105)(H,90,104)(H,91,106)(H,92,107)(H,93,103)(H,94,110)/b28-20+,48-21+/t49-,50+,51-,59+,60+,61-,62+,63+,66+,67-,68+,73+,74+,83-,84+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537067
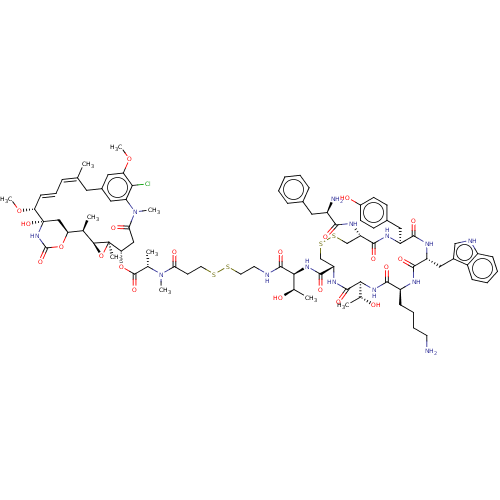
(CHEMBL4532058)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCNC(=O)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)[C@@H](C)O |r,t:17,19| Show InChI InChI=1S/C86H115ClN14O21S4/c1-46-19-18-25-67(119-10)86(117)42-66(120-84(116)99-86)47(2)74-85(6,122-74)68(41-70(106)101(8)64-38-53(35-46)39-65(118-9)71(64)87)121-83(115)48(3)100(7)69(105)30-33-123-124-34-32-90-81(113)72(49(4)102)97-80(112)63-45-126-125-44-62(95-75(107)57(89)36-51-20-12-11-13-21-51)79(111)93-60(37-52-26-28-55(104)29-27-52)77(109)94-61(40-54-43-91-58-23-15-14-22-56(54)58)78(110)92-59(24-16-17-31-88)76(108)98-73(50(5)103)82(114)96-63/h11-15,18-23,25-29,38-39,43,47-50,57,59-63,66-68,72-74,91,102-104,117H,16-17,24,30-37,40-42,44-45,88-89H2,1-10H3,(H,90,113)(H,92,110)(H,93,111)(H,94,109)(H,95,107)(H,96,114)(H,97,112)(H,98,108)(H,99,116)/b25-18+,46-19+/t47-,48+,49-,50-,57-,59+,60+,61-,62+,63+,66+,67-,68+,72+,73+,74+,85-,86+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537062

(CHEMBL4549303)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCCN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O)C(=O)N[C@H](CO)[C@@H](C)O |r,t:17,19| Show InChI InChI=1S/C87H118ClN13O20S4/c1-49-23-21-31-70(118-10)87(116)44-69(119-85(115)99-87)50(2)76-86(6,121-76)71(43-73(106)101(8)67-40-56(37-49)41-68(117-9)74(67)88)120-84(114)51(3)100(7)72(105)32-36-123-122-35-22-34-90-61(38-54-24-13-11-14-25-54)78(108)96-65-47-124-125-48-66(82(112)95-64(46-102)52(4)103)97-83(113)75(53(5)104)98-77(107)60(30-19-20-33-89)92-80(110)63(42-57-45-91-59-29-18-17-28-58(57)59)94-79(109)62(93-81(65)111)39-55-26-15-12-16-27-55/h11-18,21,23-29,31,40-41,45,50-53,60-66,69-71,75-76,90-91,102-104,116H,19-20,22,30,32-39,42-44,46-48,89H2,1-10H3,(H,92,110)(H,93,111)(H,94,109)(H,95,112)(H,96,108)(H,97,113)(H,98,107)(H,99,115)/b31-21+,49-23+/t50-,51+,52-,53-,60+,61-,62+,63-,64-,65+,66+,69+,70-,71+,75+,76+,86-,87+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537064
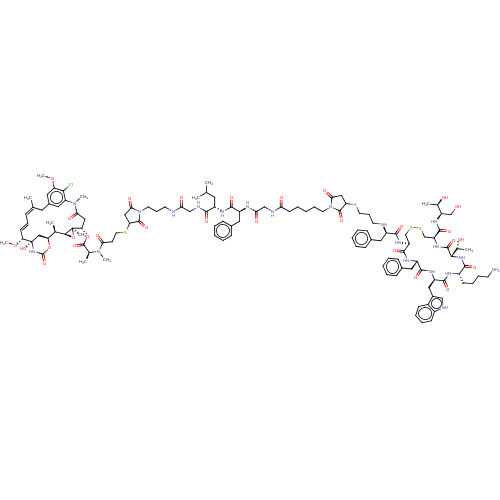
(CHEMBL4563111)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSC1CC(=O)N(CCCNC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)CCCCCN2C(=O)CC(SCCCN[C@H](Cc3ccccc3)C(=O)N[C@H]3CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](Cc4ccccc4)NC3=O)[C@@H](C)O)C(=O)N[C@H](CO)[C@@H](C)O)C2=O)C1=O |r,t:17,19| Show InChI InChI=1S/C123H167ClN20O29S4/c1-70(2)52-85(109(155)130-65-100(149)127-46-30-49-144-105(154)61-96(119(144)165)175-51-44-102(151)141(9)73(5)120(166)172-98-62-103(152)142(10)92-57-79(58-93(169-11)106(92)124)53-71(3)32-29-42-97(170-12)123(168)63-94(171-121(167)140-123)72(4)108-122(98,8)173-108)133-112(158)86(55-77-35-19-14-20-36-77)131-101(150)66-129-99(148)43-23-16-28-48-143-104(153)60-95(118(143)164)174-50-31-47-126-84(54-76-33-17-13-18-34-76)111(157)137-90-68-176-177-69-91(116(162)136-89(67-145)74(6)146)138-117(163)107(75(7)147)139-110(156)83(41-26-27-45-125)132-114(160)88(59-80-64-128-82-40-25-24-39-81(80)82)135-113(159)87(134-115(90)161)56-78-37-21-15-22-38-78/h13-15,17-22,24-25,29,32-40,42,57-58,64,70,72-75,83-91,94-98,107-108,126,128,145-147,168H,16,23,26-28,30-31,41,43-56,59-63,65-69,125H2,1-12H3,(H,127,149)(H,129,148)(H,130,155)(H,131,150)(H,132,160)(H,133,158)(H,134,161)(H,135,159)(H,136,162)(H,137,157)(H,138,163)(H,139,156)(H,140,167)/b42-29+,71-32+/t72-,73+,74-,75-,83+,84-,85+,86+,87+,88-,89-,90+,91+,94+,95?,96?,97-,98+,107+,108+,122-,123+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537075

(CHEMBL4548228)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSC1CC(=O)N(CC(=O)NC[C@H](NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](N)Cc3ccccc3)C(=O)N[C@@H](Cc3ccc(O)cc3)C(=O)N[C@H](Cc3c[nH]c4ccccc34)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N2)C(N)=O)C1=O |r,t:17,19| Show InChI InChI=1S/C89H115ClN16O23S3/c1-46-18-17-24-68(126-9)89(124)40-66(127-87(123)103-89)47(2)76-88(5,129-76)69(39-72(111)105(7)64-35-52(32-46)36-65(125-8)74(64)90)128-86(122)48(3)104(6)71(110)29-31-130-67-38-73(112)106(85(67)121)43-70(109)95-42-61(77(93)113)99-83(119)63-45-132-131-44-62(100-78(114)56(92)33-50-19-11-10-12-20-50)82(118)97-59(34-51-25-27-54(108)28-26-51)80(116)98-60(37-53-41-94-57-22-14-13-21-55(53)57)81(117)96-58(23-15-16-30-91)79(115)102-75(49(4)107)84(120)101-63/h10-14,17-22,24-28,35-36,41,47-49,56,58-63,66-69,75-76,94,107-108,124H,15-16,23,29-34,37-40,42-45,91-92H2,1-9H3,(H2,93,113)(H,95,109)(H,96,117)(H,97,118)(H,98,116)(H,99,119)(H,100,114)(H,101,120)(H,102,115)(H,103,123)/b24-17+,46-18+/t47-,48+,49-,56-,58+,59+,60-,61+,62+,63+,66+,67?,68-,69+,75+,76+,88-,89+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537071

(CHEMBL4581646)Show SMILES [H][C@]1(CCCN1C(=O)C[C@@H](OC)[C@H]([C@@H](C)CC)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(C)C(=O)OCCSSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O)C(C)C)[C@H](OC)[C@@H](C)C(=O)N[C@H](C)[C@@H](O)c1ccccc1 |r| Show InChI InChI=1S/C90H132N16O19S4/c1-14-52(6)76(71(123-12)45-72(109)106-39-25-33-70(106)78(124-13)53(7)80(112)95-54(8)77(110)58-28-19-16-20-29-58)104(10)89(121)73(50(2)3)102-88(120)75(51(4)5)105(11)90(122)125-40-41-126-127-47-67(79(93)111)99-86(118)69-49-129-128-48-68(100-81(113)62(92)42-56-26-17-15-18-27-56)85(117)97-65(43-57-34-36-60(108)37-35-57)83(115)98-66(44-59-46-94-63-31-22-21-30-61(59)63)84(116)96-64(32-23-24-38-91)82(114)103-74(55(9)107)87(119)101-69/h15-22,26-31,34-37,46,50-55,62,64-71,73-78,94,107-108,110H,14,23-25,32-33,38-45,47-49,91-92H2,1-13H3,(H2,93,111)(H,95,112)(H,96,116)(H,97,117)(H,98,115)(H,99,118)(H,100,113)(H,101,119)(H,102,120)(H,103,114)/t52-,53+,54+,55+,62+,64-,65-,66+,67-,68-,69-,70-,71+,73-,74-,75-,76-,77+,78+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537065
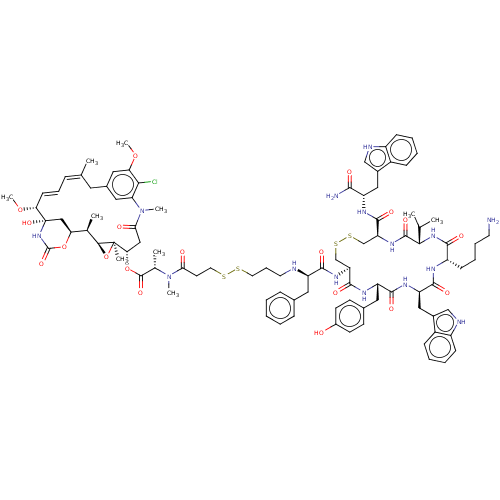
(CHEMBL4537192)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCCN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r,t:17,19| Show InChI InChI=1S/C95H122ClN15O19S4/c1-53(2)82-91(122)107-73(89(120)103-68(84(98)115)45-60-49-100-65-27-16-14-25-63(60)65)52-134-133-51-72(90(121)104-70(42-58-31-33-62(112)34-32-58)87(118)105-71(46-61-50-101-66-28-17-15-26-64(61)66)88(119)102-67(85(116)108-82)29-18-19-36-97)106-86(117)69(41-57-23-12-11-13-24-57)99-37-21-38-131-132-39-35-79(113)110(7)56(5)92(123)129-78-47-80(114)111(8)74-43-59(44-75(126-9)81(74)96)40-54(3)22-20-30-77(127-10)95(125)48-76(128-93(124)109-95)55(4)83-94(78,6)130-83/h11-17,20,22-28,30-34,43-44,49-50,53,55-56,67-73,76-78,82-83,99-101,112,125H,18-19,21,29,35-42,45-48,51-52,97H2,1-10H3,(H2,98,115)(H,102,119)(H,103,120)(H,104,121)(H,105,118)(H,106,117)(H,107,122)(H,108,116)(H,109,124)/b30-20+,54-22+/t55-,56+,67+,68+,69-,70+,71-,72+,73+,76+,77-,78+,82+,83+,94-,95+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537078

(CHEMBL4577466)Show SMILES [H][C@]([C@@H](C)CC)([C@@H](CC(=O)N1CCC[C@@]1([H])[C@H](OC)[C@@H](C)C(=O)N[C@H](C)[C@@H](O)c1ccccc1)OC)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(C)C(=O)OCCSSCCCN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O)C(=O)N[C@H](CO)[C@@H](C)O)C(C)C |r| Show InChI InChI=1S/C94H141N15O19S4/c1-15-57(6)81(76(126-13)50-77(113)109-43-29-40-75(109)83(127-14)58(7)84(115)98-59(8)82(114)64-35-23-18-24-36-64)107(11)93(124)78(55(2)3)105-92(123)80(56(4)5)108(12)94(125)128-44-46-130-129-45-30-42-96-69(47-62-31-19-16-20-32-62)86(117)103-73-53-131-132-54-74(90(121)102-72(52-110)60(9)111)104-91(122)79(61(10)112)106-85(116)68(39-27-28-41-95)99-88(119)71(49-65-51-97-67-38-26-25-37-66(65)67)101-87(118)70(100-89(73)120)48-63-33-21-17-22-34-63/h16-26,31-38,51,55-61,68-76,78-83,96-97,110-112,114H,15,27-30,39-50,52-54,95H2,1-14H3,(H,98,115)(H,99,119)(H,100,120)(H,101,118)(H,102,121)(H,103,117)(H,104,122)(H,105,123)(H,106,116)/t57-,58+,59+,60+,61+,68-,69+,70-,71+,72+,73-,74-,75-,76+,78-,79-,80-,81-,82+,83+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50058126
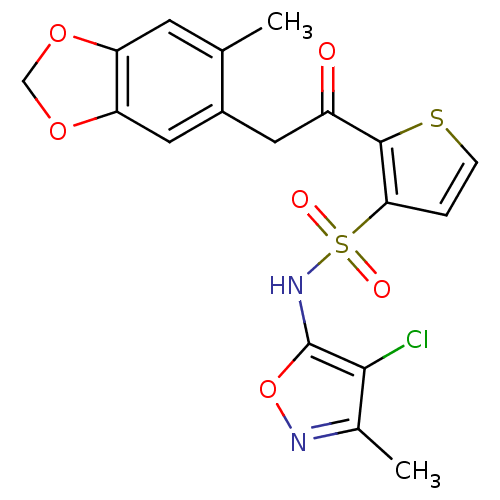
(2-[2-(6-Methyl-benzo[1,3]dioxol-5-yl)-acetyl]-thio...)Show SMILES Cc1noc(NS(=O)(=O)c2ccsc2C(=O)Cc2cc3OCOc3cc2C)c1Cl Show InChI InChI=1S/C18H15ClN2O6S2/c1-9-5-13-14(26-8-25-13)7-11(9)6-12(22)17-15(3-4-28-17)29(23,24)21-18-16(19)10(2)20-27-18/h3-5,7,21H,6,8H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards Endothelin A receptor |
J Med Chem 47: 1969-86 (2004)
Article DOI: 10.1021/jm030528p
BindingDB Entry DOI: 10.7270/Q2HD7WD6 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537073
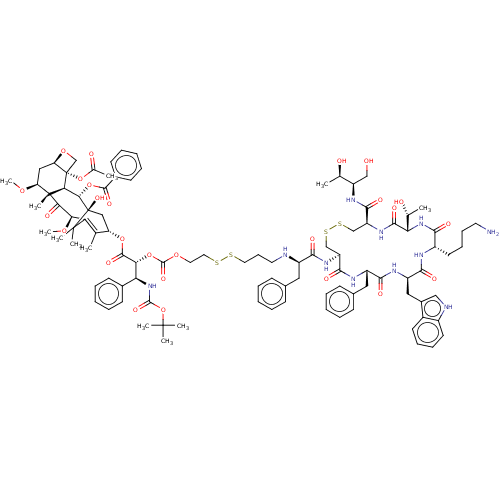
(CHEMBL4534477)Show SMILES [H][C@@]12C[C@H](OC)[C@@]3(C)C(=O)[C@H](OC)C4=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c5ccccc5)[C@]3([H])[C@@]1(CO2)OC(C)=O)C4(C)C)OC(=O)[C@H](OC(=O)OCCSSCCCN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O)C(=O)N[C@H](CO)[C@@H](C)O)[C@@H](NC(=O)OC(C)(C)C)c1ccccc1 |r,c:13| Show InChI InChI=1S/C100H131N11O26S4/c1-56-74(50-100(128)84(135-92(124)63-36-23-16-24-37-63)82-98(10,83(116)80(130-12)77(56)97(100,8)9)75(129-11)49-76-99(82,55-132-76)136-59(4)115)133-93(125)81(79(62-34-21-15-22-35-62)111-94(126)137-96(5,6)7)134-95(127)131-43-45-139-138-44-29-42-102-68(46-60-30-17-13-18-31-60)86(118)108-72-53-140-141-54-73(90(122)107-71(52-112)57(2)113)109-91(123)78(58(3)114)110-85(117)67(40-27-28-41-101)104-88(120)70(48-64-51-103-66-39-26-25-38-65(64)66)106-87(119)69(105-89(72)121)47-61-32-19-14-20-33-61/h13-26,30-39,51,57-58,67-76,78-82,84,102-103,112-114,128H,27-29,40-50,52-55,101H2,1-12H3,(H,104,120)(H,105,121)(H,106,119)(H,107,122)(H,108,118)(H,109,123)(H,110,117)(H,111,126)/t57-,58-,67+,68-,69+,70-,71-,72+,73+,74+,75+,76-,78+,79+,80-,81-,82+,84+,98-,99+,100-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537060
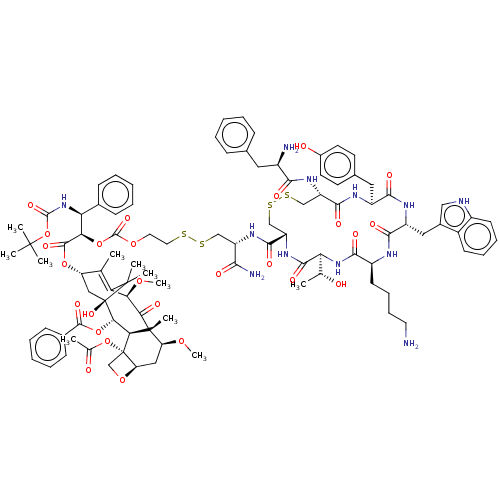
(CHEMBL4575530)Show SMILES [H][C@@]12C[C@H](OC)[C@@]3(C)C(=O)[C@H](OC)C4=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c5ccccc5)[C@]3([H])[C@@]1(CO2)OC(C)=O)C4(C)C)OC(=O)[C@H](OC(=O)OCCSSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O)[C@@H](NC(=O)OC(C)(C)C)c1ccccc1 |r,c:13| Show InChI InChI=1S/C96H122N12O26S4/c1-51-69(45-96(125)79(132-88(121)57-29-19-14-20-30-57)77-94(9,78(112)75(127-11)72(51)93(96,7)8)70(126-10)44-71-95(77,50-129-71)133-53(3)110)130-89(122)76(74(56-27-17-13-18-28-56)108-90(123)134-92(4,5)6)131-91(124)128-39-40-135-136-47-66(80(99)113)104-86(119)68-49-138-137-48-67(105-81(114)61(98)41-54-25-15-12-16-26-54)85(118)102-64(42-55-34-36-59(111)37-35-55)83(116)103-65(43-58-46-100-62-32-22-21-31-60(58)62)84(117)101-63(33-23-24-38-97)82(115)107-73(52(2)109)87(120)106-68/h12-22,25-32,34-37,46,52,61,63-71,73-77,79,100,109,111,125H,23-24,33,38-45,47-50,97-98H2,1-11H3,(H2,99,113)(H,101,117)(H,102,118)(H,103,116)(H,104,119)(H,105,114)(H,106,120)(H,107,115)(H,108,123)/t52-,61-,63+,64+,65-,66+,67+,68+,69+,70+,71-,73+,74+,75-,76-,77+,79+,94-,95+,96-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50357753
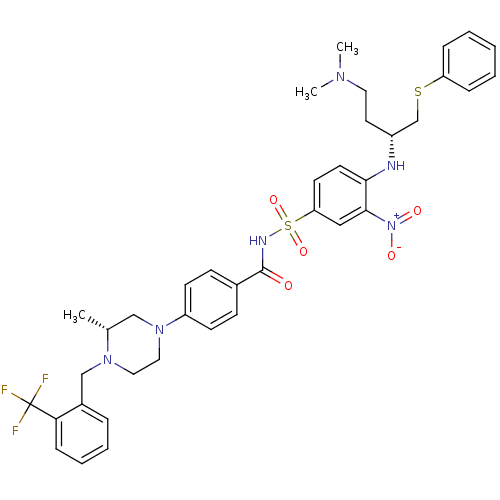
(CHEMBL1916191)Show SMILES C[C@@H]1CN(CCN1Cc1ccccc1C(F)(F)F)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN(C)C)CSc2ccccc2)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C38H43F3N6O5S2/c1-27-24-46(22-21-45(27)25-29-9-7-8-12-34(29)38(39,40)41)31-15-13-28(14-16-31)37(48)43-54(51,52)33-17-18-35(36(23-33)47(49)50)42-30(19-20-44(2)3)26-53-32-10-5-4-6-11-32/h4-18,23,27,30,42H,19-22,24-26H2,1-3H3,(H,43,48)/t27-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged human Wild type Bcl-2-like protein 1 expressed in Escherichia coli BL21 cells at 10 uM ... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50357752

(CHEMBL1916190)Show SMILES C[C@H]1CN(CCN1Cc1ccccc1C(F)(F)F)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN(C)C)CSc2ccccc2)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C38H43F3N6O5S2/c1-27-24-46(22-21-45(27)25-29-9-7-8-12-34(29)38(39,40)41)31-15-13-28(14-16-31)37(48)43-54(51,52)33-17-18-35(36(23-33)47(49)50)42-30(19-20-44(2)3)26-53-32-10-5-4-6-11-32/h4-18,23,27,30,42H,19-22,24-26H2,1-3H3,(H,43,48)/t27-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged human Wild type Bcl-2-like protein 1 expressed in Escherichia coli BL21 cells at 10 uM ... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50357758
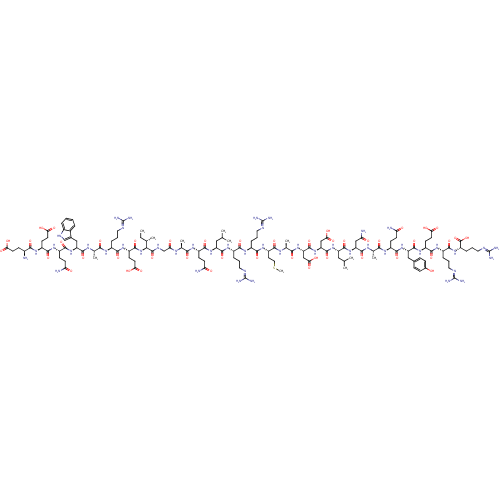
(CHEMBL1916196)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:65.67,47.54,28.28,8.13,2.2,84.85,101.102,123.124,136.137,152.153,168.170,182.183,203.205,wD:56.63,33.45,17.24,4.4,79.81,93.94,112.113,131.133,144.145,160.161,173.174,194.196,214.216,(-6.53,-14.66,;-6.53,-13.11,;-5.2,-12.34,;-3.86,-13.11,;-5.2,-10.8,;-6.53,-10.03,;-7.87,-10.81,;-7.86,-12.34,;-9.2,-10.04,;-9.2,-8.5,;-7.87,-7.73,;-7.87,-6.19,;-9.21,-5.42,;-6.54,-5.42,;-10.53,-10.81,;-11.88,-10.04,;-11.88,-8.5,;-13.21,-10.81,;-13.2,-12.35,;-11.87,-13.12,;-11.86,-14.66,;-10.52,-15.43,;-10.53,-16.97,;-11.86,-17.74,;-9.18,-17.74,;-14.55,-10.05,;-15.87,-10.82,;-15.87,-12.36,;-17.21,-10.05,;-17.21,-8.51,;-18.54,-10.82,;-19.88,-10.05,;-19.89,-8.51,;-21.21,-10.82,;-21.21,-12.37,;-19.87,-13.13,;-18.61,-12.25,;-17.37,-13.18,;-17.89,-14.63,;-17.13,-15.98,;-17.93,-17.3,;-19.47,-17.27,;-20.21,-15.92,;-19.42,-14.61,;-22.54,-10.06,;-23.88,-10.83,;-23.87,-12.37,;-25.21,-10.07,;-25.21,-8.53,;-23.88,-7.75,;-23.88,-6.22,;-25.22,-5.44,;-22.55,-5.44,;-26.55,-10.84,;-27.89,-10.07,;-27.87,-8.53,;-29.21,-10.84,;-29.21,-12.38,;-27.86,-13.15,;-27.88,-14.69,;-29.2,-15.46,;-26.54,-15.45,;-30.54,-10.07,;-31.88,-10.84,;-31.87,-12.38,;-33.22,-10.07,;-34.54,-10.84,;-33.22,-8.53,;-31.88,-7.76,;-31.88,-6.22,;-33.23,-5.46,;-30.55,-5.45,;-3.87,-10.03,;-3.87,-8.49,;-2.53,-10.8,;-1.2,-10.03,;.14,-10.79,;.14,-12.33,;1.47,-10.02,;2.8,-10.79,;2.81,-12.33,;4.14,-10.01,;4.13,-8.48,;5.47,-10.79,;6.8,-10.02,;6.8,-8.48,;8.13,-7.7,;8.13,-6.16,;6.79,-5.39,;9.47,-5.39,;8.14,-10.78,;8.14,-12.32,;9.47,-10.01,;10.8,-10.78,;10.8,-12.32,;12.14,-13.08,;12.14,-14.62,;13.47,-12.31,;12.13,-10.01,;12.13,-8.46,;13.47,-10.77,;14.8,-10,;14.8,-8.46,;16.13,-7.69,;16.13,-6.15,;17.46,-5.38,;17.46,-3.84,;16.12,-3.07,;18.79,-3.07,;16.14,-10.77,;16.14,-12.31,;17.47,-10,;18.81,-10.77,;18.81,-12.31,;20.14,-13.07,;20.14,-14.61,;21.48,-15.38,;21.48,-16.93,;20.14,-17.69,;22.82,-17.69,;20.14,-10,;20.13,-8.46,;21.47,-10.76,;22.8,-9.99,;22.8,-8.45,;24.13,-7.68,;24.13,-6.14,;25.46,-5.37,;24.13,-10.76,;24.14,-12.3,;25.47,-9.98,;26.8,-10.75,;26.81,-12.29,;28.13,-9.98,;28.14,-8.44,;29.47,-10.75,;30.8,-9.98,;30.8,-8.44,;32.13,-7.67,;33.47,-8.43,;32.13,-6.13,;32.14,-10.74,;32.14,-12.28,;33.47,-9.97,;34.8,-10.74,;34.81,-12.28,;36.14,-13.05,;37.48,-12.27,;36.15,-14.59,;36.14,-9.97,;36.14,-8.43,;37.47,-10.74,;38.8,-9.97,;38.8,-8.42,;40.13,-7.65,;40.13,-6.11,;41.47,-8.43,;40.14,-10.74,;40.14,-12.28,;41.47,-9.97,;42.81,-10.73,;42.8,-12.27,;44.14,-13.03,;45.47,-12.26,;44.14,-14.57,;44.14,-9.96,;44.14,-8.42,;45.47,-10.72,;46.8,-9.95,;46.81,-8.41,;48.14,-10.72,;48.15,-12.26,;49.47,-9.95,;50.81,-10.72,;50.81,-12.26,;52.14,-13.02,;52.14,-14.56,;50.81,-15.33,;53.48,-15.33,;52.14,-9.95,;52.13,-8.41,;53.47,-10.71,;54.8,-9.94,;54.8,-8.4,;56.13,-7.63,;57.47,-8.4,;58.81,-7.63,;58.8,-6.09,;60.13,-5.32,;57.47,-5.32,;56.13,-6.09,;56.14,-10.71,;56.14,-12.25,;57.47,-9.94,;58.81,-10.7,;58.81,-12.24,;60.14,-13.01,;60.14,-14.55,;58.81,-15.32,;61.48,-15.32,;60.14,-9.93,;60.14,-8.39,;61.48,-10.7,;62.81,-9.93,;62.8,-8.39,;64.14,-7.61,;64.13,-6.08,;65.46,-5.3,;65.46,-3.76,;64.12,-3,;66.79,-2.99,;64.14,-10.7,;64.15,-12.24,;65.48,-9.93,;66.81,-10.69,;66.81,-12.23,;68.15,-13,;68.15,-14.54,;69.48,-15.31,;69.48,-16.85,;68.15,-17.62,;70.82,-17.61,;68.14,-9.92,;69.47,-10.69,;68.14,-8.38,)| Show InChI InChI=1S/C134H214N46O44S/c1-12-63(6)104(180-120(214)83(37-44-101(193)194)169-111(205)74(22-15-46-150-130(140)141)161-106(200)65(8)159-121(215)89(55-69-59-155-73-21-14-13-20-71(69)73)177-119(213)80(34-40-95(138)184)168-115(209)81(35-42-99(189)190)164-109(203)72(135)31-41-98(187)188)128(222)156-60-97(186)157-64(7)105(199)162-78(32-38-93(136)182)117(211)174-86(52-61(2)3)123(217)167-76(24-17-48-152-132(144)145)112(206)165-75(23-16-47-151-131(142)143)113(207)171-84(45-51-225-11)110(204)158-67(10)108(202)173-91(57-102(195)196)126(220)179-92(58-103(197)198)127(221)175-87(53-62(4)5)124(218)178-90(56-96(139)185)122(216)160-66(9)107(201)163-79(33-39-94(137)183)118(212)176-88(54-68-27-29-70(181)30-28-68)125(219)170-82(36-43-100(191)192)116(210)166-77(25-18-49-153-133(146)147)114(208)172-85(129(223)224)26-19-50-154-134(148)149/h13-14,20-21,27-30,59,61-67,72,74-92,104,155,181H,12,15-19,22-26,31-58,60,135H2,1-11H3,(H2,136,182)(H2,137,183)(H2,138,184)(H2,139,185)(H,156,222)(H,157,186)(H,158,204)(H,159,215)(H,160,216)(H,161,200)(H,162,199)(H,163,201)(H,164,203)(H,165,206)(H,166,210)(H,167,217)(H,168,209)(H,169,205)(H,170,219)(H,171,207)(H,172,208)(H,173,202)(H,174,211)(H,175,221)(H,176,212)(H,177,213)(H,178,218)(H,179,220)(H,180,214)(H,187,188)(H,189,190)(H,191,192)(H,193,194)(H,195,196)(H,197,198)(H,223,224)(H4,140,141,150)(H4,142,143,151)(H4,144,145,152)(H4,146,147,153)(H4,148,149,154)/t63-,64-,65-,66-,67-,72-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,104-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged human Wild type Bcl-2-like protein 1 expressed in Escherichia coli BL21 cells at 40 uM ... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50091105

(4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...)Show SMILES Cc1noc(NS(=O)(=O)c2ccccc2-c2ccc(cc2)-c2ncco2)c1C Show InChI InChI=1S/C20H17N3O4S/c1-13-14(2)22-27-19(13)23-28(24,25)18-6-4-3-5-17(18)15-7-9-16(10-8-15)20-21-11-12-26-20/h3-12,23H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards Endothelin A receptor |
J Med Chem 47: 1969-86 (2004)
Article DOI: 10.1021/jm030528p
BindingDB Entry DOI: 10.7270/Q2HD7WD6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50357756

(CHEMBL1916194)Show SMILES CC(C)[C@@H]1CN(Cc2ccccc2C(F)(F)F)CCN1c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN(C)C)CSc2ccccc2)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C40H47F3N6O5S2/c1-28(2)38-26-47(25-30-10-8-9-13-35(30)40(41,42)43)22-23-48(38)32-16-14-29(15-17-32)39(50)45-56(53,54)34-18-19-36(37(24-34)49(51)52)44-31(20-21-46(3)4)27-55-33-11-6-5-7-12-33/h5-19,24,28,31,38,44H,20-23,25-27H2,1-4H3,(H,45,50)/t31-,38+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged human Wild type Bcl-2-like protein 1 expressed in Escherichia coli BL21 cells at 10 uM ... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50357757

(CHEMBL1916195)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CC(C)C)C(O)=O |r,wU:118.121,99.100,85.91,62.71,47.54,30.34,8.15,127.129,141.143,161.164,174.177,191.195,204.208,wD:104.117,94.95,74.82,38.43,19.26,4.4,133.135,150.152,180.183,(18.31,-39.34,;16.98,-38.56,;17,-37.02,;15.66,-36.24,;15.68,-34.69,;14.35,-33.92,;13.01,-34.68,;13,-36.22,;11.68,-33.89,;11.69,-32.35,;13.03,-31.59,;13.04,-30.05,;14.38,-29.29,;14.39,-27.75,;13.06,-26.98,;15.73,-26.99,;10.34,-34.65,;9.02,-33.88,;9.02,-32.34,;7.67,-34.64,;7.66,-36.18,;8.99,-36.95,;8.98,-38.5,;10.31,-39.28,;10.3,-40.82,;8.96,-41.58,;11.63,-41.6,;6.34,-33.86,;5.01,-34.62,;4.99,-36.16,;3.67,-33.84,;3.69,-32.3,;5.02,-31.54,;5.04,-30,;6.36,-32.32,;2.33,-34.6,;1.01,-33.82,;1.02,-32.28,;-.33,-34.58,;-.34,-36.13,;.99,-36.9,;.97,-38.44,;-.36,-39.2,;2.3,-39.22,;-1.66,-33.8,;-3,-34.56,;-3.01,-36.11,;-4.33,-33.78,;-4.32,-32.24,;-2.98,-31.48,;-2.97,-29.94,;-1.62,-29.18,;-1.62,-27.64,;-2.94,-26.86,;-.28,-26.88,;-5.66,-34.54,;-7,-33.77,;-6.98,-32.23,;-8.34,-34.53,;-9.66,-33.75,;-11,-34.51,;-11.01,-36.05,;-12.33,-33.73,;-12.32,-32.19,;-10.98,-31.43,;-9.65,-32.21,;-8.31,-31.45,;-8.31,-29.9,;-6.96,-29.15,;-9.63,-29.13,;-10.97,-29.89,;-13.68,-34.49,;-15.01,-33.71,;-15,-32.17,;-16.34,-34.47,;-16.35,-36.01,;-15.03,-36.79,;-15.04,-38.33,;-13.71,-39.11,;-13.72,-40.65,;-15.05,-41.42,;-12.4,-41.43,;-17.69,-33.69,;-19.02,-34.45,;-19.02,-35.99,;-20.34,-33.67,;-20.33,-32.13,;-19,-31.37,;-18.99,-29.83,;-20.31,-29.06,;-17.64,-29.07,;-21.67,-34.43,;-23.02,-33.66,;-23,-32.12,;-24.35,-34.42,;-24.37,-35.96,;-25.67,-33.63,;-27.02,-34.39,;-27.02,-35.94,;-28.35,-33.62,;-28.34,-32.08,;-29.68,-34.38,;-31.01,-33.6,;-30.99,-32.06,;-32.34,-34.36,;-32.36,-35.9,;-31.04,-36.68,;-29.78,-35.79,;-28.54,-36.7,;-29.03,-38.17,;-28.27,-39.51,;-29.06,-40.83,;-30.6,-40.82,;-31.35,-39.48,;-30.58,-38.15,;-33.68,-33.58,;-35.02,-34.34,;-35.03,-35.88,;-36.34,-33.56,;-37.69,-34.32,;-36.34,-32.03,;-35,-31.26,;-34.98,-29.72,;-33.66,-32.04,;17.02,-33.93,;17.03,-32.39,;18.34,-34.71,;19.69,-33.95,;19.69,-32.41,;21.03,-31.66,;21.01,-34.73,;21,-36.27,;22.35,-33.97,;23.69,-34.75,;23.67,-36.29,;25,-37.07,;26.33,-36.31,;24.99,-38.61,;25.02,-33.99,;25.03,-32.45,;26.35,-34.76,;27.69,-34,;27.7,-32.46,;29.04,-31.7,;29.05,-30.16,;27.72,-29.39,;30.39,-29.4,;29.01,-34.79,;29.01,-36.33,;30.36,-34.03,;31.68,-34.8,;31.67,-36.35,;33.01,-37.13,;34.34,-36.37,;35.67,-37.15,;35.66,-38.68,;34.32,-39.44,;32.99,-38.67,;33.02,-34.04,;33.04,-32.5,;34.36,-34.83,;35.69,-34.07,;35.7,-32.53,;37.04,-31.77,;37.05,-30.22,;35.73,-29.44,;38.39,-29.46,;37.02,-34.84,;37.01,-36.38,;38.36,-34.08,;39.69,-34.86,;41.03,-34.1,;41.04,-32.56,;42.36,-34.87,;43.69,-34.12,;43.71,-32.57,;45.05,-31.82,;45.03,-34.9,;45.01,-36.44,;46.37,-34.14,;47.69,-34.91,;47.68,-36.45,;49.01,-37.24,;50.35,-36.48,;51.68,-37.25,;51.67,-38.79,;50.33,-39.55,;49,-38.78,;49.03,-34.15,;49.04,-32.61,;50.36,-34.94,;51.7,-34.18,;51.72,-32.64,;53.05,-31.87,;53.06,-30.34,;54.4,-29.57,;54.41,-28.03,;53.03,-34.95,;53.02,-36.49,;54.36,-34.19,;55.7,-34.97,;57.04,-34.21,;57.04,-32.67,;58.36,-34.99,;59.7,-34.23,;59.72,-32.69,;61.05,-31.93,;61.07,-30.39,;62.39,-32.71,;61.04,-35.01,;62.37,-34.25,;61.02,-36.55,)| Show InChI InChI=1S/C133H204N40O38S/c1-68(2)54-79(135)110(191)167-95(60-76-62-149-80-29-17-16-28-78(76)80)122(203)154-71(7)108(189)153-72(8)109(190)158-87(39-43-100(136)177)118(199)161-85(34-23-52-148-133(143)144)117(198)169-92(59-75-35-37-77(176)38-36-75)113(194)152-63-101(178)155-82(31-20-49-145-130(137)138)114(195)164-88(41-45-105(183)184)119(200)168-91(55-69(3)4)123(204)162-84(33-22-51-147-132(141)142)115(196)160-83(32-21-50-146-131(139)140)116(197)166-90(47-53-212-9)121(202)173-99(67-175)128(209)172-96(61-107(187)188)126(207)165-89(42-46-106(185)186)120(201)170-93(57-73-24-12-10-13-25-73)125(206)163-86(40-44-104(181)182)112(193)151-65-103(180)157-98(66-174)127(208)171-94(58-74-26-14-11-15-27-74)124(205)159-81(30-18-19-48-134)111(192)150-64-102(179)156-97(129(210)211)56-70(5)6/h10-17,24-29,35-38,62,68-72,79,81-99,149,174-176H,18-23,30-34,39-61,63-67,134-135H2,1-9H3,(H2,136,177)(H,150,192)(H,151,193)(H,152,194)(H,153,189)(H,154,203)(H,155,178)(H,156,179)(H,157,180)(H,158,190)(H,159,205)(H,160,196)(H,161,199)(H,162,204)(H,163,206)(H,164,195)(H,165,207)(H,166,197)(H,167,191)(H,168,200)(H,169,198)(H,170,201)(H,171,208)(H,172,209)(H,173,202)(H,181,182)(H,183,184)(H,185,186)(H,187,188)(H,210,211)(H4,137,138,145)(H4,139,140,146)(H4,141,142,147)(H4,143,144,148)/t71-,72-,79-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged human Wild type Bcl-2-like protein 1 expressed in Escherichia coli BL21 cells at 40 uM ... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50357755
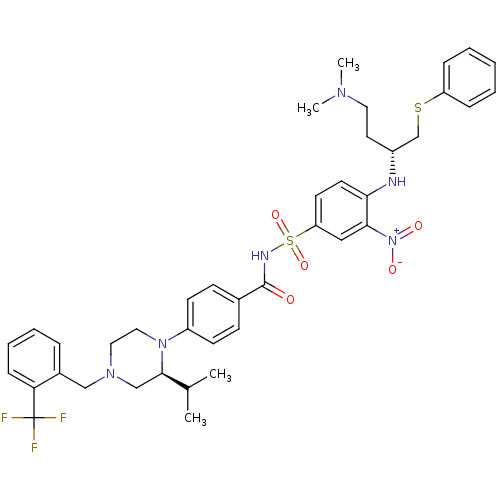
(CHEMBL1916193)Show SMILES CC(C)[C@H]1CN(Cc2ccccc2C(F)(F)F)CCN1c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN(C)C)CSc2ccccc2)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C40H47F3N6O5S2/c1-28(2)38-26-47(25-30-10-8-9-13-35(30)40(41,42)43)22-23-48(38)32-16-14-29(15-17-32)39(50)45-56(53,54)34-18-19-36(37(24-34)49(51)52)44-31(20-21-46(3)4)27-55-33-11-6-5-7-12-33/h5-19,24,28,31,38,44H,20-23,25-27H2,1-4H3,(H,45,50)/t31-,38-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged human Wild type Bcl-2-like protein 1 expressed in Escherichia coli BL21 cells at 10 uM ... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM21447

(4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C42H45ClN6O5S2/c1-46(2)23-22-35(30-55-37-9-4-3-5-10-37)44-40-21-20-38(28-41(40)49(51)52)56(53,54)45-42(50)32-14-18-36(19-15-32)48-26-24-47(25-27-48)29-33-8-6-7-11-39(33)31-12-16-34(43)17-13-31/h3-21,28,35,44H,22-27,29-30H2,1-2H3,(H,45,50)/t35-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged human Wild type Bcl-2-like protein 1 expressed in Escherichia coli BL21 cells at 10 uM ... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM21434
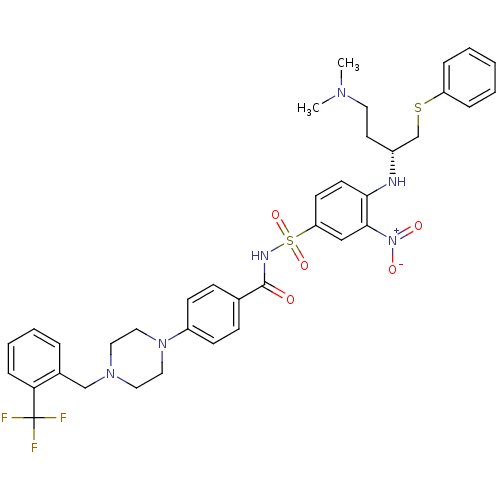
(N-Benylpiperazine derivative, 23j | N-[(4-{[(2R)-4...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2C(F)(F)F)CC1 |r| Show InChI InChI=1S/C37H41F3N6O5S2/c1-43(2)19-18-29(26-52-31-9-4-3-5-10-31)41-34-17-16-32(24-35(34)46(48)49)53(50,51)42-36(47)27-12-14-30(15-13-27)45-22-20-44(21-23-45)25-28-8-6-7-11-33(28)37(38,39)40/h3-17,24,29,41H,18-23,25-26H2,1-2H3,(H,42,47)/t29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged human Wild type Bcl-2-like protein 1 expressed in Escherichia coli BL21 cells at 10 uM ... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50108202
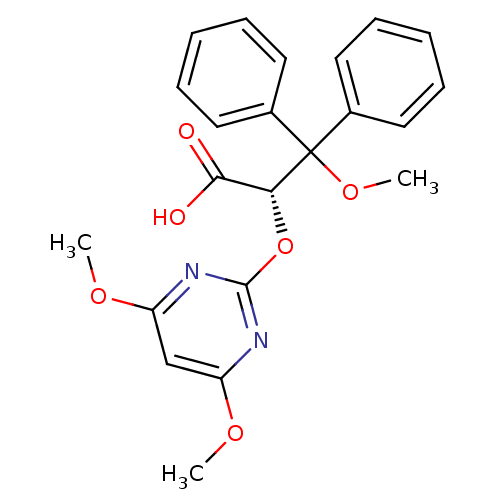
((S)-2-(4,6-Dimethoxy-pyrimidin-2-yloxy)-3-methoxy-...)Show SMILES COc1cc(OC)nc(O[C@H](C(O)=O)C(OC)(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C22H22N2O6/c1-27-17-14-18(28-2)24-21(23-17)30-19(20(25)26)22(29-3,15-10-6-4-7-11-15)16-12-8-5-9-13-16/h4-14,19H,1-3H3,(H,25,26)/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards Endothelin A receptor |
J Med Chem 47: 1969-86 (2004)
Article DOI: 10.1021/jm030528p
BindingDB Entry DOI: 10.7270/Q2HD7WD6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM21400
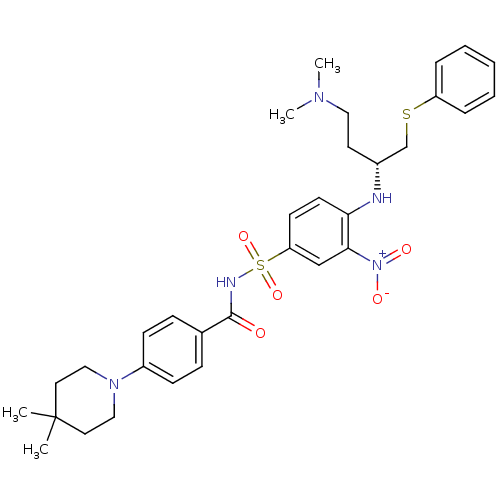
(CHEMBL192571 | N-[(4-{[(2R)-4-(dimethylamino)-1-(p...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCC(C)(C)CC1 |r| Show InChI InChI=1S/C32H41N5O5S2/c1-32(2)17-20-36(21-18-32)26-12-10-24(11-13-26)31(38)34-44(41,42)28-14-15-29(30(22-28)37(39)40)33-25(16-19-35(3)4)23-43-27-8-6-5-7-9-27/h5-15,22,25,33H,16-21,23H2,1-4H3,(H,34,38)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged human Wild type Bcl-2-like protein 1 expressed in Escherichia coli BL21 cells at 10 uM ... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50061101
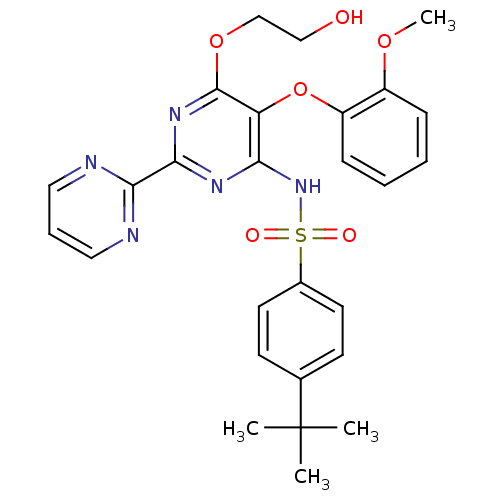
(4-(1,1-Dimethylethyl)-N-(6-(2-hydroxyethoxy)-5-(2-...)Show SMILES COc1ccccc1Oc1c(NS(=O)(=O)c2ccc(cc2)C(C)(C)C)nc(nc1OCCO)-c1ncccn1 Show InChI InChI=1S/C27H29N5O6S/c1-27(2,3)18-10-12-19(13-11-18)39(34,35)32-23-22(38-21-9-6-5-8-20(21)36-4)26(37-17-16-33)31-25(30-23)24-28-14-7-15-29-24/h5-15,33H,16-17H2,1-4H3,(H,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards Endothelin A receptor |
J Med Chem 47: 1969-86 (2004)
Article DOI: 10.1021/jm030528p
BindingDB Entry DOI: 10.7270/Q2HD7WD6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50357759

(CHEMBL1916197)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CCSC)[C@@H](C)CC)[C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(O)=O |r,wU:110.113,99.109,92.93,79.81,61.61,47.52,8.15,2.2,139.141,159.162,wD:83.90,116.119,65.77,120.123,56.56,38.43,30.34,19.26,4.4,131.133,148.150,167.171,(21.53,-21.17,;20.2,-20.4,;20.2,-18.85,;21.53,-18.08,;18.86,-18.09,;17.52,-18.86,;17.53,-20.4,;18.86,-21.16,;16.19,-21.17,;14.86,-20.4,;14.86,-18.85,;13.53,-18.09,;13.53,-16.55,;12.2,-15.78,;10.87,-16.55,;12.2,-14.24,;16.19,-22.7,;14.86,-23.47,;13.52,-22.71,;14.85,-25.01,;16.19,-25.78,;17.53,-25.01,;18.86,-25.78,;20.19,-25.01,;21.53,-25.78,;21.53,-27.32,;22.86,-25.01,;13.52,-25.78,;13.53,-27.32,;14.86,-28.09,;12.19,-28.09,;12.19,-29.63,;10.86,-30.4,;9.52,-29.63,;10.86,-31.93,;10.86,-27.32,;10.86,-25.78,;12.19,-25.01,;9.53,-25.01,;8.19,-25.78,;6.85,-25.01,;5.53,-25.78,;5.53,-27.31,;4.19,-25.01,;9.53,-23.46,;8.2,-22.7,;6.86,-23.47,;8.2,-21.15,;9.53,-20.39,;10.87,-21.16,;12.2,-20.39,;12.19,-18.86,;13.53,-21.16,;6.87,-20.39,;6.86,-18.85,;8.2,-18.08,;5.53,-18.08,;4.2,-18.85,;5.53,-16.54,;4.2,-15.77,;2.87,-16.55,;4.2,-14.23,;2.87,-13.46,;2.87,-11.92,;4.2,-11.15,;1.54,-11.15,;.2,-11.92,;.21,-13.46,;1.1,-14.7,;.19,-15.94,;-1.27,-15.46,;-2.6,-16.23,;-3.93,-15.45,;-3.93,-13.91,;-2.59,-13.16,;-1.26,-13.93,;1.54,-9.61,;.21,-8.85,;-1.13,-9.61,;.2,-7.3,;-1.13,-6.53,;-1.13,-4.99,;.2,-4.22,;-2.46,-4.22,;-3.79,-4.99,;-3.79,-6.53,;-5.13,-7.3,;-6.46,-6.54,;-5.13,-8.84,;-2.46,-2.68,;-3.79,-1.91,;-5.12,-2.68,;-3.8,-.37,;-2.41,.33,;-2.94,1.92,;-4.61,1.91,;-5.13,.31,;-6.42,-.53,;-6.34,-2.07,;-7.75,.25,;-7.73,1.8,;-6.39,2.55,;-6.37,4.09,;-7.7,4.87,;-9.04,4.11,;-10.37,4.9,;-9.05,2.57,;-9.08,-.5,;-10.41,.28,;-10.4,1.82,;-11.76,-.48,;-11.78,-2.02,;-13.09,.3,;-14.43,-.45,;-14.44,-1.99,;-15.76,-2.75,;1.54,-6.53,;2.87,-7.3,;1.54,-4.99,;2.87,-4.22,;5.53,-13.46,;6.87,-14.23,;5.53,-11.92,;6.86,-11.15,;18.86,-16.55,;17.53,-15.78,;20.2,-15.78,;20.19,-14.24,;21.53,-13.47,;22.87,-14.24,;21.53,-11.93,;22.86,-11.16,;24.2,-11.93,;25.53,-11.16,;25.53,-9.63,;26.87,-11.93,;22.86,-9.62,;21.53,-8.85,;24.2,-8.85,;24.2,-7.31,;22.87,-6.54,;22.87,-5,;21.53,-4.24,;20.2,-5.01,;21.53,-2.7,;25.53,-6.54,;26.87,-7.31,;25.53,-5,;26.86,-4.23,;28.2,-5,;29.54,-4.23,;29.54,-2.7,;30.88,-1.93,;32.21,-2.7,;32.2,-4.24,;30.87,-5.01,;26.87,-2.7,;25.53,-1.93,;28.2,-1.93,;28.2,-.39,;26.87,.38,;26.87,1.92,;28.21,2.69,;25.54,2.69,;29.54,.38,;30.89,-.38,;29.54,1.92,;30.88,2.7,;32.22,1.92,;30.87,4.23,;32.21,5.01,;29.55,5,)| Show InChI InChI=1S/C108H170N32O31S/c1-12-54(6)84(101(166)122-52-79(143)125-75(50-83(150)151)98(163)130-68(34-38-81(146)147)92(157)134-72(47-59-24-16-15-17-25-59)97(162)135-74(49-78(111)142)95(160)124-58(10)105(170)171)137-93(158)65(29-21-42-119-107(114)115)127-89(154)64(28-20-41-118-106(112)113)128-96(161)71(46-53(4)5)133-91(156)67(33-37-80(144)145)129-90(155)66(32-36-77(110)141)126-87(152)57(9)123-102(167)85(55(7)13-2)139-99(164)73(48-60-51-121-63-27-19-18-26-61(60)63)136-103(168)86(56(8)14-3)138-94(159)69(35-39-82(148)149)131-100(165)76-31-23-44-140(76)104(169)70(30-22-43-120-108(116)117)132-88(153)62(109)40-45-172-11/h15-19,24-27,51,53-58,62,64-76,84-86,121H,12-14,20-23,28-50,52,109H2,1-11H3,(H2,110,141)(H2,111,142)(H,122,166)(H,123,167)(H,124,160)(H,125,143)(H,126,152)(H,127,154)(H,128,161)(H,129,155)(H,130,163)(H,131,165)(H,132,153)(H,133,156)(H,134,157)(H,135,162)(H,136,168)(H,137,158)(H,138,159)(H,139,164)(H,144,145)(H,146,147)(H,148,149)(H,150,151)(H,170,171)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120)/t54-,55-,56-,57-,58-,62-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,84-,85-,86-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged human Wild type Bcl-2-like protein 1 expressed in Escherichia coli BL21 cells at 40 uM ... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 2
(Homo sapiens (Human)) | BDBM50357756

(CHEMBL1916194)Show SMILES CC(C)[C@@H]1CN(Cc2ccccc2C(F)(F)F)CCN1c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN(C)C)CSc2ccccc2)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C40H47F3N6O5S2/c1-28(2)38-26-47(25-30-10-8-9-13-35(30)40(41,42)43)22-23-48(38)32-16-14-29(15-17-32)39(50)45-56(53,54)34-18-19-36(37(24-34)49(51)52)44-31(20-21-46(3)4)27-55-33-11-6-5-7-12-33/h5-19,24,28,31,38,44H,20-23,25-27H2,1-4H3,(H,45,50)/t31-,38+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged human wild type Bcl-W expressed in Escherichia coli BL21 cells at 10 uM after 3 hrs by ... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 2
(Homo sapiens (Human)) | BDBM21434

(N-Benylpiperazine derivative, 23j | N-[(4-{[(2R)-4...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2C(F)(F)F)CC1 |r| Show InChI InChI=1S/C37H41F3N6O5S2/c1-43(2)19-18-29(26-52-31-9-4-3-5-10-31)41-34-17-16-32(24-35(34)46(48)49)53(50,51)42-36(47)27-12-14-30(15-13-27)45-22-20-44(21-23-45)25-28-8-6-7-11-33(28)37(38,39)40/h3-17,24,29,41H,18-23,25-26H2,1-2H3,(H,42,47)/t29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged human wild type Bcl-W expressed in Escherichia coli BL21 cells at 10 uM after 3 hrs by ... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 2
(Homo sapiens (Human)) | BDBM50357753

(CHEMBL1916191)Show SMILES C[C@@H]1CN(CCN1Cc1ccccc1C(F)(F)F)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN(C)C)CSc2ccccc2)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C38H43F3N6O5S2/c1-27-24-46(22-21-45(27)25-29-9-7-8-12-34(29)38(39,40)41)31-15-13-28(14-16-31)37(48)43-54(51,52)33-17-18-35(36(23-33)47(49)50)42-30(19-20-44(2)3)26-53-32-10-5-4-6-11-32/h4-18,23,27,30,42H,19-22,24-26H2,1-3H3,(H,43,48)/t27-,30-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged human wild type Bcl-W expressed in Escherichia coli BL21 cells at 10 uM after 3 hrs by ... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 2
(Homo sapiens (Human)) | BDBM50357755

(CHEMBL1916193)Show SMILES CC(C)[C@H]1CN(Cc2ccccc2C(F)(F)F)CCN1c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN(C)C)CSc2ccccc2)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C40H47F3N6O5S2/c1-28(2)38-26-47(25-30-10-8-9-13-35(30)40(41,42)43)22-23-48(38)32-16-14-29(15-17-32)39(50)45-56(53,54)34-18-19-36(37(24-34)49(51)52)44-31(20-21-46(3)4)27-55-33-11-6-5-7-12-33/h5-19,24,28,31,38,44H,20-23,25-27H2,1-4H3,(H,45,50)/t31-,38-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged human wild type Bcl-W expressed in Escherichia coli BL21 cells at 10 uM after 3 hrs by ... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 2
(Homo sapiens (Human)) | BDBM50357752

(CHEMBL1916190)Show SMILES C[C@H]1CN(CCN1Cc1ccccc1C(F)(F)F)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN(C)C)CSc2ccccc2)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C38H43F3N6O5S2/c1-27-24-46(22-21-45(27)25-29-9-7-8-12-34(29)38(39,40)41)31-15-13-28(14-16-31)37(48)43-54(51,52)33-17-18-35(36(23-33)47(49)50)42-30(19-20-44(2)3)26-53-32-10-5-4-6-11-32/h4-18,23,27,30,42H,19-22,24-26H2,1-3H3,(H,43,48)/t27-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged human wild type Bcl-W expressed in Escherichia coli BL21 cells at 10 uM after 3 hrs by ... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50357754

(CHEMBL1916192)Show SMILES CC1CN(CC(C)N1Cc1ccccc1C(F)(F)F)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN(C)C)CSc2ccccc2)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C39H45F3N6O5S2/c1-27-23-46(24-28(2)47(27)25-30-10-8-9-13-35(30)39(40,41)42)32-16-14-29(15-17-32)38(49)44-55(52,53)34-18-19-36(37(22-34)48(50)51)43-31(20-21-45(3)4)26-54-33-11-6-5-7-12-33/h5-19,22,27-28,31,43H,20-21,23-26H2,1-4H3,(H,44,49)/t27?,28?,31-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged human Wild type Bcl-2-like protein 1 expressed in Escherichia coli BL21 cells at 10 uM ... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 2
(Homo sapiens (Human)) | BDBM50357754

(CHEMBL1916192)Show SMILES CC1CN(CC(C)N1Cc1ccccc1C(F)(F)F)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN(C)C)CSc2ccccc2)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C39H45F3N6O5S2/c1-27-23-46(24-28(2)47(27)25-30-10-8-9-13-35(30)39(40,41)42)32-16-14-29(15-17-32)38(49)44-55(52,53)34-18-19-36(37(22-34)48(50)51)43-31(20-21-45(3)4)26-54-33-11-6-5-7-12-33/h5-19,22,27-28,31,43H,20-21,23-26H2,1-4H3,(H,44,49)/t27?,28?,31-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged human wild type Bcl-W expressed in Escherichia coli BL21 cells at 10 uM after 3 hrs by ... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 homolog
(Mus musculus (Mouse)) | BDBM50357753

(CHEMBL1916191)Show SMILES C[C@@H]1CN(CCN1Cc1ccccc1C(F)(F)F)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN(C)C)CSc2ccccc2)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C38H43F3N6O5S2/c1-27-24-46(22-21-45(27)25-29-9-7-8-12-34(29)38(39,40)41)31-15-13-28(14-16-31)37(48)43-54(51,52)33-17-18-35(36(23-33)47(49)50)42-30(19-20-44(2)3)26-53-32-10-5-4-6-11-32/h4-18,23,27,30,42H,19-22,24-26H2,1-3H3,(H,43,48)/t27-,30-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged mouse wild type MCL1 expressed in Escherichia coli BL21 cells at 10 uM after 3 hrs by f... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 homolog
(Mus musculus (Mouse)) | BDBM50357756

(CHEMBL1916194)Show SMILES CC(C)[C@@H]1CN(Cc2ccccc2C(F)(F)F)CCN1c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN(C)C)CSc2ccccc2)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C40H47F3N6O5S2/c1-28(2)38-26-47(25-30-10-8-9-13-35(30)40(41,42)43)22-23-48(38)32-16-14-29(15-17-32)39(50)45-56(53,54)34-18-19-36(37(24-34)49(51)52)44-31(20-21-46(3)4)27-55-33-11-6-5-7-12-33/h5-19,24,28,31,38,44H,20-23,25-27H2,1-4H3,(H,45,50)/t31-,38+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged mouse wild type MCL1 expressed in Escherichia coli BL21 cells at 10 uM after 3 hrs by f... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 homolog
(Mus musculus (Mouse)) | BDBM50357755

(CHEMBL1916193)Show SMILES CC(C)[C@H]1CN(Cc2ccccc2C(F)(F)F)CCN1c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN(C)C)CSc2ccccc2)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C40H47F3N6O5S2/c1-28(2)38-26-47(25-30-10-8-9-13-35(30)40(41,42)43)22-23-48(38)32-16-14-29(15-17-32)39(50)45-56(53,54)34-18-19-36(37(24-34)49(51)52)44-31(20-21-46(3)4)27-55-33-11-6-5-7-12-33/h5-19,24,28,31,38,44H,20-23,25-27H2,1-4H3,(H,45,50)/t31-,38-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged mouse wild type MCL1 expressed in Escherichia coli BL21 cells at 10 uM after 3 hrs by f... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 homolog
(Mus musculus (Mouse)) | BDBM50357752

(CHEMBL1916190)Show SMILES C[C@H]1CN(CCN1Cc1ccccc1C(F)(F)F)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN(C)C)CSc2ccccc2)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C38H43F3N6O5S2/c1-27-24-46(22-21-45(27)25-29-9-7-8-12-34(29)38(39,40)41)31-15-13-28(14-16-31)37(48)43-54(51,52)33-17-18-35(36(23-33)47(49)50)42-30(19-20-44(2)3)26-53-32-10-5-4-6-11-32/h4-18,23,27,30,42H,19-22,24-26H2,1-3H3,(H,43,48)/t27-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged mouse wild type MCL1 expressed in Escherichia coli BL21 cells at 10 uM after 3 hrs by f... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537079
(CHEMBL4587303)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCNC(=O)NCCCCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O |r,t:17,19| Show InChI InChI=1S/C90H123ClN16O21S4/c1-50-22-21-28-71(125-9)90(123)45-70(126-88(122)105-90)51(2)77-89(5,128-77)72(44-74(111)107(7)68-41-56(38-50)42-69(124-8)75(68)91)127-86(120)52(3)106(6)73(110)33-37-129-130-47-65(78(94)112)101-84(118)67-49-132-131-48-66(102-79(113)60(93)39-54-23-13-12-14-24-54)83(117)99-63(40-55-29-31-58(109)32-30-55)81(115)100-64(43-57-46-97-61-26-16-15-25-59(57)61)82(116)98-62(80(114)104-76(53(4)108)85(119)103-67)27-17-20-36-96-87(121)95-35-19-11-10-18-34-92/h12-16,21-26,28-32,41-42,46,51-53,60,62-67,70-72,76-77,97,108-109,123H,10-11,17-20,27,33-40,43-45,47-49,92-93H2,1-9H3,(H2,94,112)(H,98,116)(H,99,117)(H,100,115)(H,101,118)(H,102,113)(H,103,119)(H,104,114)(H,105,122)(H2,95,96,121)/b28-21+,50-22+/t51-,52+,53-,60-,62+,63+,64-,65+,66+,67+,70+,71-,72+,76+,77+,89-,90+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 homolog
(Mus musculus (Mouse)) | BDBM21434

(N-Benylpiperazine derivative, 23j | N-[(4-{[(2R)-4...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2C(F)(F)F)CC1 |r| Show InChI InChI=1S/C37H41F3N6O5S2/c1-43(2)19-18-29(26-52-31-9-4-3-5-10-31)41-34-17-16-32(24-35(34)46(48)49)53(50,51)42-36(47)27-12-14-30(15-13-27)45-22-20-44(21-23-45)25-28-8-6-7-11-33(28)37(38,39)40/h3-17,24,29,41H,18-23,25-26H2,1-2H3,(H,42,47)/t29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged mouse wild type MCL1 expressed in Escherichia coli BL21 cells at 10 uM after 3 hrs by f... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 homolog
(Mus musculus (Mouse)) | BDBM50357754

(CHEMBL1916192)Show SMILES CC1CN(CC(C)N1Cc1ccccc1C(F)(F)F)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN(C)C)CSc2ccccc2)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C39H45F3N6O5S2/c1-27-23-46(24-28(2)47(27)25-30-10-8-9-13-35(30)39(40,41)42)32-16-14-29(15-17-32)38(49)44-55(52,53)34-18-19-36(37(22-34)48(50)51)43-31(20-21-45(3)4)26-54-33-11-6-5-7-12-33/h5-19,22,27-28,31,43H,20-21,23-26H2,1-4H3,(H,44,49)/t27?,28?,31-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged mouse wild type MCL1 expressed in Escherichia coli BL21 cells at 10 uM after 3 hrs by f... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50357751

(CHEMBL1916198)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#16])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1)-[#6](-[#6])-[#6])-[#6@@H](-[#6])-[#8])-[#6](-[#8])=O |r| Show InChI InChI=1S/C136H224N40O39S/c1-67(2)56-89(168-119(200)85(41-46-100(140)179)165-132(213)108(75(15)177)176-110(191)74(14)154-130(211)98(66-216)174-121(202)87(43-48-104(184)185)164-131(212)107(72(11)12)175-122(203)88(44-49-105(186)187)163-124(205)90(57-68(3)4)169-120(201)86(42-47-103(182)183)156-109(190)73(13)153-112(193)78-36-26-52-148-78)123(204)160-81(37-27-53-149-134(142)143)113(194)158-83(39-29-55-151-136(146)147)117(198)170-93(61-76-30-18-16-19-31-76)111(192)152-65-102(181)155-96(64-106(188)189)129(210)159-80(35-23-25-51-138)116(197)166-91(58-69(5)6)125(206)172-95(63-101(141)180)128(209)171-94(62-77-32-20-17-21-33-77)127(208)161-82(38-28-54-150-135(144)145)114(195)162-84(40-45-99(139)178)118(199)157-79(34-22-24-50-137)115(196)167-92(59-70(7)8)126(207)173-97(133(214)215)60-71(9)10/h16-21,30-33,67-75,78-98,107-108,148,177,216H,22-29,34-66,137-138H2,1-15H3,(H2,139,178)(H2,140,179)(H2,141,180)(H,152,192)(H,153,193)(H,154,211)(H,155,181)(H,156,190)(H,157,199)(H,158,194)(H,159,210)(H,160,204)(H,161,208)(H,162,195)(H,163,205)(H,164,212)(H,165,213)(H,166,197)(H,167,196)(H,168,200)(H,169,201)(H,170,198)(H,171,209)(H,172,206)(H,173,207)(H,174,202)(H,175,203)(H,176,191)(H,182,183)(H,184,185)(H,186,187)(H,188,189)(H,214,215)(H4,142,143,149)(H4,144,145,150)(H4,146,147,151)/t73-,74-,75+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,107-,108-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-Bak conjugated peptide from GST-tagged human Wild type Bcl-2-like protein 1 expressed in Escherichia coli BL21 cells at 40 uM ... |
Bioorg Med Chem Lett 21: 4951-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.134
BindingDB Entry DOI: 10.7270/Q2DB8287 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































