

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205093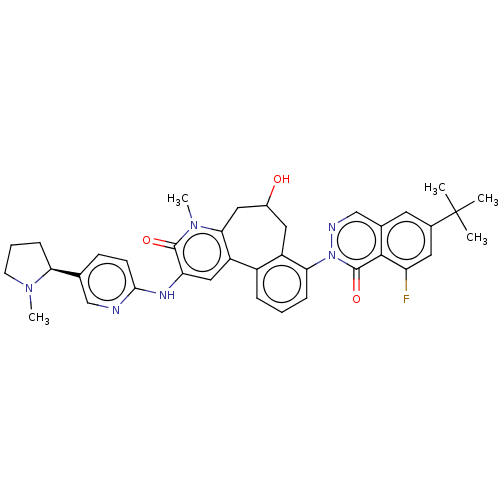 (US9556147, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... | US Patent US9556147 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205094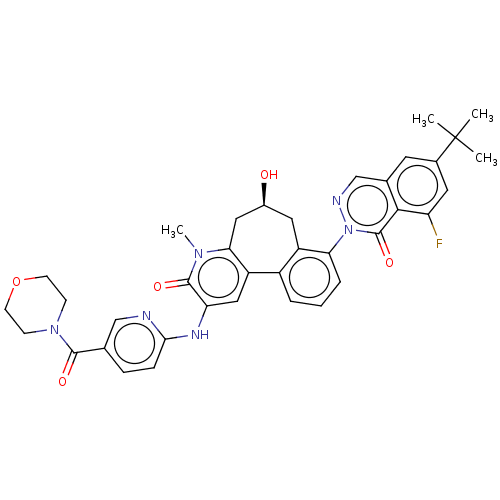 (US9556147, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... | US Patent US9556147 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FP9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205082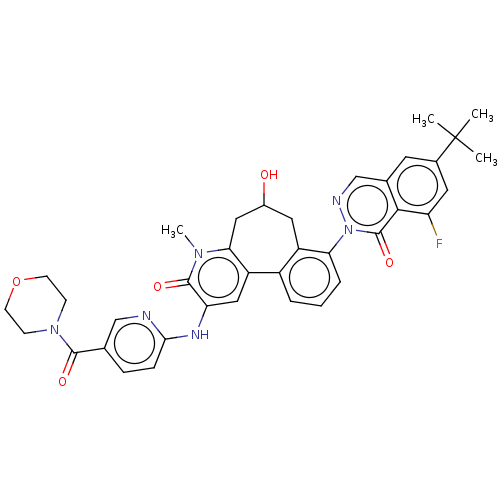 (US9556147, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... | US Patent US9556147 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FP9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205095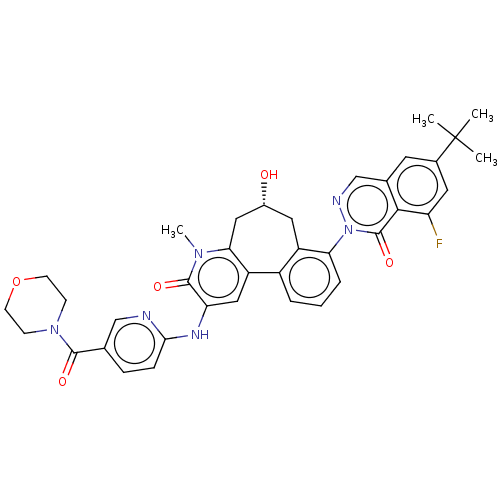 (US9556147, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... | US Patent US9556147 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM291171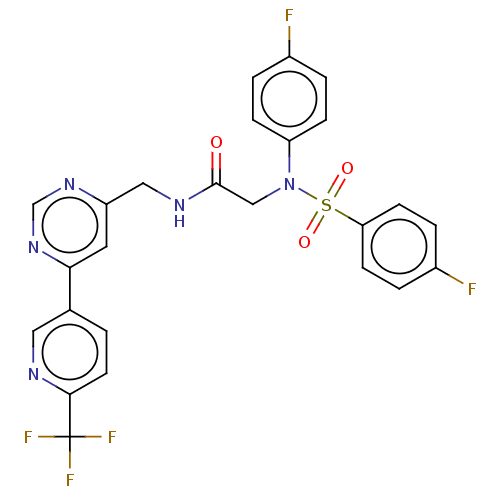 (US9580411, Example 86) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e... | US Patent US9580411 (2017) BindingDB Entry DOI: 10.7270/Q25T3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM291170 (2-[N-(Propan-2-yl)(4-fluorobenzene)sulfonamido]-N-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e... | US Patent US9580411 (2017) BindingDB Entry DOI: 10.7270/Q25T3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM291169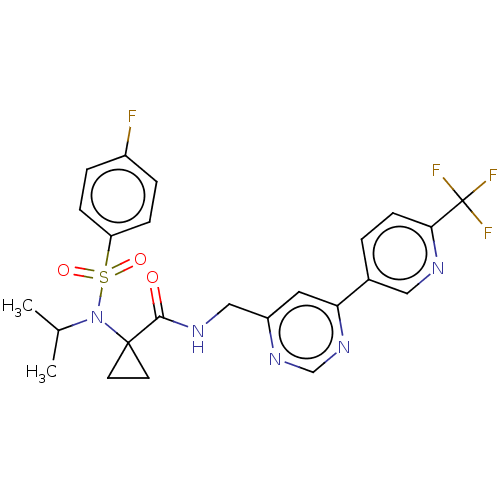 (1-(4-Fluoro-N-isopropylphenylsulfonamido)-N-((6-(6...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e... | US Patent US9580411 (2017) BindingDB Entry DOI: 10.7270/Q25T3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415550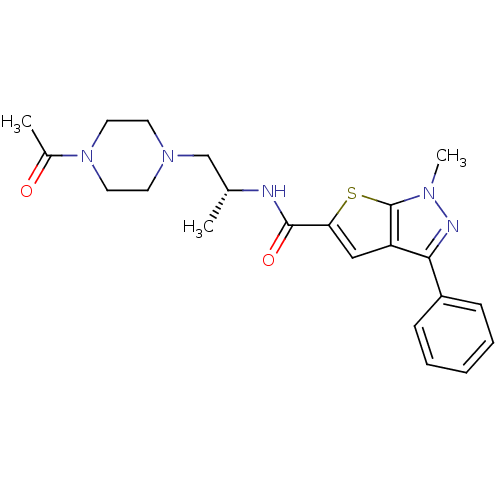 (CHEMBL599760 | Ro-85) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM291163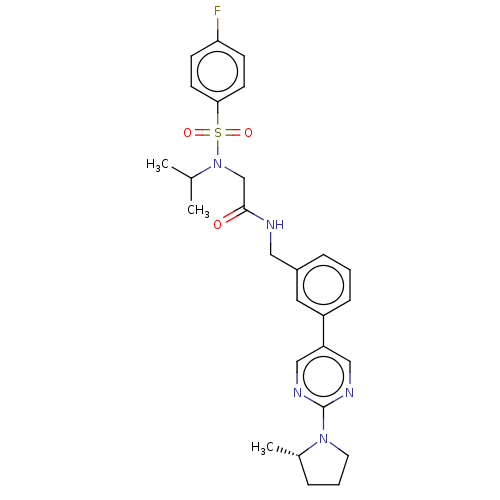 (US9580411, Example 79-(R) | US9580411, Example 79-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e... | US Patent US9580411 (2017) BindingDB Entry DOI: 10.7270/Q25T3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415553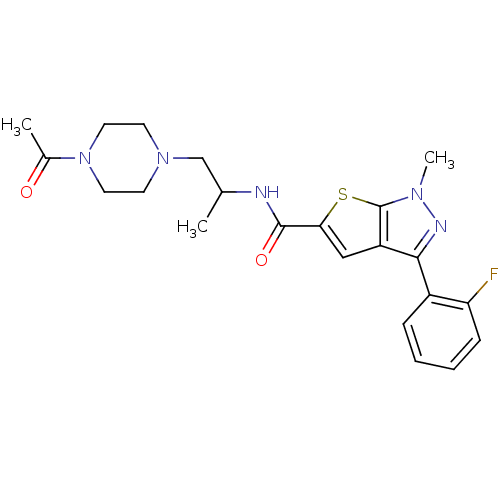 (CHEMBL605130) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415601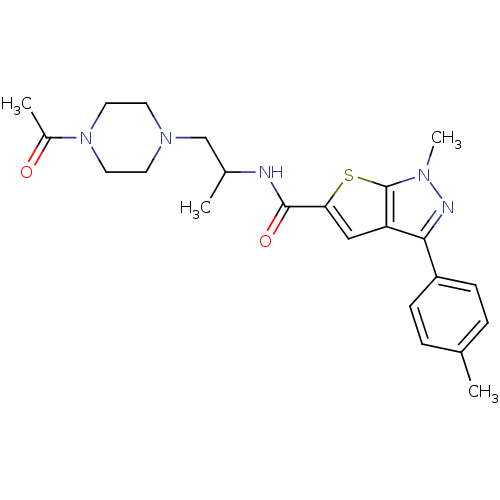 (CHEMBL598103) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415574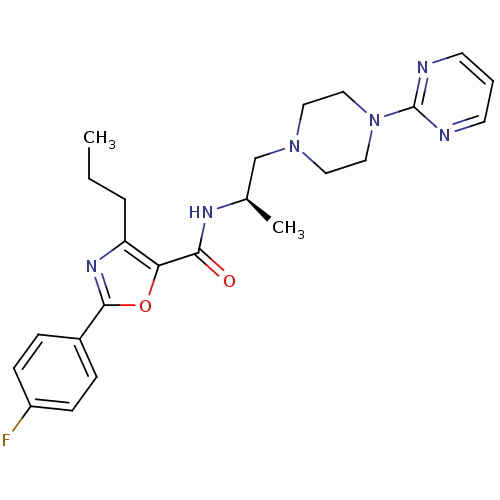 (CHEMBL597486) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM291163 (US9580411, Example 79-(R) | US9580411, Example 79-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e... | US Patent US9580411 (2017) BindingDB Entry DOI: 10.7270/Q25T3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM291168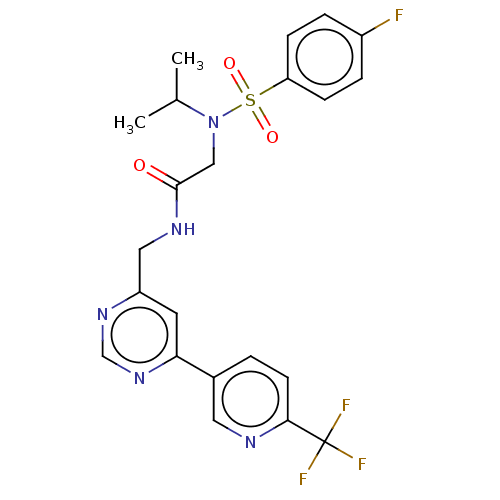 (2,2-Dideuterio-2-(4-fluoro-N-isopropylphenylsulfon...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e... | US Patent US9580411 (2017) BindingDB Entry DOI: 10.7270/Q25T3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415599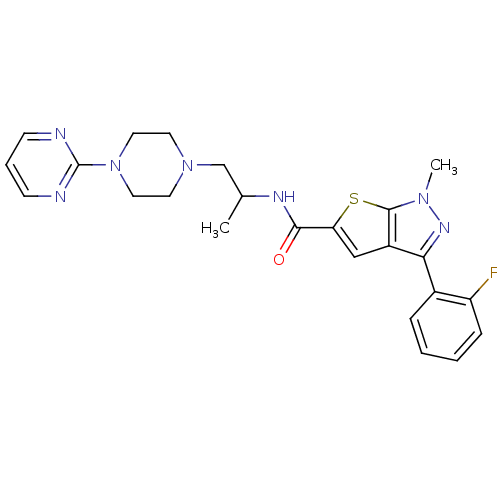 (CHEMBL604740) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415550 (CHEMBL599760 | Ro-85) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415564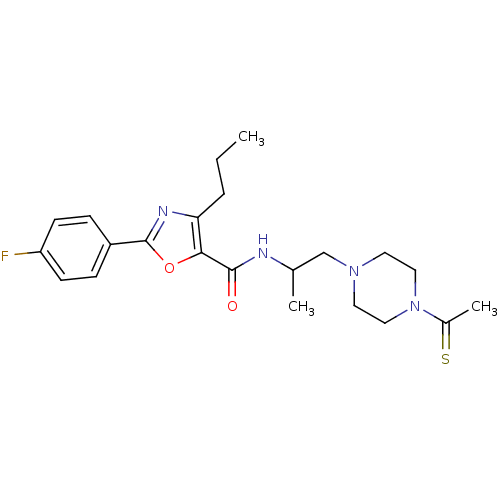 (CHEMBL598728) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415575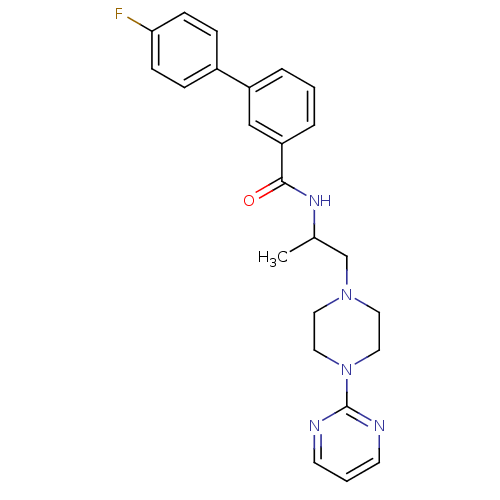 (CHEMBL597485) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM234880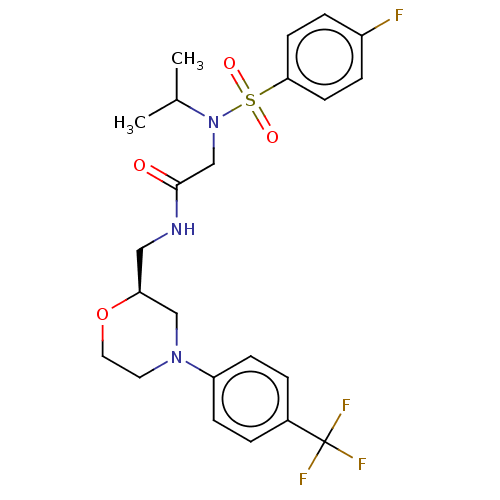 (US9359337, 11) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-... | US Patent US9359337 (2016) BindingDB Entry DOI: 10.7270/Q2Q81BZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM234877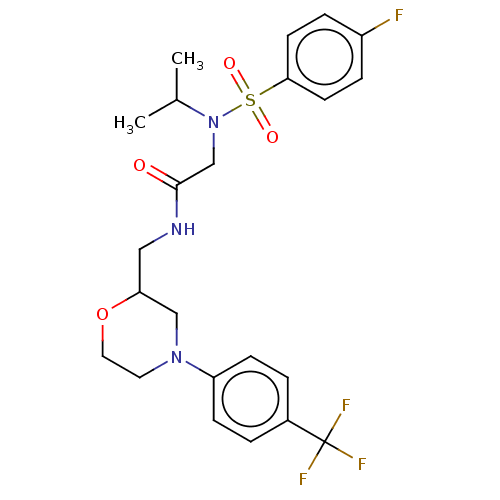 (US9359337, 8) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-... | US Patent US9359337 (2016) BindingDB Entry DOI: 10.7270/Q2Q81BZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415583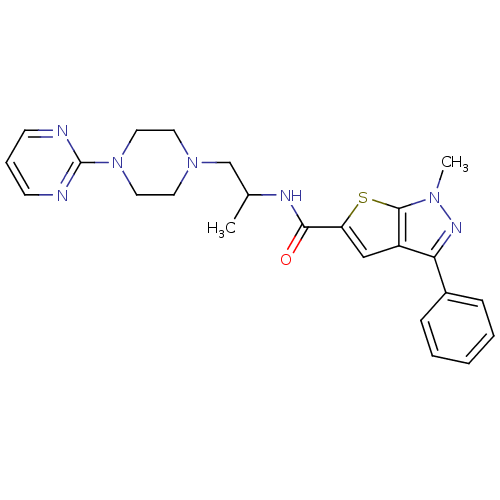 (CHEMBL597110) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM234878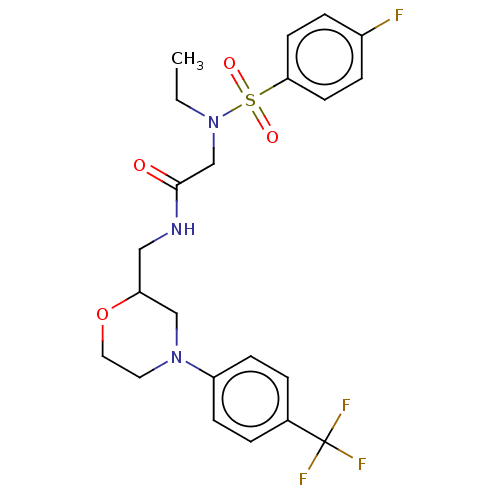 (US9359337, 9) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-... | US Patent US9359337 (2016) BindingDB Entry DOI: 10.7270/Q2Q81BZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM234871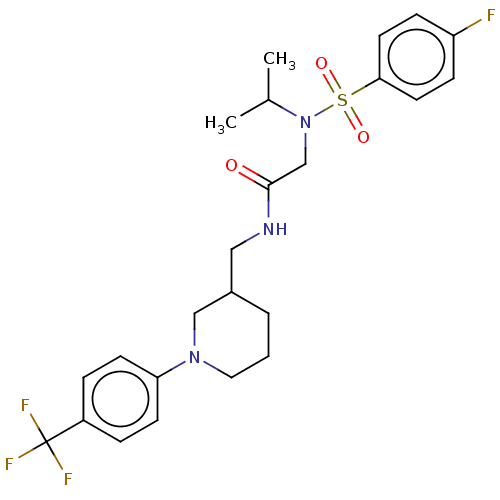 (US9359337, 2) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-... | US Patent US9359337 (2016) BindingDB Entry DOI: 10.7270/Q2Q81BZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205097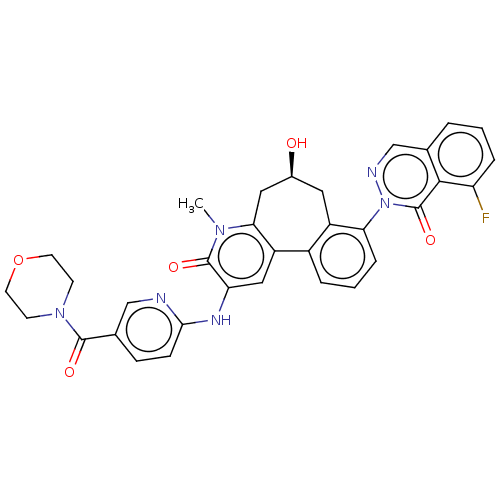 (US9556147, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 235 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... | US Patent US9556147 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM291165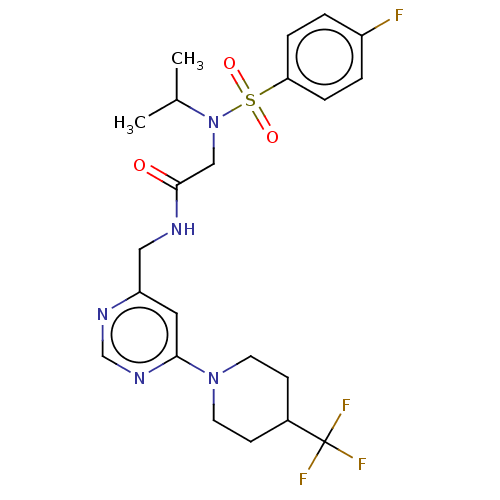 (2-(4-Fluoro-N-isopropylphenylsulfonamido)-N-((6-(4...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e... | US Patent US9580411 (2017) BindingDB Entry DOI: 10.7270/Q25T3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415563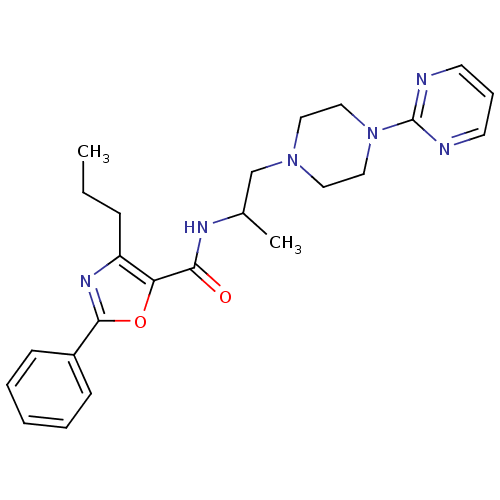 (CHEMBL605325) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM234874 (US9359337, 5) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 307 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-... | US Patent US9359337 (2016) BindingDB Entry DOI: 10.7270/Q2Q81BZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM234881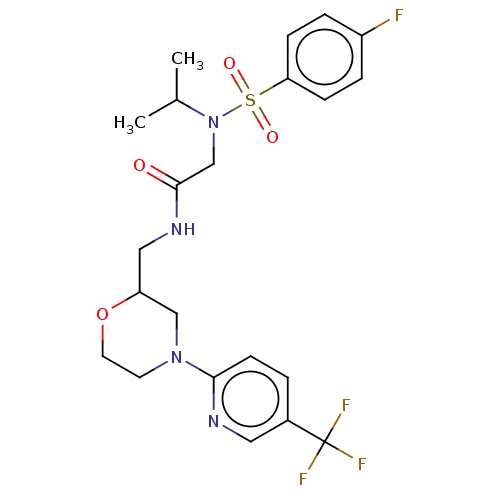 (US9359337, 12) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 385 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-... | US Patent US9359337 (2016) BindingDB Entry DOI: 10.7270/Q2Q81BZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415578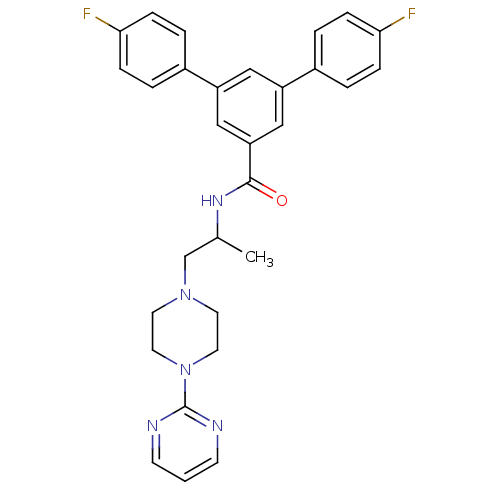 (CHEMBL603453) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415566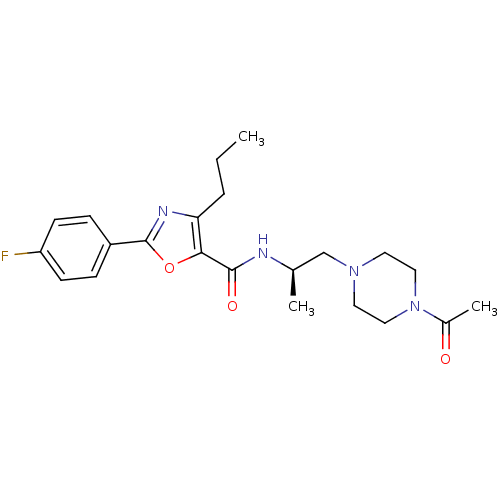 (CHEMBL604571) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM50415550 (CHEMBL599760 | Ro-85) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at human P2X3 receptor | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205096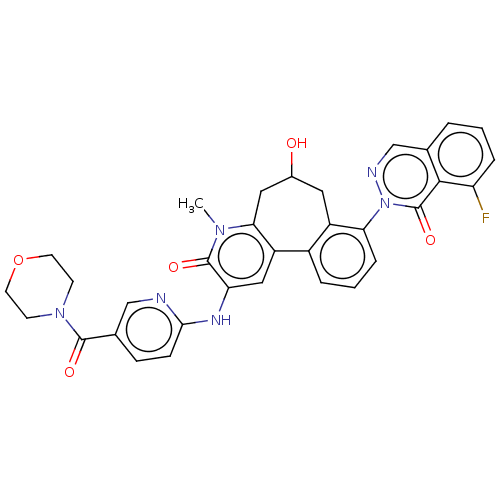 (US9556147, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 414 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... | US Patent US9556147 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM234873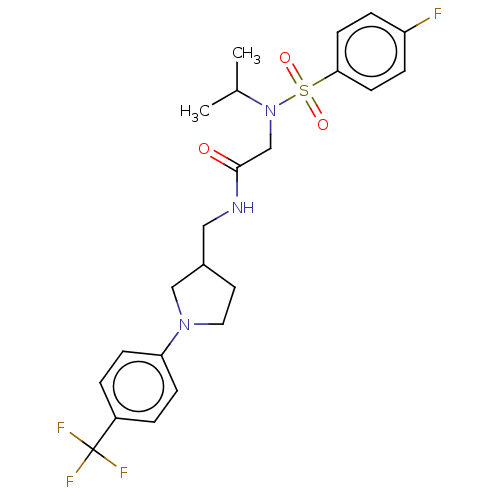 (US9359337, 4) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 425 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-... | US Patent US9359337 (2016) BindingDB Entry DOI: 10.7270/Q2Q81BZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM234870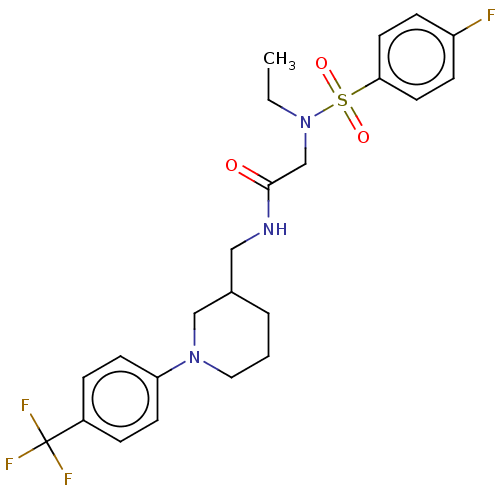 (US9359337, 1) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 494 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-... | US Patent US9359337 (2016) BindingDB Entry DOI: 10.7270/Q2Q81BZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415571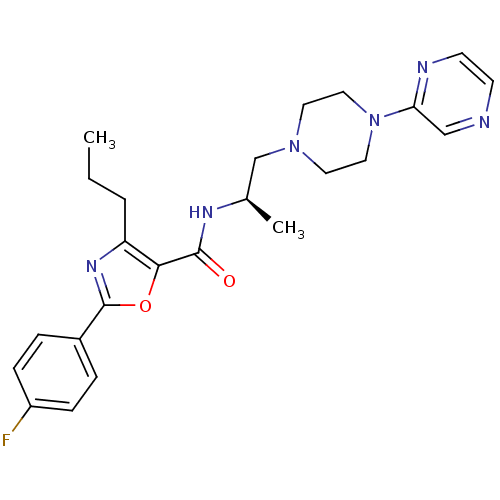 (CHEMBL598498) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415576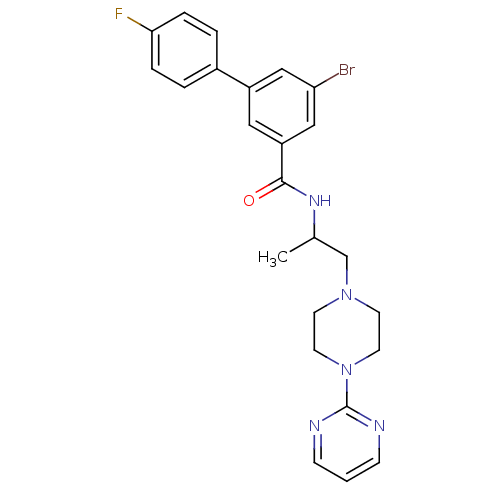 (CHEMBL597285) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415577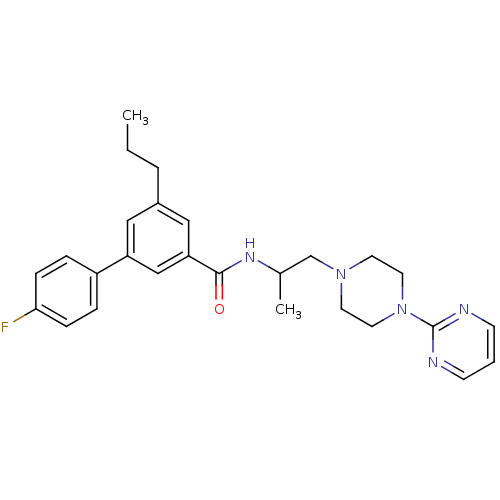 (CHEMBL597480) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415593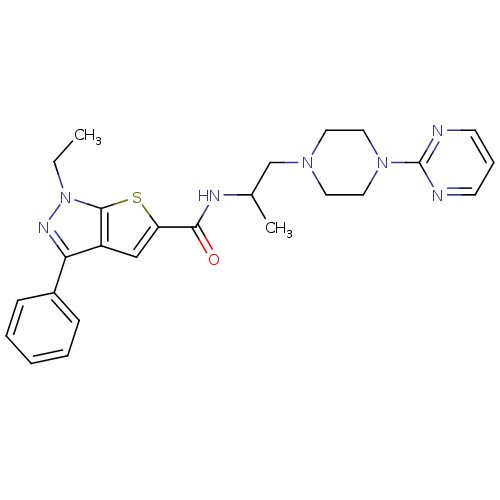 (CHEMBL599348) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM234875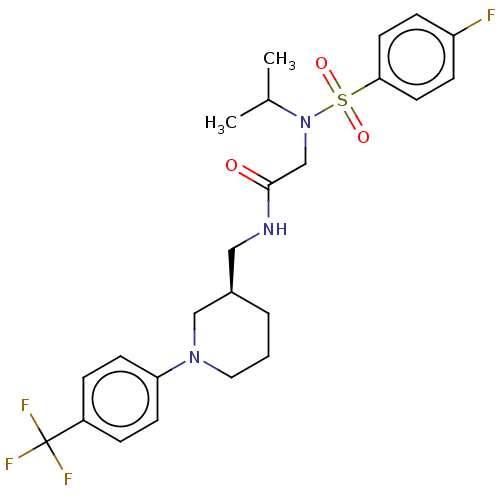 (US9359337, 6) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 504 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-... | US Patent US9359337 (2016) BindingDB Entry DOI: 10.7270/Q2Q81BZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415586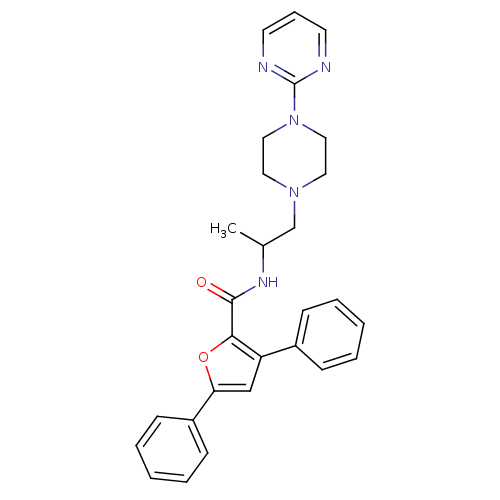 (CHEMBL603254) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415569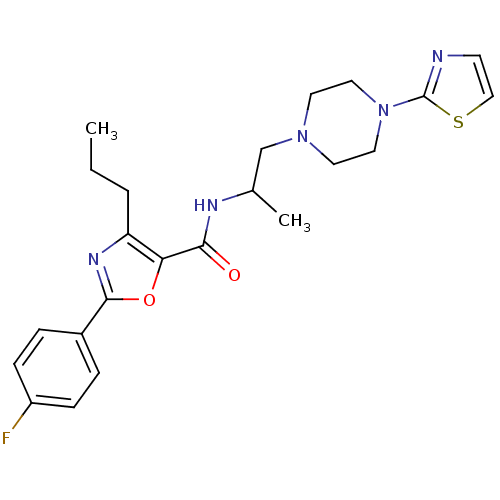 (CHEMBL605156) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM234872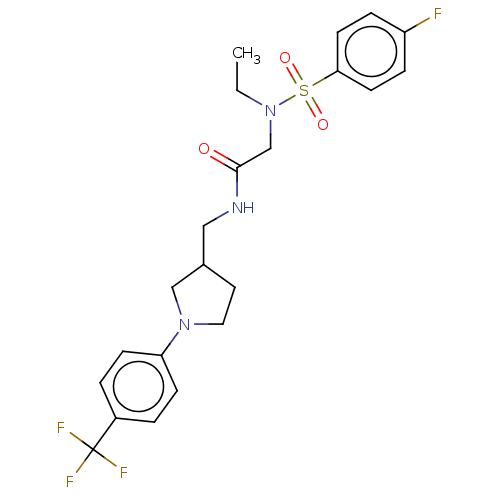 (US9359337, 3) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 634 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-... | US Patent US9359337 (2016) BindingDB Entry DOI: 10.7270/Q2Q81BZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM291161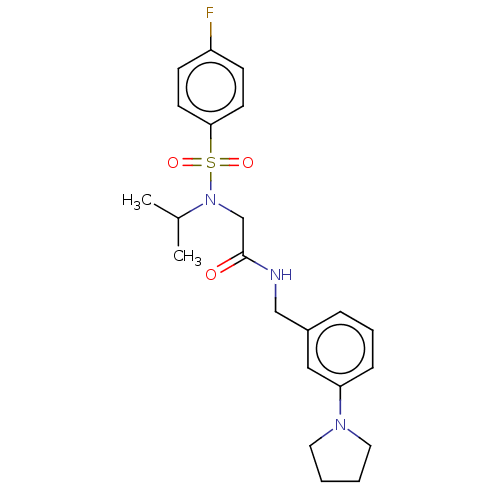 (2-(4-fluoro-N-isopropylphenylsulfonamido)-N-(3-(py...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 883 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e... | US Patent US9580411 (2017) BindingDB Entry DOI: 10.7270/Q25T3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM291162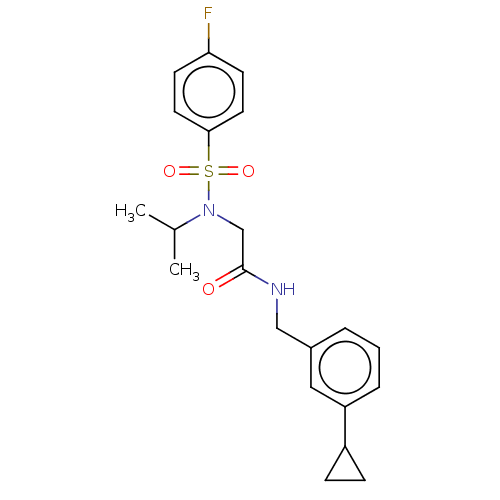 (N-(3-cyclopropylbenzyl)-2-(4-fluoro-N-isopropylphe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e... | US Patent US9580411 (2017) BindingDB Entry DOI: 10.7270/Q25T3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415596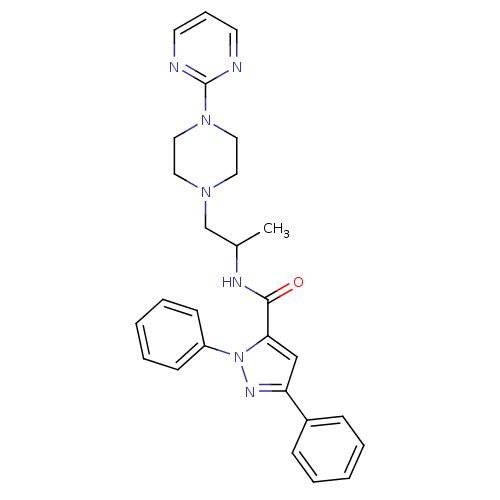 (CHEMBL596814) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415597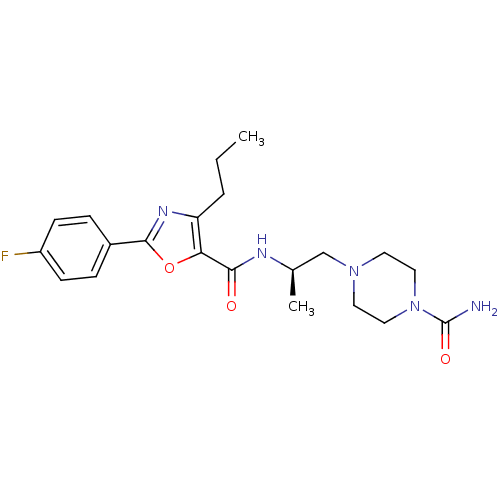 (CHEMBL598729) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415587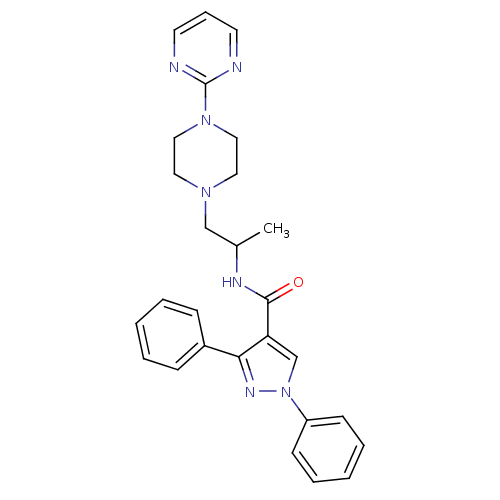 (CHEMBL598865) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415580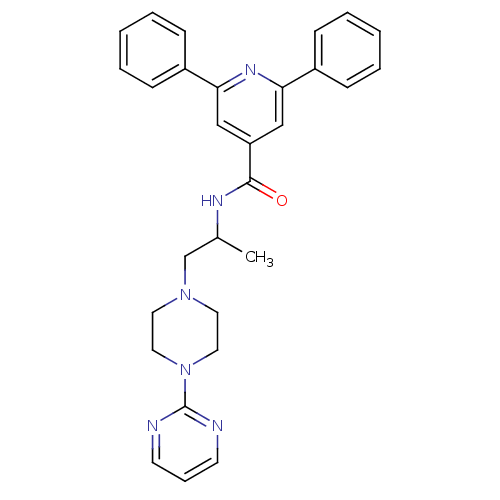 (CHEMBL598471) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415588 (CHEMBL605322) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50415570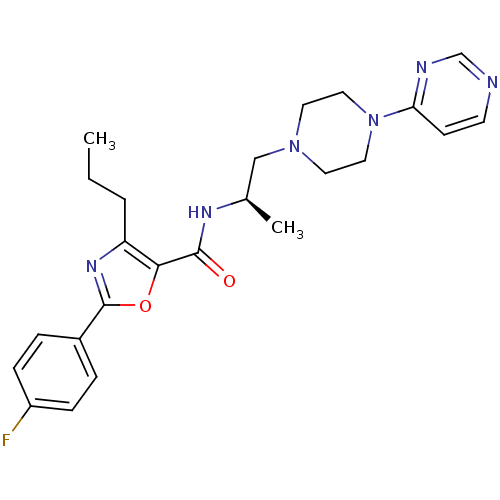 (CHEMBL590972) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at rat P2X3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1031-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.044 BindingDB Entry DOI: 10.7270/Q22V2HCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 96 total ) | Next | Last >> |