 Found 214 hits with Last Name = 'brown' and Initial = 'cj'
Found 214 hits with Last Name = 'brown' and Initial = 'cj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072078
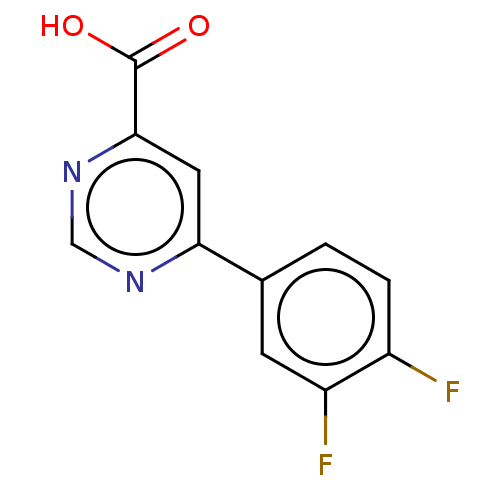
(CHEMBL3407905)Show InChI InChI=1S/C11H6F2N2O2/c12-7-2-1-6(3-8(7)13)9-4-10(11(16)17)15-5-14-9/h1-5H,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072123
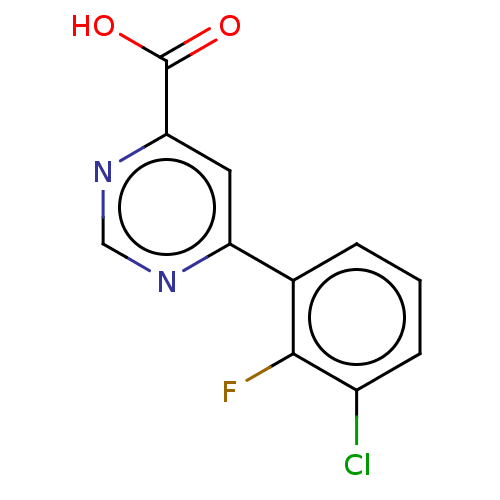
(CHEMBL3407904)Show InChI InChI=1S/C11H6ClFN2O2/c12-7-3-1-2-6(10(7)13)8-4-9(11(16)17)15-5-14-8/h1-5H,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072120
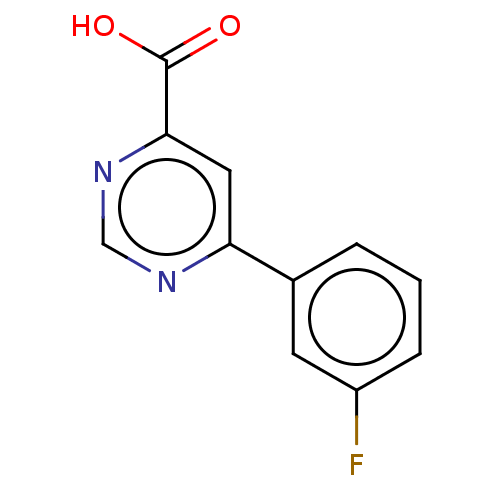
(CHEMBL3407901)Show InChI InChI=1S/C11H7FN2O2/c12-8-3-1-2-7(4-8)9-5-10(11(15)16)14-6-13-9/h1-6H,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072122
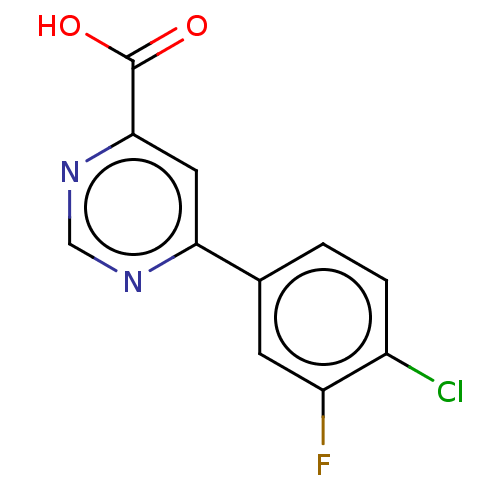
(CHEMBL3407903)Show InChI InChI=1S/C11H6ClFN2O2/c12-7-2-1-6(3-8(7)13)9-4-10(11(16)17)15-5-14-9/h1-5H,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072081
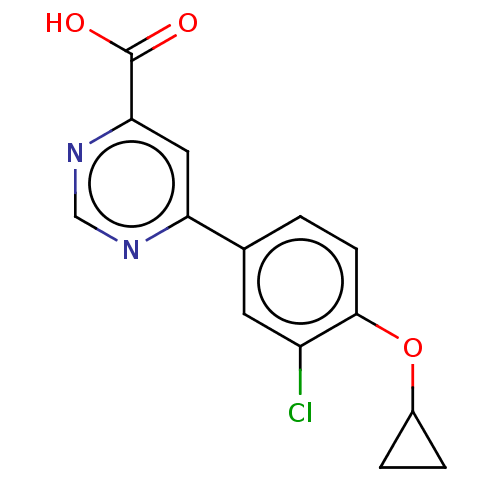
(CHEMBL3407922)Show InChI InChI=1S/C14H11ClN2O3/c15-10-5-8(1-4-13(10)20-9-2-3-9)11-6-12(14(18)19)17-7-16-11/h1,4-7,9H,2-3H2,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072082
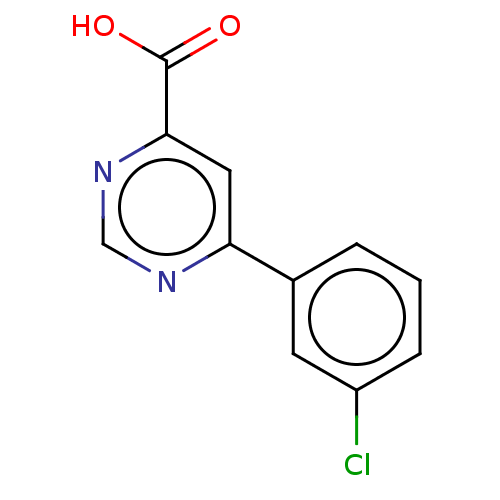
(CHEMBL3407865)Show InChI InChI=1S/C11H7ClN2O2/c12-8-3-1-2-7(4-8)9-5-10(11(15)16)14-6-13-9/h1-6H,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072077

(CHEMBL3407866)Show InChI InChI=1S/C11H6Cl2N2O2/c12-7-2-1-6(3-8(7)13)9-4-10(11(16)17)15-5-14-9/h1-5H,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072079
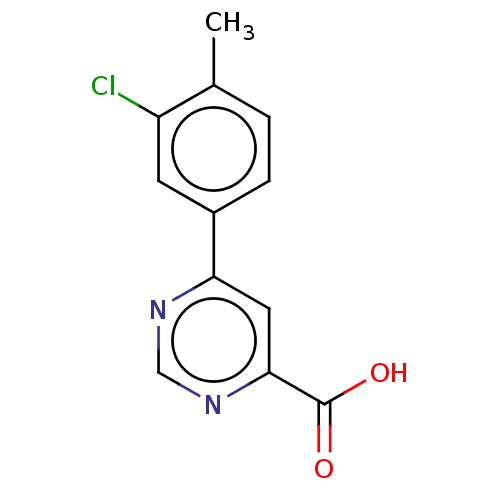
(CHEMBL3407913)Show InChI InChI=1S/C12H9ClN2O2/c1-7-2-3-8(4-9(7)13)10-5-11(12(16)17)15-6-14-10/h2-6H,1H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072131
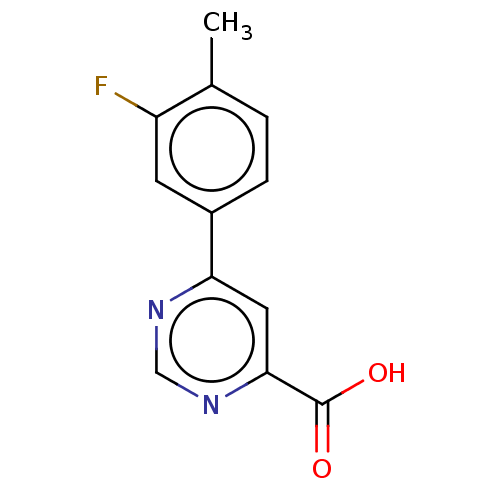
(CHEMBL3407914)Show InChI InChI=1S/C12H9FN2O2/c1-7-2-3-8(4-9(7)13)10-5-11(12(16)17)15-6-14-10/h2-6H,1H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072121
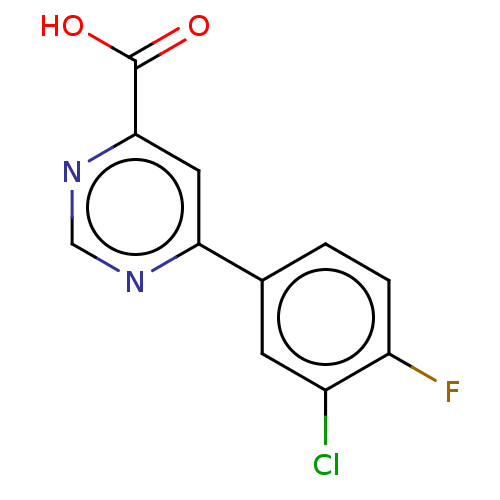
(CHEMBL3407902)Show InChI InChI=1S/C11H6ClFN2O2/c12-7-3-6(1-2-8(7)13)9-4-10(11(16)17)15-5-14-9/h1-5H,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072136
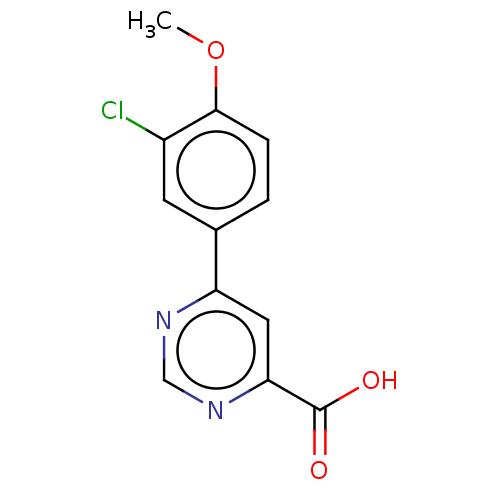
(CHEMBL3407920)Show InChI InChI=1S/C12H9ClN2O3/c1-18-11-3-2-7(4-8(11)13)9-5-10(12(16)17)15-6-14-9/h2-6H,1H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50072078

(CHEMBL3407905)Show InChI InChI=1S/C11H6F2N2O2/c12-7-2-1-6(3-8(7)13)9-4-10(11(16)17)15-5-14-9/h1-5H,(H,16,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of mouse KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072143
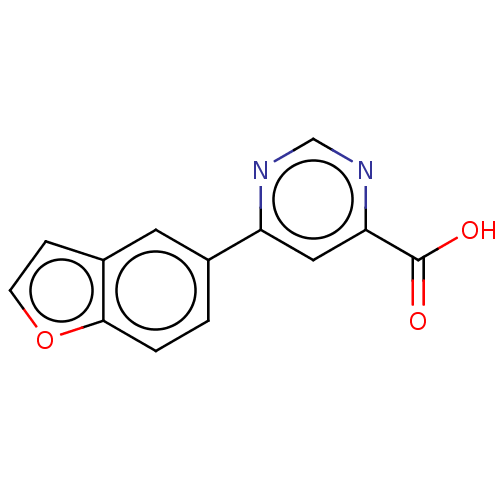
(CHEMBL3407924)Show InChI InChI=1S/C13H8N2O3/c16-13(17)11-6-10(14-7-15-11)8-1-2-12-9(5-8)3-4-18-12/h1-7H,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Rattus norvegicus) | BDBM50072078

(CHEMBL3407905)Show InChI InChI=1S/C11H6F2N2O2/c12-7-2-1-6(3-8(7)13)9-4-10(11(16)17)15-5-14-9/h1-5H,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of rat KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072101
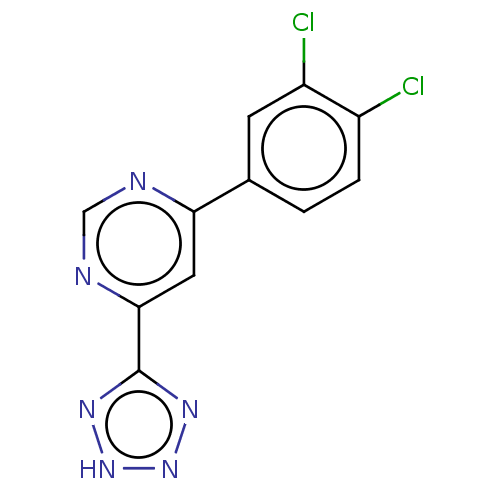
(CHEMBL3407883)Show InChI InChI=1S/C11H6Cl2N6/c12-7-2-1-6(3-8(7)13)9-4-10(15-5-14-9)11-16-18-19-17-11/h1-5H,(H,16,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072175
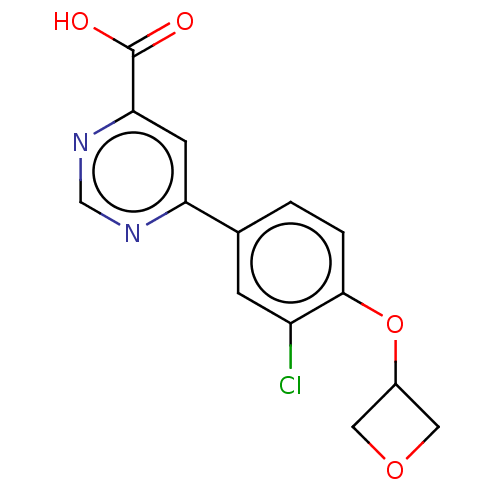
(CHEMBL3407926)Show InChI InChI=1S/C14H11ClN2O4/c15-10-3-8(1-2-13(10)21-9-5-20-6-9)11-4-12(14(18)19)17-7-16-11/h1-4,7,9H,5-6H2,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072080
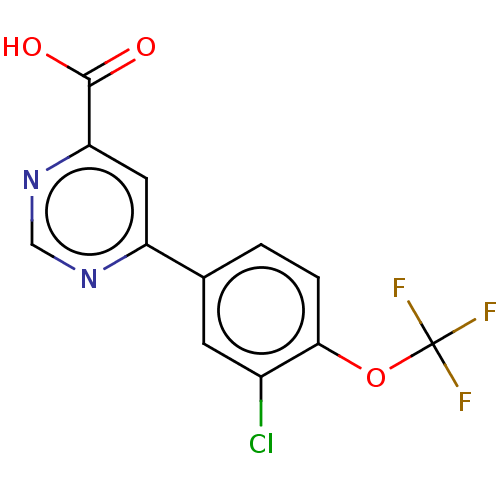
(CHEMBL3407919)Show InChI InChI=1S/C12H6ClF3N2O3/c13-7-3-6(1-2-10(7)21-12(14,15)16)8-4-9(11(19)20)18-5-17-8/h1-5H,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM224572
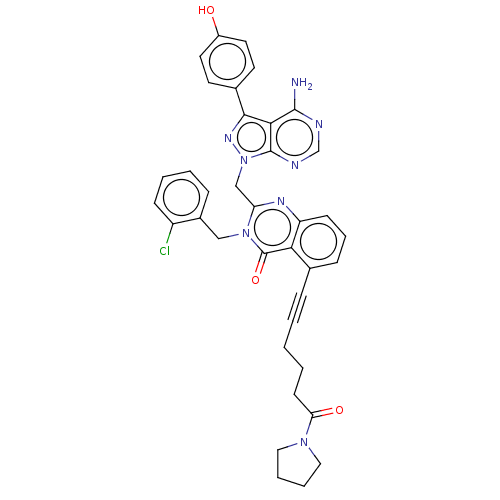
(US9321773, 89)Show SMILES Nc1ncnc2n(Cc3nc4cccc(C#CCCCC(=O)N5CCCC5)c4c(=O)n3Cc3ccccc3Cl)nc(-c3ccc(O)cc3)c12 Show InChI InChI=1S/C37H33ClN8O3/c38-28-12-5-4-10-26(28)21-45-30(22-46-36-33(35(39)40-23-41-36)34(43-46)25-15-17-27(47)18-16-25)42-29-13-8-11-24(32(29)37(45)49)9-2-1-3-14-31(48)44-19-6-7-20-44/h4-5,8,10-13,15-18,23,47H,1,3,6-7,14,19-22H2,(H2,39,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd.
US Patent
| Assay Description
PI3 kinases catalyse the phosphorylation of phosphatidylinositol 4,5-biphosphate (PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3) in the pres... |
US Patent US9321773 (2016)
BindingDB Entry DOI: 10.7270/Q2MG7NCG |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Rattus norvegicus) | BDBM50072077

(CHEMBL3407866)Show InChI InChI=1S/C11H6Cl2N2O2/c12-7-2-1-6(3-8(7)13)9-4-10(11(16)17)15-5-14-9/h1-5H,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of rat KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072092
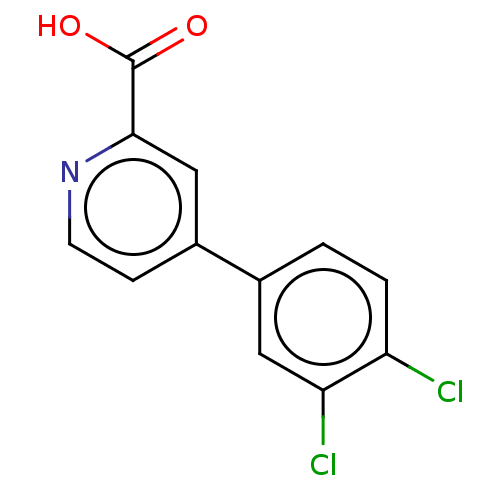
(CHEMBL3407874)Show InChI InChI=1S/C12H7Cl2NO2/c13-9-2-1-7(5-10(9)14)8-3-4-15-11(6-8)12(16)17/h1-6H,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403063
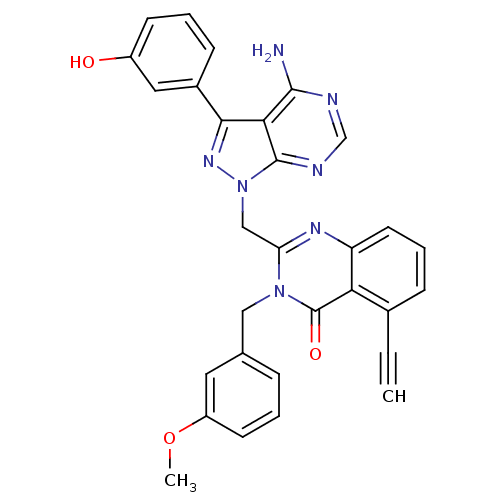
(CHEMBL2216862 | US9321773, 15)Show SMILES COc1cccc(Cn2c(Cn3nc(-c4cccc(O)c4)c4c(N)ncnc34)nc3cccc(C#C)c3c2=O)c1 Show InChI InChI=1S/C30H23N7O3/c1-3-19-8-6-12-23-25(19)30(39)36(15-18-7-4-11-22(13-18)40-2)24(34-23)16-37-29-26(28(31)32-17-33-29)27(35-37)20-9-5-10-21(38)14-20/h1,4-14,17,38H,15-16H2,2H3,(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd.
US Patent
| Assay Description
PI3 kinases catalyse the phosphorylation of phosphatidylinositol 4,5-biphosphate (PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3) in the pres... |
US Patent US9321773 (2016)
BindingDB Entry DOI: 10.7270/Q2MG7NCG |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Rattus norvegicus) | BDBM50072081

(CHEMBL3407922)Show InChI InChI=1S/C14H11ClN2O3/c15-10-5-8(1-4-13(10)20-9-2-3-9)11-6-12(14(18)19)17-7-16-11/h1,4-7,9H,2-3H2,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of rat KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Rattus norvegicus) | BDBM50072079

(CHEMBL3407913)Show InChI InChI=1S/C12H9ClN2O2/c1-7-2-3-8(4-9(7)13)10-5-11(12(16)17)15-6-14-10/h2-6H,1H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of rat KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072126
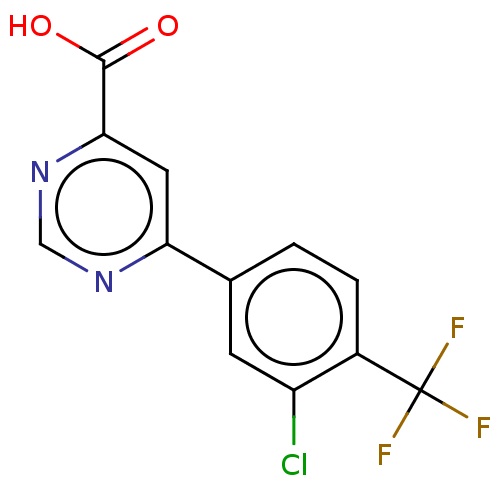
(CHEMBL3407908)Show InChI InChI=1S/C12H6ClF3N2O2/c13-8-3-6(1-2-7(8)12(14,15)16)9-4-10(11(19)20)18-5-17-9/h1-5H,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50072081

(CHEMBL3407922)Show InChI InChI=1S/C14H11ClN2O3/c15-10-5-8(1-4-13(10)20-9-2-3-9)11-6-12(14(18)19)17-7-16-11/h1,4-7,9H,2-3H2,(H,18,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of mouse KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072095
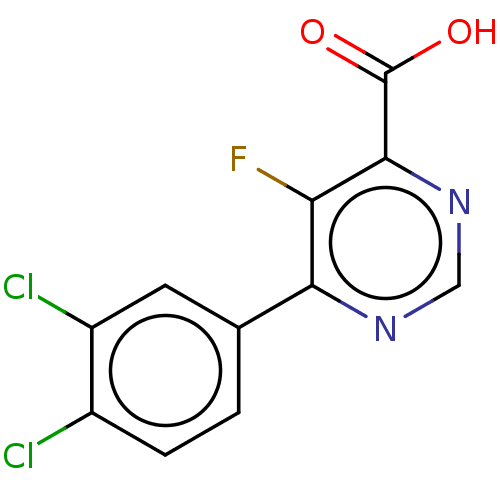
(CHEMBL3407877)Show InChI InChI=1S/C11H5Cl2FN2O2/c12-6-2-1-5(3-7(6)13)9-8(14)10(11(17)18)16-4-15-9/h1-4H,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072141
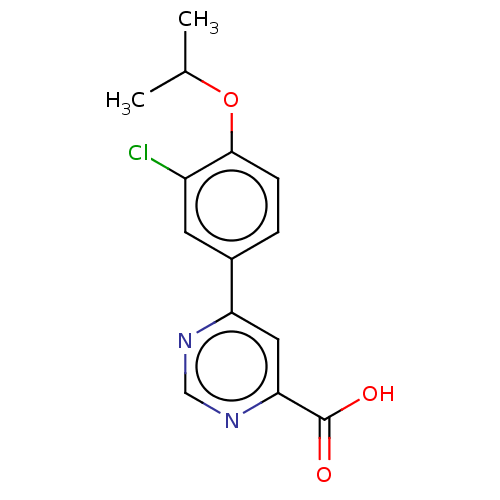
(CHEMBL3407921)Show InChI InChI=1S/C14H13ClN2O3/c1-8(2)20-13-4-3-9(5-10(13)15)11-6-12(14(18)19)17-7-16-11/h3-8H,1-2H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50072077

(CHEMBL3407866)Show InChI InChI=1S/C11H6Cl2N2O2/c12-7-2-1-6(3-8(7)13)9-4-10(11(16)17)15-5-14-9/h1-5H,(H,16,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of mouse KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50072079

(CHEMBL3407913)Show InChI InChI=1S/C12H9ClN2O2/c1-7-2-3-8(4-9(7)13)10-5-11(12(16)17)15-6-14-10/h2-6H,1H3,(H,16,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of mouse KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM224567

(US9321773, 1)Show SMILES Nc1ncnc2n(Cc3nc4cccc(C#C)c4c(=O)n3Cc3ccccc3Cl)nc(-c3cccc(O)c3)c12 Show InChI InChI=1S/C29H20ClN7O2/c1-2-17-8-6-12-22-24(17)29(39)36(14-19-7-3-4-11-21(19)30)23(34-22)15-37-28-25(27(31)32-16-33-28)26(35-37)18-9-5-10-20(38)13-18/h1,3-13,16,38H,14-15H2,(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd.
US Patent
| Assay Description
PI3 kinases catalyse the phosphorylation of phosphatidylinositol 4,5-biphosphate (PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3) in the pres... |
US Patent US9321773 (2016)
BindingDB Entry DOI: 10.7270/Q2MG7NCG |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072081

(CHEMBL3407922)Show InChI InChI=1S/C14H11ClN2O3/c15-10-5-8(1-4-13(10)20-9-2-3-9)11-6-12(14(18)19)17-7-16-11/h1,4-7,9H,2-3H2,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO transfected in CHO cells assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072098
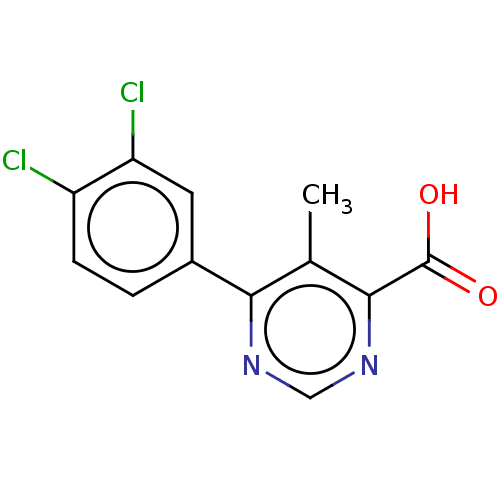
(CHEMBL3407880)Show InChI InChI=1S/C12H8Cl2N2O2/c1-6-10(15-5-16-11(6)12(17)18)7-2-3-8(13)9(14)4-7/h2-5H,1H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072118
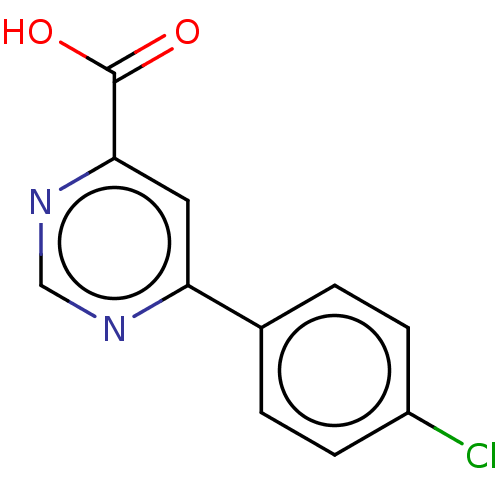
(CHEMBL3407899)Show InChI InChI=1S/C11H7ClN2O2/c12-8-3-1-7(2-4-8)9-5-10(11(15)16)14-6-13-9/h1-6H,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072080

(CHEMBL3407919)Show InChI InChI=1S/C12H6ClF3N2O3/c13-7-3-6(1-2-10(7)21-12(14,15)16)8-4-9(11(19)20)18-5-17-8/h1-5H,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO transfected in CHO cells assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50072080

(CHEMBL3407919)Show InChI InChI=1S/C12H6ClF3N2O3/c13-7-3-6(1-2-10(7)21-12(14,15)16)8-4-9(11(19)20)18-5-17-8/h1-5H,(H,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of mouse KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM224570
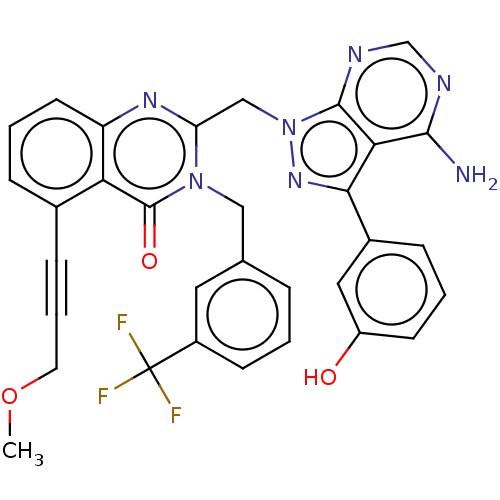
(US9321773, 37)Show SMILES COCC#Cc1cccc2nc(Cn3nc(-c4cccc(O)c4)c4c(N)ncnc34)n(Cc3cccc(c3)C(F)(F)F)c(=O)c12 Show InChI InChI=1S/C32H24F3N7O3/c1-45-13-5-9-20-7-4-12-24-26(20)31(44)41(16-19-6-2-10-22(14-19)32(33,34)35)25(39-24)17-42-30-27(29(36)37-18-38-30)28(40-42)21-8-3-11-23(43)15-21/h2-4,6-8,10-12,14-15,18,43H,13,16-17H2,1H3,(H2,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd.
US Patent
| Assay Description
PI3 kinases catalyse the phosphorylation of phosphatidylinositol 4,5-biphosphate (PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3) in the pres... |
US Patent US9321773 (2016)
BindingDB Entry DOI: 10.7270/Q2MG7NCG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM224568
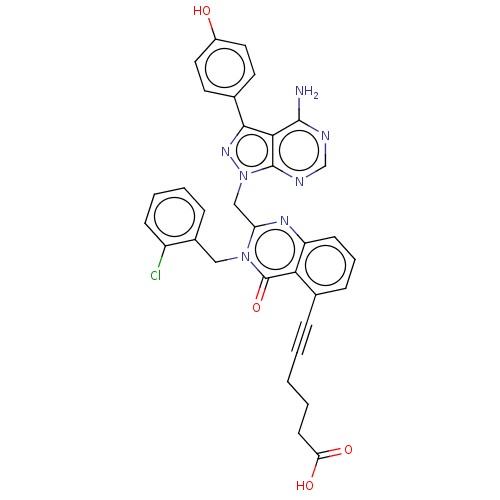
(US9321773, 4)Show SMILES Nc1ncnc2n(Cc3nc4cccc(C#CCCCC(O)=O)c4c(=O)n3Cc3ccccc3Cl)nc(-c3ccc(O)cc3)c12 Show InChI InChI=1S/C33H26ClN7O4/c34-24-10-5-4-8-22(24)17-40-26(38-25-11-6-9-20(28(25)33(40)45)7-2-1-3-12-27(43)44)18-41-32-29(31(35)36-19-37-32)30(39-41)21-13-15-23(42)16-14-21/h4-6,8-11,13-16,19,42H,1,3,12,17-18H2,(H,43,44)(H2,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd.
US Patent
| Assay Description
PI3 kinases catalyse the phosphorylation of phosphatidylinositol 4,5-biphosphate (PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3) in the pres... |
US Patent US9321773 (2016)
BindingDB Entry DOI: 10.7270/Q2MG7NCG |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072167
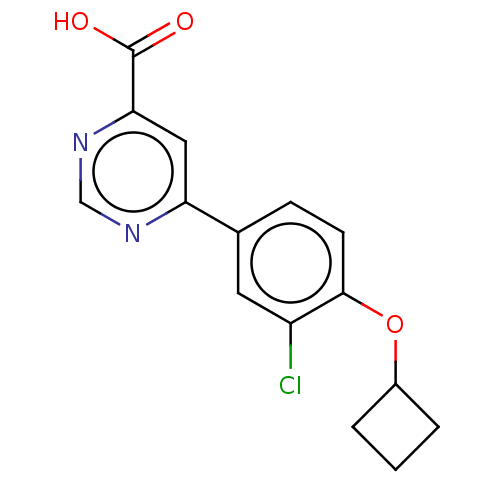
(CHEMBL3407925)Show InChI InChI=1S/C15H13ClN2O3/c16-11-6-9(4-5-14(11)21-10-2-1-3-10)12-7-13(15(19)20)18-8-17-12/h4-8,10H,1-3H2,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072102
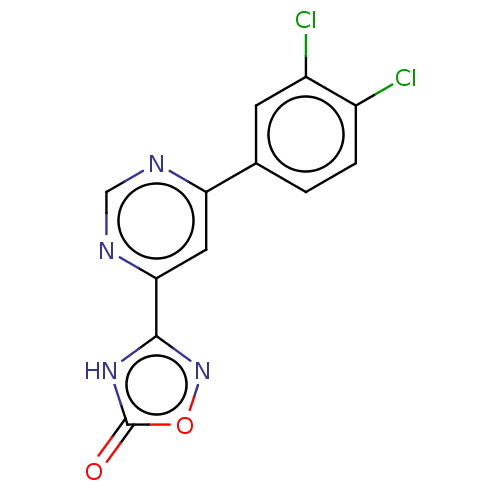
(CHEMBL3407884)Show InChI InChI=1S/C12H6Cl2N4O2/c13-7-2-1-6(3-8(7)14)9-4-10(16-5-15-9)11-17-12(19)20-18-11/h1-5H,(H,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072094
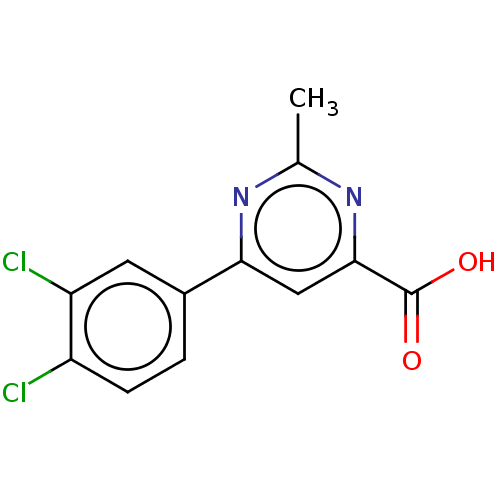
(CHEMBL3407876)Show InChI InChI=1S/C12H8Cl2N2O2/c1-6-15-10(5-11(16-6)12(17)18)7-2-3-8(13)9(14)4-7/h2-5H,1H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072122

(CHEMBL3407903)Show InChI InChI=1S/C11H6ClFN2O2/c12-7-2-1-6(3-8(7)13)9-4-10(11(16)17)15-5-14-9/h1-5H,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO transfected in CHO cells assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072136

(CHEMBL3407920)Show InChI InChI=1S/C12H9ClN2O3/c1-18-11-3-2-7(4-8(11)13)9-5-10(12(16)17)15-6-14-9/h2-6H,1H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO transfected in CHO cells assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM224569
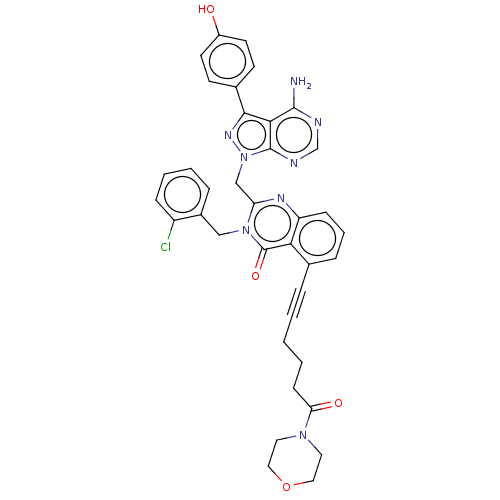
(US9321773, 5)Show SMILES Nc1ncnc2n(Cc3nc4cccc(C#CCCCC(=O)N5CCOCC5)c4c(=O)n3Cc3ccccc3Cl)nc(-c3ccc(O)cc3)c12 Show InChI InChI=1S/C37H33ClN8O4/c38-28-10-5-4-8-26(28)21-45-30(22-46-36-33(35(39)40-23-41-36)34(43-46)25-13-15-27(47)16-14-25)42-29-11-6-9-24(32(29)37(45)49)7-2-1-3-12-31(48)44-17-19-50-20-18-44/h4-6,8-11,13-16,23,47H,1,3,12,17-22H2,(H2,39,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd.
US Patent
| Assay Description
PI3 kinases catalyse the phosphorylation of phosphatidylinositol 4,5-biphosphate (PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3) in the pres... |
US Patent US9321773 (2016)
BindingDB Entry DOI: 10.7270/Q2MG7NCG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha
(Homo sapiens (Human)) | BDBM224568

(US9321773, 4)Show SMILES Nc1ncnc2n(Cc3nc4cccc(C#CCCCC(O)=O)c4c(=O)n3Cc3ccccc3Cl)nc(-c3ccc(O)cc3)c12 Show InChI InChI=1S/C33H26ClN7O4/c34-24-10-5-4-8-22(24)17-40-26(38-25-11-6-9-20(28(25)33(40)45)7-2-1-3-12-27(43)44)18-41-32-29(31(35)36-19-37-32)30(39-41)21-13-15-23(42)16-14-21/h4-6,8-11,13-16,19,42H,1,3,12,17-18H2,(H,43,44)(H2,35,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd.
US Patent
| Assay Description
PI3 kinases catalyse the phosphorylation of phosphatidylinositol 4,5-biphosphate (PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3) in the pres... |
US Patent US9321773 (2016)
BindingDB Entry DOI: 10.7270/Q2MG7NCG |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Rattus norvegicus) | BDBM50072080

(CHEMBL3407919)Show InChI InChI=1S/C12H6ClF3N2O3/c13-7-3-6(1-2-10(7)21-12(14,15)16)8-4-9(11(19)20)18-5-17-8/h1-5H,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of rat KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072129
(CHEMBL3407911)Show InChI InChI=1S/C12H10N2O2/c1-8-3-2-4-9(5-8)10-6-11(12(15)16)14-7-13-10/h2-7H,1H3,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072119
(CHEMBL3407900)Show InChI InChI=1S/C11H6Cl2N2O2/c12-7-1-6(2-8(13)3-7)9-4-10(11(16)17)15-5-14-9/h1-5H,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM224568

(US9321773, 4)Show SMILES Nc1ncnc2n(Cc3nc4cccc(C#CCCCC(O)=O)c4c(=O)n3Cc3ccccc3Cl)nc(-c3ccc(O)cc3)c12 Show InChI InChI=1S/C33H26ClN7O4/c34-24-10-5-4-8-22(24)17-40-26(38-25-11-6-9-20(28(25)33(40)45)7-2-1-3-12-27(43)44)18-41-32-29(31(35)36-19-37-32)30(39-41)21-13-15-23(42)16-14-21/h4-6,8-11,13-16,19,42H,1,3,12,17-18H2,(H,43,44)(H2,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd.
US Patent
| Assay Description
PI3 kinases catalyse the phosphorylation of phosphatidylinositol 4,5-biphosphate (PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3) in the pres... |
US Patent US9321773 (2016)
BindingDB Entry DOI: 10.7270/Q2MG7NCG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha
(Homo sapiens (Human)) | BDBM224569

(US9321773, 5)Show SMILES Nc1ncnc2n(Cc3nc4cccc(C#CCCCC(=O)N5CCOCC5)c4c(=O)n3Cc3ccccc3Cl)nc(-c3ccc(O)cc3)c12 Show InChI InChI=1S/C37H33ClN8O4/c38-28-10-5-4-8-26(28)21-45-30(22-46-36-33(35(39)40-23-41-36)34(43-46)25-13-15-27(47)16-14-25)42-29-11-6-9-24(32(29)37(45)49)7-2-1-3-12-31(48)44-17-19-50-20-18-44/h4-6,8-11,13-16,23,47H,1,3,12,17-22H2,(H2,39,40,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd.
US Patent
| Assay Description
PI3 kinases catalyse the phosphorylation of phosphatidylinositol 4,5-biphosphate (PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3) in the pres... |
US Patent US9321773 (2016)
BindingDB Entry DOI: 10.7270/Q2MG7NCG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM224569

(US9321773, 5)Show SMILES Nc1ncnc2n(Cc3nc4cccc(C#CCCCC(=O)N5CCOCC5)c4c(=O)n3Cc3ccccc3Cl)nc(-c3ccc(O)cc3)c12 Show InChI InChI=1S/C37H33ClN8O4/c38-28-10-5-4-8-26(28)21-45-30(22-46-36-33(35(39)40-23-41-36)34(43-46)25-13-15-27(47)16-14-25)42-29-11-6-9-24(32(29)37(45)49)7-2-1-3-12-31(48)44-17-19-50-20-18-44/h4-6,8-11,13-16,23,47H,1,3,12,17-22H2,(H2,39,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd.
US Patent
| Assay Description
PI3 kinases catalyse the phosphorylation of phosphatidylinositol 4,5-biphosphate (PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3) in the pres... |
US Patent US9321773 (2016)
BindingDB Entry DOI: 10.7270/Q2MG7NCG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data