 Found 35 hits with Last Name = 'brocklehurst' and Initial = 'ce'
Found 35 hits with Last Name = 'brocklehurst' and Initial = 'ce' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase WNK1 [166-489]
(Homo sapiens (Human)) | BDBM203827
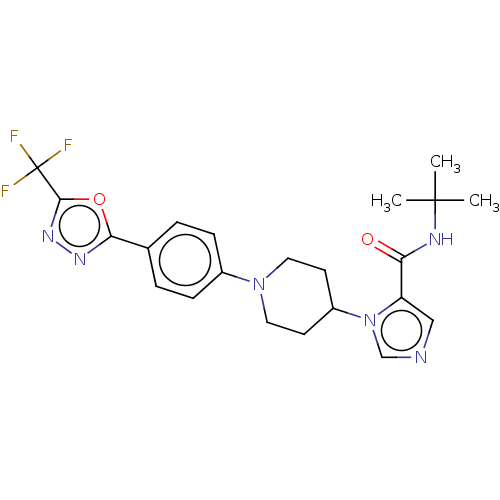
(N-(tert-butyl)-1-(1-(5-(5-(trifluoromethyl)-1,3,4-...)Show SMILES CC(C)(C)NC(=O)c1cncn1C1CCN(CC1)c1ccc(cc1)-c1nnc(o1)C(F)(F)F Show InChI InChI=1S/C22H25F3N6O2/c1-21(2,3)27-18(32)17-12-26-13-31(17)16-8-10-30(11-9-16)15-6-4-14(5-7-15)19-28-29-20(33-19)22(23,24)25/h4-7,12-13,16H,8-11H2,1-3H3,(H,27,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Novartis Institutes
| Assay Description
Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... |
Nat Chem Biol 12: 896-898 (2016)
Article DOI: 10.1038/nchembio.2168
BindingDB Entry DOI: 10.7270/Q2ZK5FH5 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187693

(CHEMBL3186509)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCN(CCC(=O)c3ccc4[nH]c(=O)oc4c3)CC2)c1 Show InChI InChI=1S/C22H21Cl2N3O5/c23-16-9-14(10-17(24)12-16)13-31-22(30)27-7-5-26(6-8-27)4-3-19(28)15-1-2-18-20(11-15)32-21(29)25-18/h1-2,9-12H,3-8,13H2,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM241106
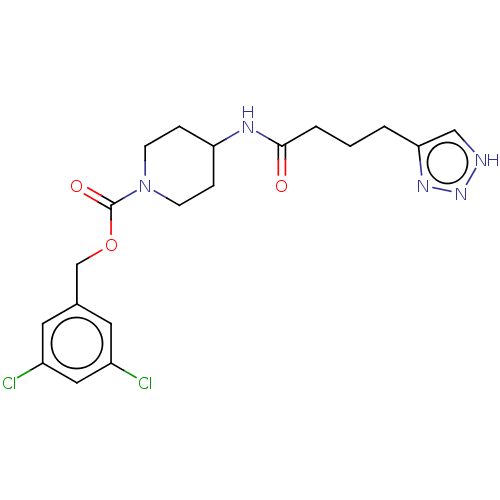
(US9409895, 17 | US9630945, 17)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCC(CC2)NC(=O)CCCc2c[nH]nn2)c1 Show InChI InChI=1S/C19H23Cl2N5O3/c20-14-8-13(9-15(21)10-14)12-29-19(28)26-6-4-16(5-7-26)23-18(27)3-1-2-17-11-22-25-24-17/h8-11,16H,1-7,12H2,(H,23,27)(H,22,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM241109
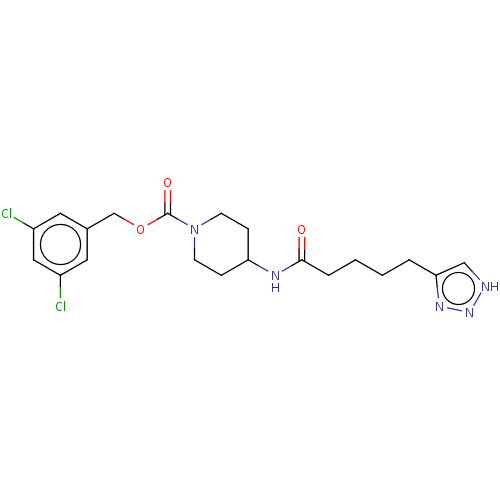
(US9409895, 20 | US9630945, 20)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCC(CC2)NC(=O)CCCCc2c[nH]nn2)c1 Show InChI InChI=1S/C20H25Cl2N5O3/c21-15-9-14(10-16(22)11-15)13-30-20(29)27-7-5-17(6-8-27)24-19(28)4-2-1-3-18-12-23-26-25-18/h9-12,17H,1-8,13H2,(H,24,28)(H,23,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50274089
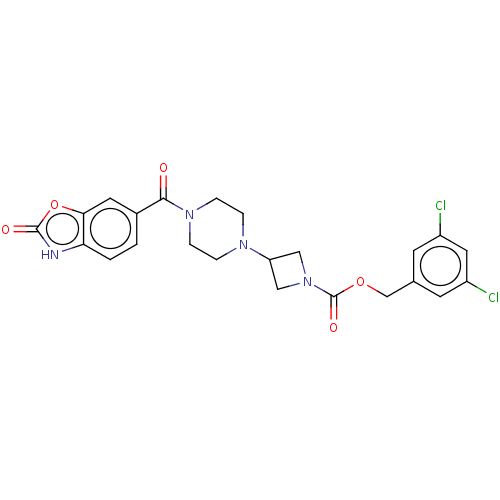
(CHEMBL4128124)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CC(C2)N2CCN(CC2)C(=O)c2ccc3[nH]c(=O)oc3c2)c1 Show InChI InChI=1S/C23H22Cl2N4O5/c24-16-7-14(8-17(25)10-16)13-33-23(32)29-11-18(12-29)27-3-5-28(6-4-27)21(30)15-1-2-19-20(9-15)34-22(31)26-19/h1-2,7-10,18H,3-6,11-13H2,(H,26,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM241108
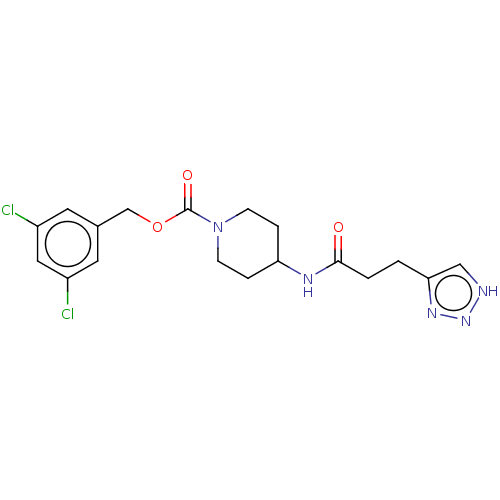
(US9409895, 19 | US9630945, 19)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCC(CC2)NC(=O)CCc2c[nH]nn2)c1 Show InChI InChI=1S/C18H21Cl2N5O3/c19-13-7-12(8-14(20)9-13)11-28-18(27)25-5-3-15(4-6-25)22-17(26)2-1-16-10-21-24-23-16/h7-10,15H,1-6,11H2,(H,22,26)(H,21,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase WNK1 [1-491]
(Homo sapiens (Human)) | BDBM203827

(N-(tert-butyl)-1-(1-(5-(5-(trifluoromethyl)-1,3,4-...)Show SMILES CC(C)(C)NC(=O)c1cncn1C1CCN(CC1)c1ccc(cc1)-c1nnc(o1)C(F)(F)F Show InChI InChI=1S/C22H25F3N6O2/c1-21(2,3)27-18(32)17-12-26-13-31(17)16-8-10-30(11-9-16)15-6-4-14(5-7-15)19-28-29-20(33-19)22(23,24)25/h4-7,12-13,16H,8-11H2,1-3H3,(H,27,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Novartis Institutes
| Assay Description
Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... |
Nat Chem Biol 12: 896-898 (2016)
Article DOI: 10.1038/nchembio.2168
BindingDB Entry DOI: 10.7270/Q2ZK5FH5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase WNK1 [1-434]
(Homo sapiens (Human)) | BDBM203827

(N-(tert-butyl)-1-(1-(5-(5-(trifluoromethyl)-1,3,4-...)Show SMILES CC(C)(C)NC(=O)c1cncn1C1CCN(CC1)c1ccc(cc1)-c1nnc(o1)C(F)(F)F Show InChI InChI=1S/C22H25F3N6O2/c1-21(2,3)27-18(32)17-12-26-13-31(17)16-8-10-30(11-9-16)15-6-4-14(5-7-15)19-28-29-20(33-19)22(23,24)25/h4-7,12-13,16H,8-11H2,1-3H3,(H,27,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Novartis Institutes
| Assay Description
Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... |
Nat Chem Biol 12: 896-898 (2016)
Article DOI: 10.1038/nchembio.2168
BindingDB Entry DOI: 10.7270/Q2ZK5FH5 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM241133
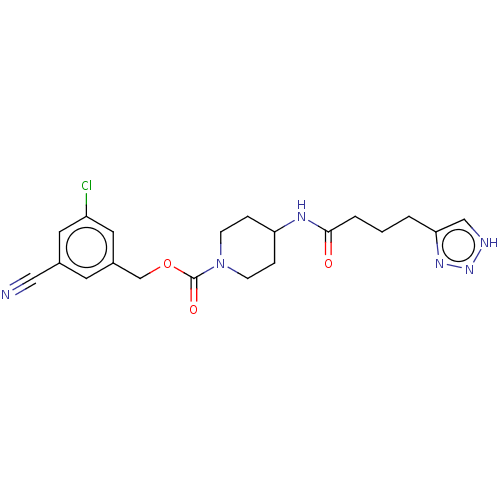
(US9409895, 44 | US9630945, 44)Show SMILES Clc1cc(COC(=O)N2CCC(CC2)NC(=O)CCCc2c[nH]nn2)cc(c1)C#N Show InChI InChI=1S/C20H23ClN6O3/c21-16-9-14(11-22)8-15(10-16)13-30-20(29)27-6-4-17(5-7-27)24-19(28)3-1-2-18-12-23-26-25-18/h8-10,12,17H,1-7,13H2,(H,24,28)(H,23,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50274085
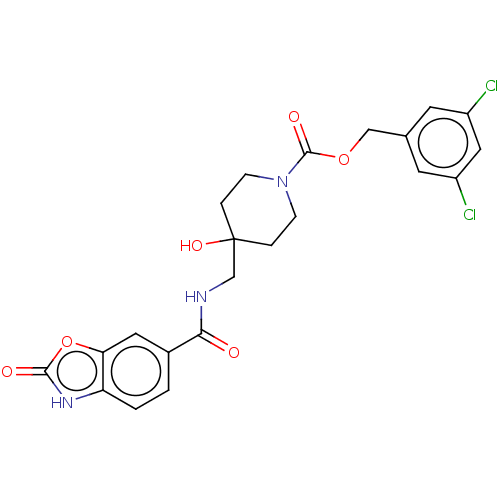
(CHEMBL4129507)Show SMILES OC1(CNC(=O)c2ccc3[nH]c(=O)oc3c2)CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C22H21Cl2N3O6/c23-15-7-13(8-16(24)10-15)11-32-21(30)27-5-3-22(31,4-6-27)12-25-19(28)14-1-2-17-18(9-14)33-20(29)26-17/h1-2,7-10,31H,3-6,11-12H2,(H,25,28)(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM241106

(US9409895, 17 | US9630945, 17)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCC(CC2)NC(=O)CCCc2c[nH]nn2)c1 Show InChI InChI=1S/C19H23Cl2N5O3/c20-14-8-13(9-15(21)10-14)12-29-19(28)26-6-4-16(5-7-26)23-18(27)3-1-2-17-11-22-25-24-17/h8-11,16H,1-7,12H2,(H,23,27)(H,22,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX in human serum assessed as reduction in LPA 18:1 levels after 3 hrs by LC-MS/MS analysis |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase WNK1 [1-444]
(Homo sapiens (Human)) | BDBM203827

(N-(tert-butyl)-1-(1-(5-(5-(trifluoromethyl)-1,3,4-...)Show SMILES CC(C)(C)NC(=O)c1cncn1C1CCN(CC1)c1ccc(cc1)-c1nnc(o1)C(F)(F)F Show InChI InChI=1S/C22H25F3N6O2/c1-21(2,3)27-18(32)17-12-26-13-31(17)16-8-10-30(11-9-16)15-6-4-14(5-7-15)19-28-29-20(33-19)22(23,24)25/h4-7,12-13,16H,8-11H2,1-3H3,(H,27,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Novartis Institutes
| Assay Description
Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... |
Nat Chem Biol 12: 896-898 (2016)
Article DOI: 10.1038/nchembio.2168
BindingDB Entry DOI: 10.7270/Q2ZK5FH5 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50274084
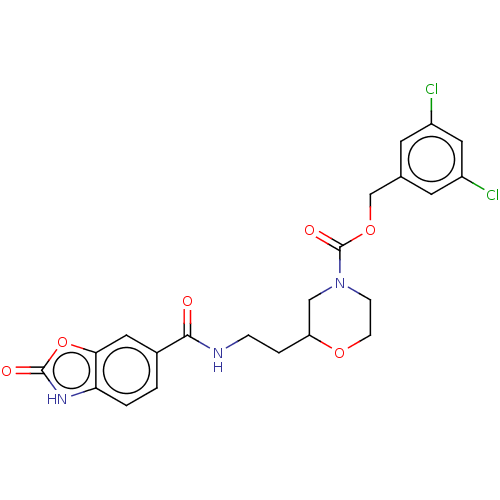
(CHEMBL4126051)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCOC(CCNC(=O)c3ccc4[nH]c(=O)oc4c3)C2)c1 Show InChI InChI=1S/C22H21Cl2N3O6/c23-15-7-13(8-16(24)10-15)12-32-22(30)27-5-6-31-17(11-27)3-4-25-20(28)14-1-2-18-19(9-14)33-21(29)26-18/h1-2,7-10,17H,3-6,11-12H2,(H,25,28)(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50274081

(CHEMBL4127145)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCC(C2)N2CCN(CC2)C(=O)c2ccc3[nH]c(=O)oc3c2)c1 Show InChI InChI=1S/C24H24Cl2N4O5/c25-17-9-15(10-18(26)12-17)14-34-24(33)30-4-3-19(13-30)28-5-7-29(8-6-28)22(31)16-1-2-20-21(11-16)35-23(32)27-20/h1-2,9-12,19H,3-8,13-14H2,(H,27,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM241121
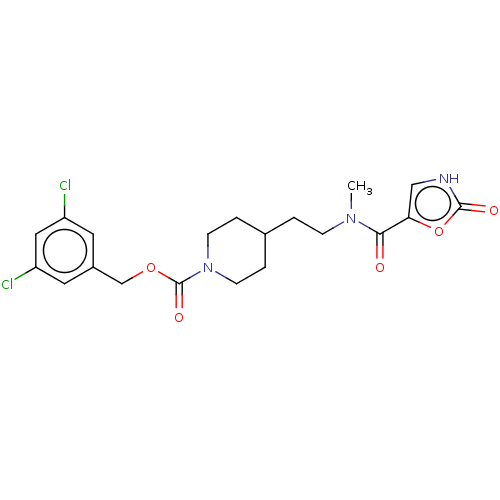
(US9409895, 32 | US9630945, 32)Show SMILES CN(CCC1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1)C(=O)c1c[nH]c(=O)o1 Show InChI InChI=1S/C20H23Cl2N3O5/c1-24(18(26)17-11-23-19(27)30-17)5-2-13-3-6-25(7-4-13)20(28)29-12-14-8-15(21)10-16(22)9-14/h8-11,13H,2-7,12H2,1H3,(H,23,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM241143
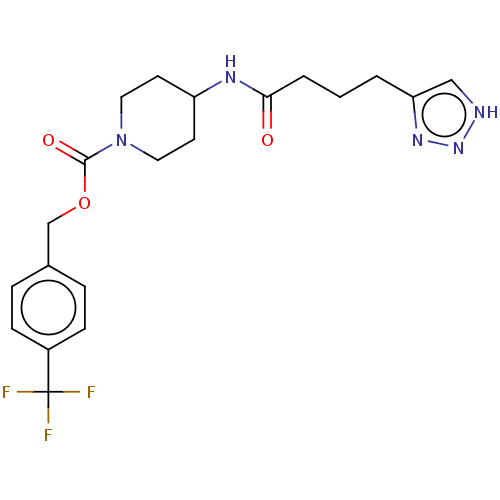
(US9409895, 53 | US9630945, 53)Show SMILES FC(F)(F)c1ccc(COC(=O)N2CCC(CC2)NC(=O)CCCc2c[nH]nn2)cc1 Show InChI InChI=1S/C20H24F3N5O3/c21-20(22,23)15-6-4-14(5-7-15)13-31-19(30)28-10-8-16(9-11-28)25-18(29)3-1-2-17-12-24-27-26-17/h4-7,12,16H,1-3,8-11,13H2,(H,25,29)(H,24,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase WNK1 [1-491]
(Homo sapiens (Human)) | BDBM203827

(N-(tert-butyl)-1-(1-(5-(5-(trifluoromethyl)-1,3,4-...)Show SMILES CC(C)(C)NC(=O)c1cncn1C1CCN(CC1)c1ccc(cc1)-c1nnc(o1)C(F)(F)F Show InChI InChI=1S/C22H25F3N6O2/c1-21(2,3)27-18(32)17-12-26-13-31(17)16-8-10-30(11-9-16)15-6-4-14(5-7-15)19-28-29-20(33-19)22(23,24)25/h4-7,12-13,16H,8-11H2,1-3H3,(H,27,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.3 | 4 |
Novartis Institutes
| Assay Description
Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... |
Nat Chem Biol 12: 896-898 (2016)
Article DOI: 10.1038/nchembio.2168
BindingDB Entry DOI: 10.7270/Q2ZK5FH5 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM241126
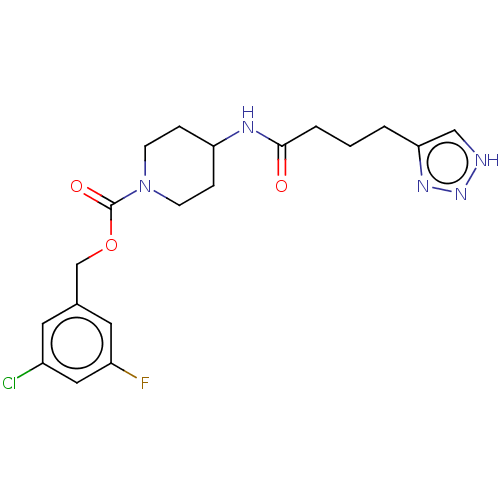
(US9409895, 37 | US9630945, 37)Show SMILES Fc1cc(Cl)cc(COC(=O)N2CCC(CC2)NC(=O)CCCc2c[nH]nn2)c1 Show InChI InChI=1S/C19H23ClFN5O3/c20-14-8-13(9-15(21)10-14)12-29-19(28)26-6-4-16(5-7-26)23-18(27)3-1-2-17-11-22-25-24-17/h8-11,16H,1-7,12H2,(H,23,27)(H,22,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM241110
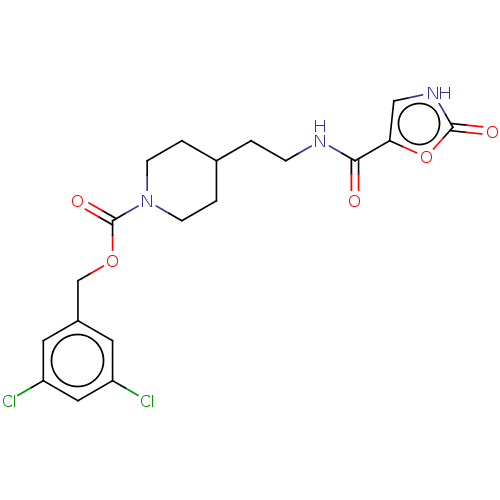
(US9409895, 21 | US9630945, 21)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCC(CCNC(=O)c3c[nH]c(=O)o3)CC2)c1 Show InChI InChI=1S/C19H21Cl2N3O5/c20-14-7-13(8-15(21)9-14)11-28-19(27)24-5-2-12(3-6-24)1-4-22-17(25)16-10-23-18(26)29-16/h7-10,12H,1-6,11H2,(H,22,25)(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM241107
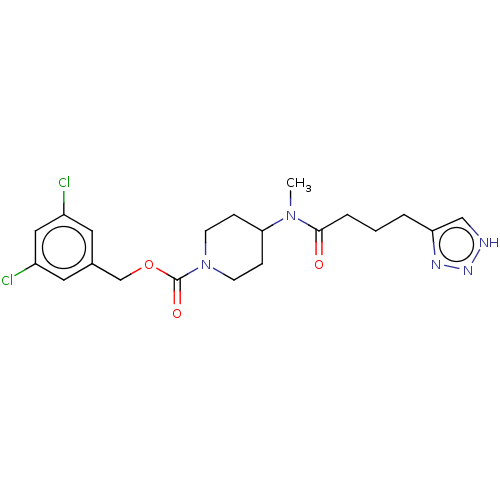
(US9409895, 18 | US9630945, 18)Show SMILES CN(C1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1)C(=O)CCCc1c[nH]nn1 Show InChI InChI=1S/C20H25Cl2N5O3/c1-26(19(28)4-2-3-17-12-23-25-24-17)18-5-7-27(8-6-18)20(29)30-13-14-9-15(21)11-16(22)10-14/h9-12,18H,2-8,13H2,1H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50274086

(CHEMBL4130315)Show SMILES O=C(OCc1ccccc1)N1CCN(CCC(=O)c2ccc3[nH]c(=O)oc3c2)CC1 Show InChI InChI=1S/C22H23N3O5/c26-19(17-6-7-18-20(14-17)30-21(27)23-18)8-9-24-10-12-25(13-11-24)22(28)29-15-16-4-2-1-3-5-16/h1-7,14H,8-13,15H2,(H,23,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM241124
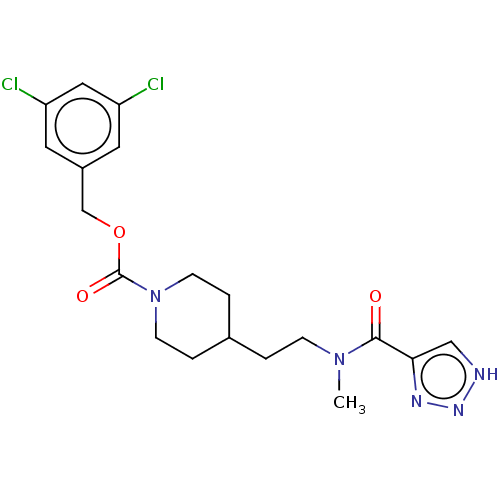
(US9409895, 35 | US9630945, 35)Show SMILES CN(CCC1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1)C(=O)c1c[nH]nn1 Show InChI InChI=1S/C19H23Cl2N5O3/c1-25(18(27)17-11-22-24-23-17)5-2-13-3-6-26(7-4-13)19(28)29-12-14-8-15(20)10-16(21)9-14/h8-11,13H,2-7,12H2,1H3,(H,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50274087
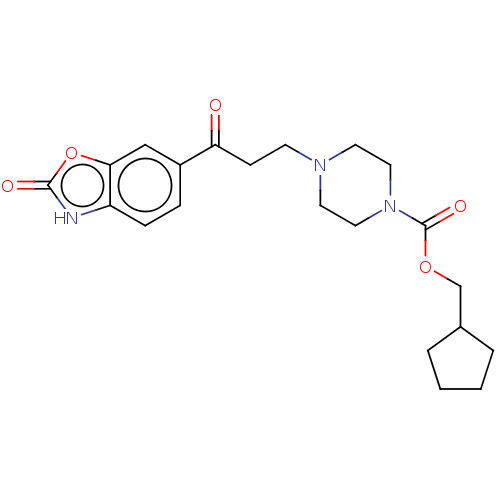
(CHEMBL4129365)Show SMILES O=C(CCN1CCN(CC1)C(=O)OCC1CCCC1)c1ccc2[nH]c(=O)oc2c1 Show InChI InChI=1S/C21H27N3O5/c25-18(16-5-6-17-19(13-16)29-20(26)22-17)7-8-23-9-11-24(12-10-23)21(27)28-14-15-3-1-2-4-15/h5-6,13,15H,1-4,7-12,14H2,(H,22,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM241148
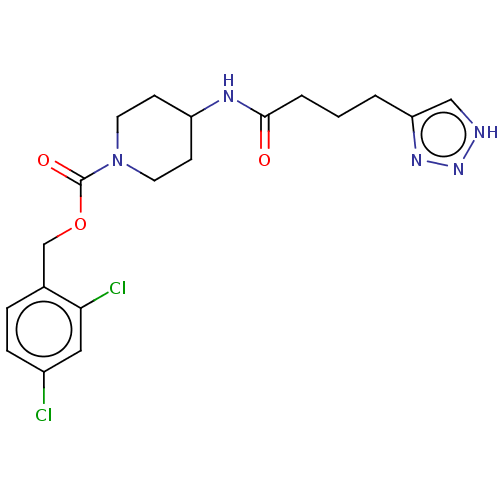
(US9409895, 58 | US9630945, 58)Show SMILES Clc1ccc(COC(=O)N2CCC(CC2)NC(=O)CCCc2c[nH]nn2)c(Cl)c1 Show InChI InChI=1S/C19H23Cl2N5O3/c20-14-5-4-13(17(21)10-14)12-29-19(28)26-8-6-15(7-9-26)23-18(27)3-1-2-16-11-22-25-24-16/h4-5,10-11,15H,1-3,6-9,12H2,(H,23,27)(H,22,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50274088

(CHEMBL4127038)Show SMILES O=C(CCN1CCN(CC1)C(=O)OCC1CCCCC1)c1ccc2[nH]c(=O)oc2c1 Show InChI InChI=1S/C22H29N3O5/c26-19(17-6-7-18-20(14-17)30-21(27)23-18)8-9-24-10-12-25(13-11-24)22(28)29-15-16-4-2-1-3-5-16/h6-7,14,16H,1-5,8-13,15H2,(H,23,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM241116
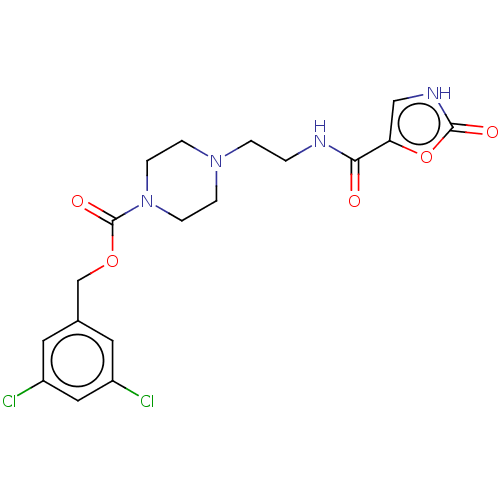
(US9409895, 27 | US9630945, 27)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCN(CCNC(=O)c3c[nH]c(=O)o3)CC2)c1 Show InChI InChI=1S/C18H20Cl2N4O5/c19-13-7-12(8-14(20)9-13)11-28-18(27)24-5-3-23(4-6-24)2-1-21-16(25)15-10-22-17(26)29-15/h7-10H,1-6,11H2,(H,21,25)(H,22,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase WNK1 [1-491]
(Homo sapiens (Human)) | BDBM203826
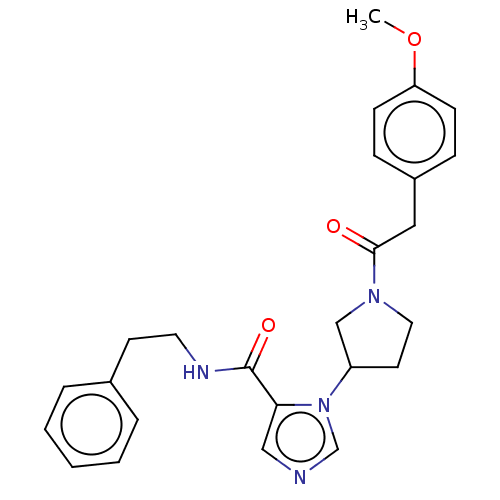
((R)-1-(1-(2-(4-methoxyphenyl)acetyl)pyrrolidin-3-y...)Show SMILES COc1ccc(CC(=O)N2CCC(C2)n2cncc2C(=O)NCCc2ccccc2)cc1 Show InChI InChI=1S/C25H28N4O3/c1-32-22-9-7-20(8-10-22)15-24(30)28-14-12-21(17-28)29-18-26-16-23(29)25(31)27-13-11-19-5-3-2-4-6-19/h2-10,16,18,21H,11-15,17H2,1H3,(H,27,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 856 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Novartis Institutes
| Assay Description
Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... |
Nat Chem Biol 12: 896-898 (2016)
Article DOI: 10.1038/nchembio.2168
BindingDB Entry DOI: 10.7270/Q2ZK5FH5 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50274112
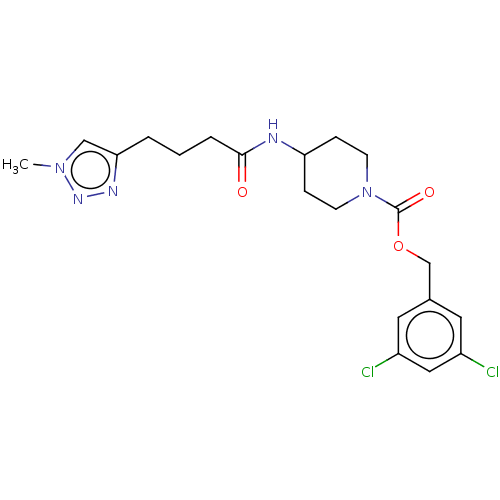
(CHEMBL4127963)Show SMILES Cn1cc(CCCC(=O)NC2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)nn1 Show InChI InChI=1S/C20H25Cl2N5O3/c1-26-12-18(24-25-26)3-2-4-19(28)23-17-5-7-27(8-6-17)20(29)30-13-14-9-15(21)11-16(22)10-14/h9-12,17H,2-8,13H2,1H3,(H,23,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50187693

(CHEMBL3186509)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCN(CCC(=O)c3ccc4[nH]c(=O)oc4c3)CC2)c1 Show InChI InChI=1S/C22H21Cl2N3O5/c23-16-9-14(10-17(24)12-16)13-31-22(30)27-7-5-26(6-8-27)4-3-19(28)15-1-2-18-20(11-15)32-21(29)25-18/h1-2,9-12H,3-8,13H2,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM241121

(US9409895, 32 | US9630945, 32)Show SMILES CN(CCC1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1)C(=O)c1c[nH]c(=O)o1 Show InChI InChI=1S/C20H23Cl2N3O5/c1-24(18(26)17-11-23-19(27)30-17)5-2-13-3-6-25(7-4-13)20(28)29-12-14-8-15(21)10-16(22)9-14/h8-11,13H,2-7,12H2,1H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells at -90 mV holding potential by Qpatch clamp assay |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM241124

(US9409895, 35 | US9630945, 35)Show SMILES CN(CCC1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1)C(=O)c1c[nH]nn1 Show InChI InChI=1S/C19H23Cl2N5O3/c1-25(18(27)17-11-22-24-23-17)5-2-13-3-6-26(7-4-13)19(28)29-12-14-8-15(20)10-16(21)9-14/h8-11,13H,2-7,12H2,1H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells at -90 mV holding potential by Qpatch clamp assay |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM241108

(US9409895, 19 | US9630945, 19)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCC(CC2)NC(=O)CCc2c[nH]nn2)c1 Show InChI InChI=1S/C18H21Cl2N5O3/c19-13-7-12(8-14(20)9-13)11-28-18(27)25-5-3-15(4-6-25)22-17(26)2-1-16-10-21-24-23-16/h7-10,15H,1-6,11H2,(H,22,26)(H,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells at -90 mV holding potential by Qpatch clamp assay |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM241109

(US9409895, 20 | US9630945, 20)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCC(CC2)NC(=O)CCCCc2c[nH]nn2)c1 Show InChI InChI=1S/C20H25Cl2N5O3/c21-15-9-14(10-16(22)11-15)13-30-20(29)27-7-5-17(6-8-27)24-19(28)4-2-1-3-18-12-23-26-25-18/h9-12,17H,1-8,13H2,(H,24,28)(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells at -90 mV holding potential by Qpatch clamp assay |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM241106

(US9409895, 17 | US9630945, 17)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCC(CC2)NC(=O)CCCc2c[nH]nn2)c1 Show InChI InChI=1S/C19H23Cl2N5O3/c20-14-8-13(9-15(21)10-14)12-29-19(28)26-6-4-16(5-7-26)23-18(27)3-1-2-17-11-22-25-24-17/h8-11,16H,1-7,12H2,(H,23,27)(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells at -90 mV holding potential by Qpatch clamp assay |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM241121

(US9409895, 32 | US9630945, 32)Show SMILES CN(CCC1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1)C(=O)c1c[nH]c(=O)o1 Show InChI InChI=1S/C20H23Cl2N3O5/c1-24(18(26)17-11-23-19(27)30-17)5-2-13-3-6-25(7-4-13)20(28)29-12-14-8-15(21)10-16(22)9-14/h8-11,13H,2-7,12H2,1H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data