

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrase (Human immunodeficiency virus 1) | BDBM50170870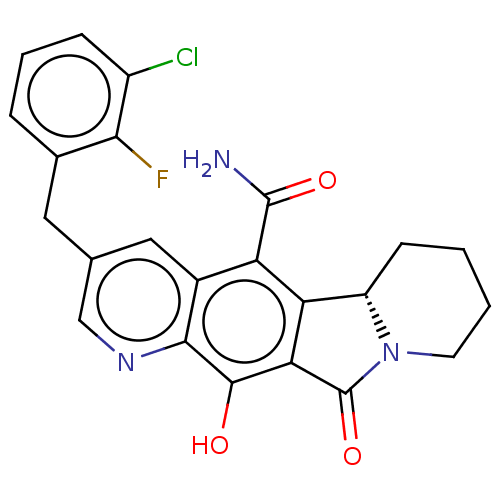 (CHEMBL3805182) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170867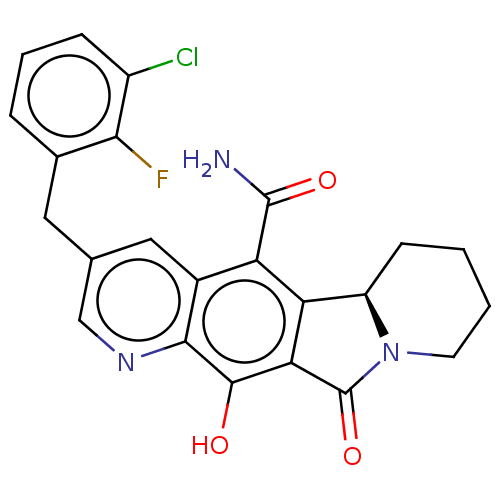 (CHEMBL3806067) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170867 (CHEMBL3806067) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in in HIV1 infected human CIP4 cells afte... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170591 (CHEMBL2403116) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170870 (CHEMBL3805182) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in in HIV1 infected human CIP4 cells afte... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170604 (CHEMBL3805585) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50483553 (CHEBI:76007 | Dolutegravir Sodium | GSK1349572 | G...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of Human immunodeficiency virus 1 Integrase using [3H]labeled target DNA as substrate after 45 mins by scintil... | Antimicrob Agents Chemother 55: 813-21 (2011) Article DOI: 10.1128/AAC.01209-10 BindingDB Entry DOI: 10.7270/Q2RX9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50045107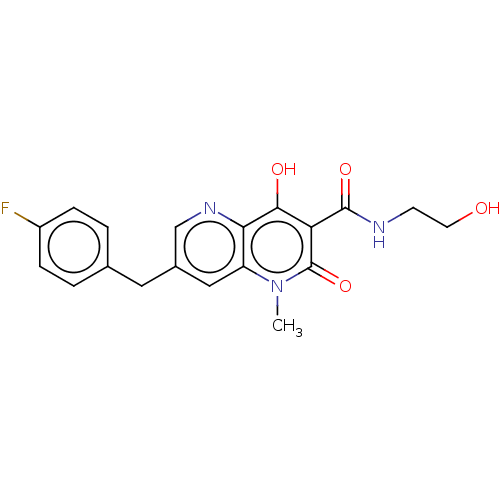 (CHEMBL1256978 | GSK364735) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170865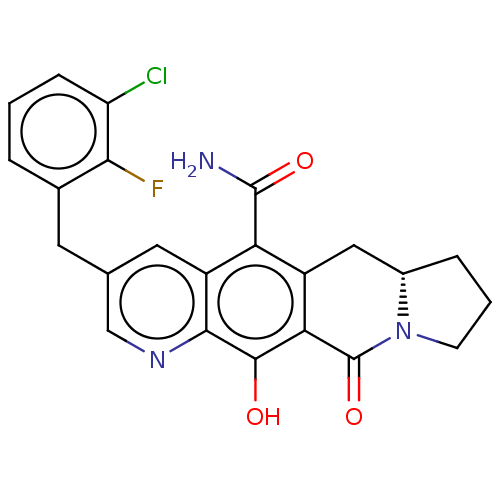 (CHEMBL3805145) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480673 (Isentress | Isentress hd | MK-0518 | MK-0518 POTAS...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of Human immunodeficiency virus 1 Integrase using [3H]labeled target DNA as substrate after 45 mins by scintil... | Antimicrob Agents Chemother 55: 813-21 (2011) Article DOI: 10.1128/AAC.01209-10 BindingDB Entry DOI: 10.7270/Q2RX9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170819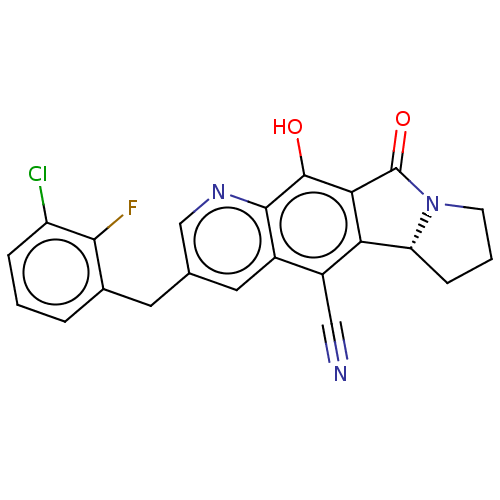 (CHEMBL3805531) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50183273 ((S)-6-(3-chloro-2-fluorobenzyl)-1-(1-hydroxy-3-met...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of Human immunodeficiency virus 1 Integrase using [3H]labeled target DNA as substrate after 45 mins by scintil... | Antimicrob Agents Chemother 55: 813-21 (2011) Article DOI: 10.1128/AAC.01209-10 BindingDB Entry DOI: 10.7270/Q2RX9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170607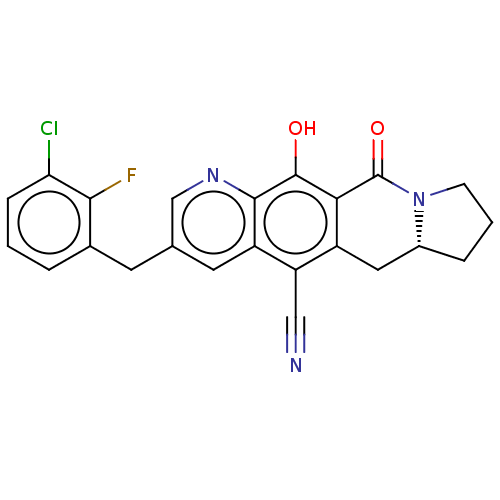 (CHEMBL3805498) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170864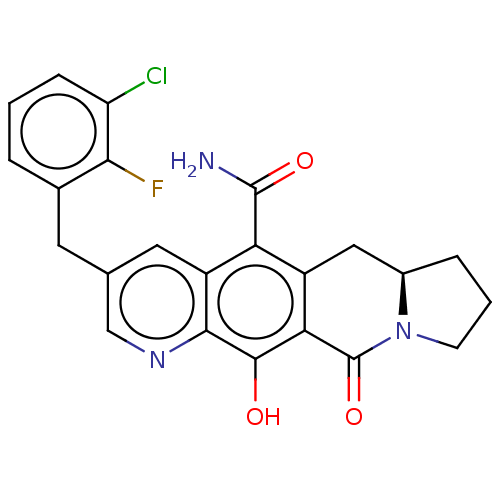 (CHEMBL3805726) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170835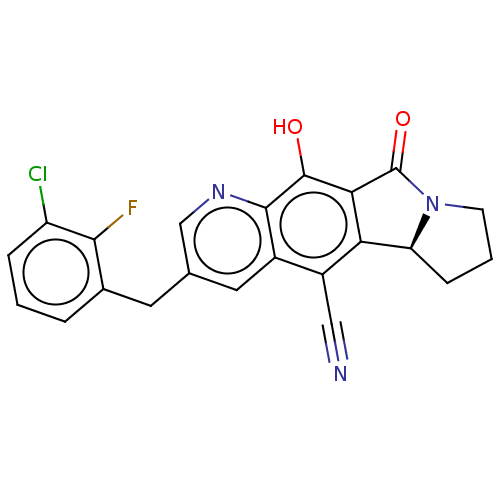 (CHEMBL3804966) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170725 (CHEMBL3805132) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170772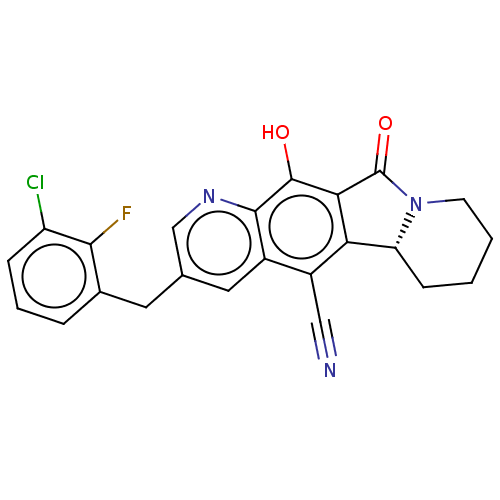 (CHEMBL3805269) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170865 (CHEMBL3805145) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in in HIV1 infected human CIP4 cells afte... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170596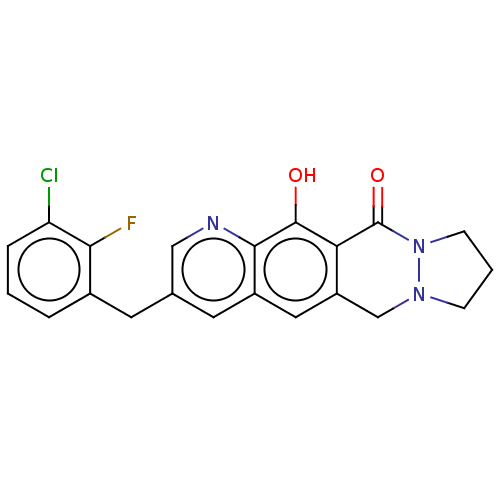 (CHEMBL3805532) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170778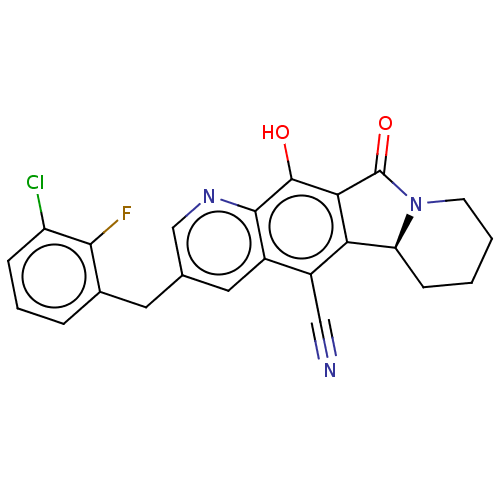 (CHEMBL3806308) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170864 (CHEMBL3805726) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in in HIV1 infected human CIP4 cells afte... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170725 (CHEMBL3805132) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in in HIV1 infected human CIP4 cells afte... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170596 (CHEMBL3805532) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in in HIV1 infected human CIP4 cells afte... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50269061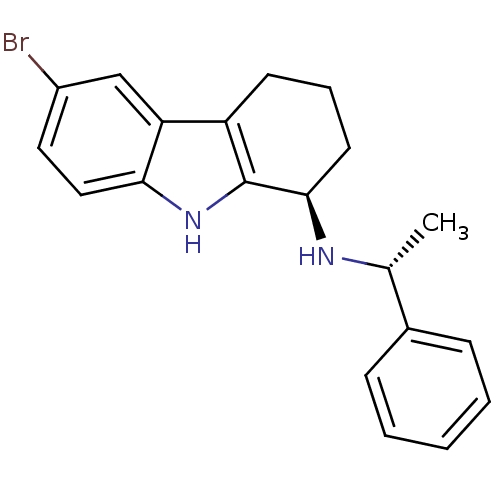 ((1R)-6-Bromo-N-[(1R)-1-phenylethyl]-2,3,4,9-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170819 (CHEMBL3805531) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 294 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in in HIV1 infected human CIP4 cells afte... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170604 (CHEMBL3805585) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 415 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in in HIV1 infected human CIP4 cells afte... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170772 (CHEMBL3805269) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 457 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in in HIV1 infected human CIP4 cells afte... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170778 (CHEMBL3806308) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in in HIV1 infected human CIP4 cells afte... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50269061 ((1R)-6-Bromo-N-[(1R)-1-phenylethyl]-2,3,4,9-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170835 (CHEMBL3804966) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in in HIV1 infected human CIP4 cells afte... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50170607 (CHEMBL3805498) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in in HIV1 infected human CIP4 cells afte... | Eur J Med Chem 117: 99-112 (2016) Article DOI: 10.1016/j.ejmech.2016.03.038 BindingDB Entry DOI: 10.7270/Q2G162R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50295257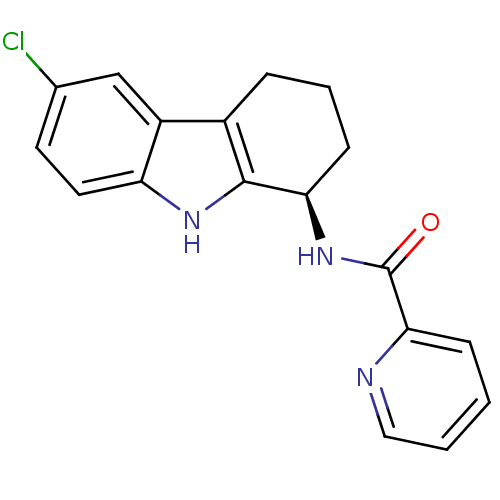 ((R)-N-(6-chloro-2,3,4,9-tetrahydro-1H-carbazol-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50295256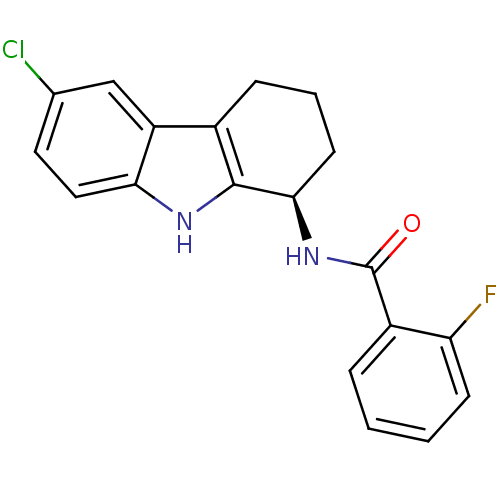 ((R)-N-(6-chloro-2,3,4,9-tetrahydro-1H-carbazol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50295257 ((R)-N-(6-chloro-2,3,4,9-tetrahydro-1H-carbazol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50295256 ((R)-N-(6-chloro-2,3,4,9-tetrahydro-1H-carbazol-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50295255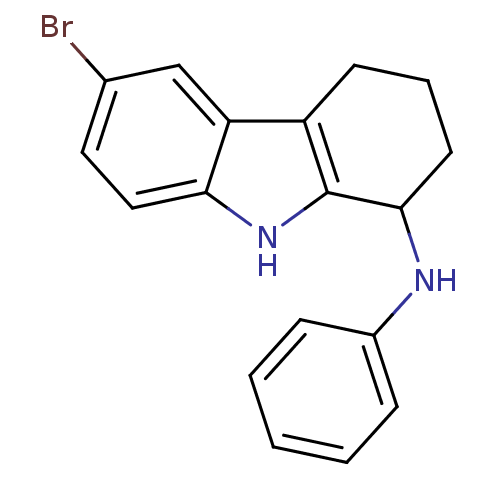 (6-bromo-N-phenyl-2,3,4,9-tetrahydro-1H-carbazol-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50295255 (6-bromo-N-phenyl-2,3,4,9-tetrahydro-1H-carbazol-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||