

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50140689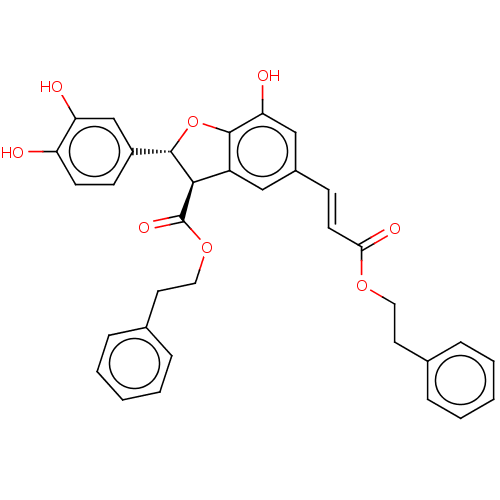 (CHEMBL3752809) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno Curated by ChEMBL | Assay Description Inhibition of PGES-1 in IL-1beta stimulated human A549 cell microsomes using PGH2 as substrate assessed as suppression of PGE2 formation preincubated... | Bioorg Med Chem 24: 820-6 (2016) Article DOI: 10.1016/j.bmc.2016.01.002 BindingDB Entry DOI: 10.7270/Q2JH3P1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno Curated by ChEMBL | Assay Description Inhibition of PGES-1 in IL-1beta stimulated human A549 cell microsomes using PGH2 as substrate assessed as suppression of PGE2 formation preincubated... | Bioorg Med Chem 24: 820-6 (2016) Article DOI: 10.1016/j.bmc.2016.01.002 BindingDB Entry DOI: 10.7270/Q2JH3P1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50140689 (CHEMBL3752809) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno Curated by ChEMBL | Assay Description Inhibition of PGES-1 in IL-1beta stimulated human A549 cell microsomes using PGH2 as substrate assessed as suppression of PGE2 formation preincubated... | Bioorg Med Chem 24: 820-6 (2016) Article DOI: 10.1016/j.bmc.2016.01.002 BindingDB Entry DOI: 10.7270/Q2JH3P1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50140688 (CHEMBL3754377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno Curated by ChEMBL | Assay Description Inhibition of PGES-1 in IL-1beta stimulated human A549 cell microsomes using PGH2 as substrate assessed as suppression of PGE2 formation preincubated... | Bioorg Med Chem 24: 820-6 (2016) Article DOI: 10.1016/j.bmc.2016.01.002 BindingDB Entry DOI: 10.7270/Q2JH3P1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50140690 (CHEMBL3752963) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno Curated by ChEMBL | Assay Description Inhibition of PGES-1 in IL-1beta stimulated human A549 cell microsomes using PGH2 as substrate assessed as suppression of PGE2 formation preincubated... | Bioorg Med Chem 24: 820-6 (2016) Article DOI: 10.1016/j.bmc.2016.01.002 BindingDB Entry DOI: 10.7270/Q2JH3P1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50140687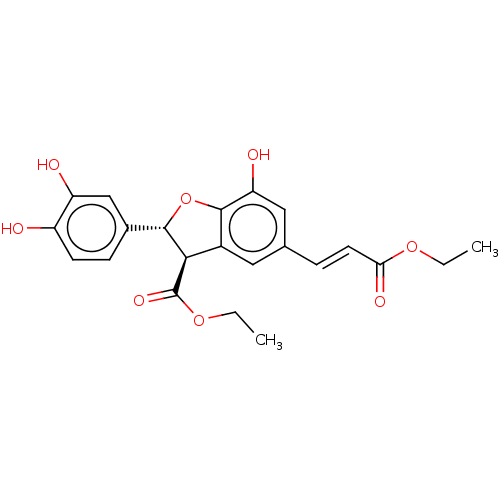 (CHEMBL3752388) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno Curated by ChEMBL | Assay Description Inhibition of PGES-1 in IL-1beta stimulated human A549 cell microsomes using PGH2 as substrate assessed as suppression of PGE2 formation preincubated... | Bioorg Med Chem 24: 820-6 (2016) Article DOI: 10.1016/j.bmc.2016.01.002 BindingDB Entry DOI: 10.7270/Q2JH3P1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50140686 (CHEMBL538661) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno Curated by ChEMBL | Assay Description Inhibition of PGES-1 in IL-1beta stimulated human A549 cell microsomes using PGH2 as substrate assessed as suppression of PGE2 formation preincubated... | Bioorg Med Chem 24: 820-6 (2016) Article DOI: 10.1016/j.bmc.2016.01.002 BindingDB Entry DOI: 10.7270/Q2JH3P1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Aspergillus oryzae) | BDBM50499768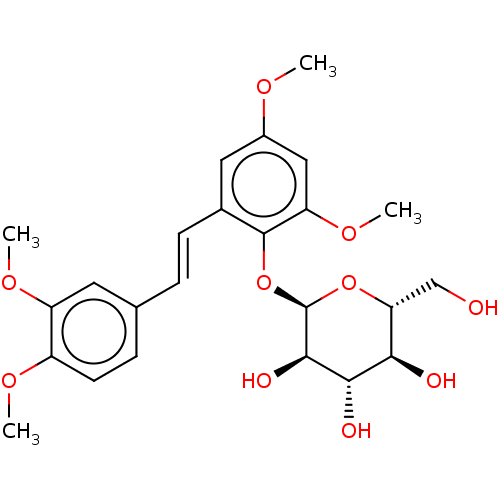 (CHEMBL3740658) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Catania Curated by ChEMBL | Assay Description Inhibition of Aspergillus oryzae beta-galactosidase using oNP-beta-Gal as substrate assessed as release of o-nitrophenol incubated for 30 mins by spe... | J Nat Prod 78: 2675-83 (2015) Article DOI: 10.1021/acs.jnatprod.5b00619 BindingDB Entry DOI: 10.7270/Q2NZ8BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Aspergillus oryzae) | BDBM50499767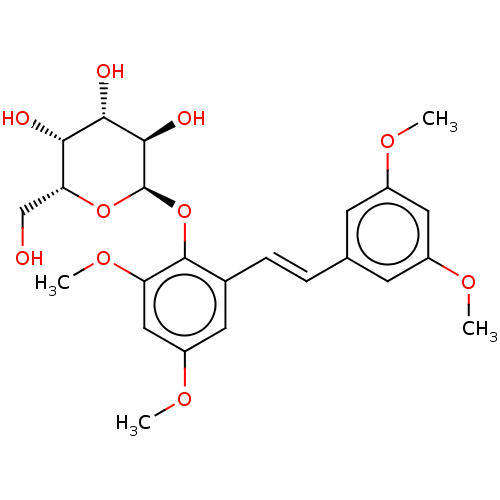 (CHEMBL3741440) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.96E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Catania Curated by ChEMBL | Assay Description Inhibition of Aspergillus oryzae beta-galactosidase using oNP-beta-Gal as substrate assessed as release of o-nitrophenol incubated for 30 mins by spe... | J Nat Prod 78: 2675-83 (2015) Article DOI: 10.1021/acs.jnatprod.5b00619 BindingDB Entry DOI: 10.7270/Q2NZ8BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Aspergillus oryzae) | BDBM50499771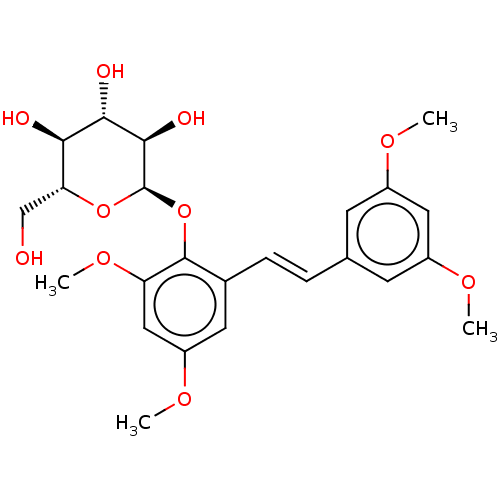 (CHEMBL3740283) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.97E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Catania Curated by ChEMBL | Assay Description Inhibition of Aspergillus oryzae beta-galactosidase using oNP-beta-Gal as substrate assessed as release of o-nitrophenol incubated for 30 mins by spe... | J Nat Prod 78: 2675-83 (2015) Article DOI: 10.1021/acs.jnatprod.5b00619 BindingDB Entry DOI: 10.7270/Q2NZ8BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Aspergillus oryzae) | BDBM50499769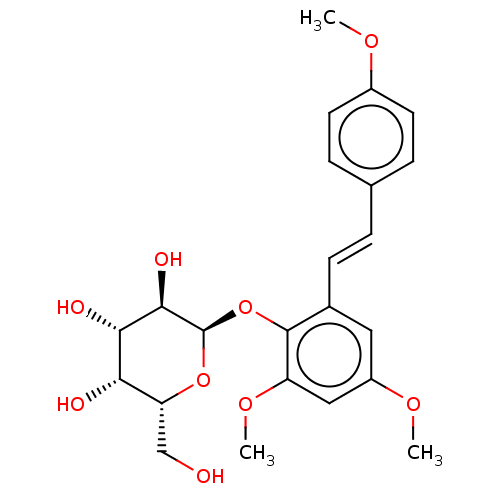 (CHEMBL3739825) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Catania Curated by ChEMBL | Assay Description Inhibition of Aspergillus oryzae beta-galactosidase using oNP-beta-Gal as substrate assessed as release of o-nitrophenol incubated for 30 mins by spe... | J Nat Prod 78: 2675-83 (2015) Article DOI: 10.1021/acs.jnatprod.5b00619 BindingDB Entry DOI: 10.7270/Q2NZ8BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Aspergillus oryzae) | BDBM50499770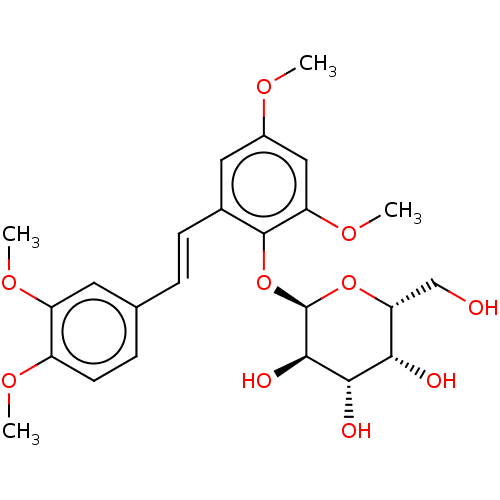 (CHEMBL3742045) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.99E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Catania Curated by ChEMBL | Assay Description Inhibition of Aspergillus oryzae beta-galactosidase using oNP-beta-Gal as substrate assessed as release of o-nitrophenol incubated for 30 mins by spe... | J Nat Prod 78: 2675-83 (2015) Article DOI: 10.1021/acs.jnatprod.5b00619 BindingDB Entry DOI: 10.7270/Q2NZ8BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50462628 (CHEMBL4239155) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal His-tagged TNKS2 (Ser959 to Gly1166 residues) expressed in Escherichia coli by surface plasmon reson... | Bioorg Med Chem 26: 3953-3957 (2018) Article DOI: 10.1016/j.bmc.2018.06.019 BindingDB Entry DOI: 10.7270/Q2KH0R05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50462626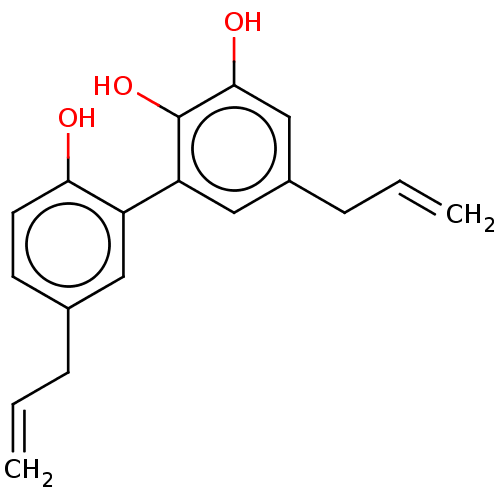 (CHEMBL4127893) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Binding affinity to recombinant human PARP1 expressed in baculovirus infected Sf9 cells by surface plasmon resonance analysis | Bioorg Med Chem 26: 3953-3957 (2018) Article DOI: 10.1016/j.bmc.2018.06.019 BindingDB Entry DOI: 10.7270/Q2KH0R05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50462629 (CHEMBL3594248) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal His-tagged TNKS2 (Ser959 to Gly1166 residues) expressed in Escherichia coli by surface plasmon reson... | Bioorg Med Chem 26: 3953-3957 (2018) Article DOI: 10.1016/j.bmc.2018.06.019 BindingDB Entry DOI: 10.7270/Q2KH0R05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha/beta (Homo sapiens (Human)-Rattus norvegicus) | BDBM11323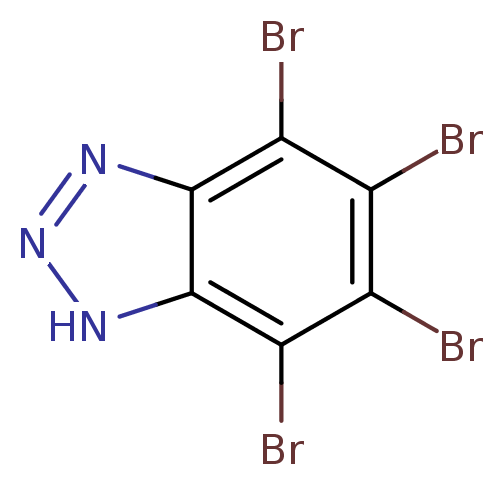 (4,5,6,7-tetrabromo-1H-1,2,3-benzotriazole | 4,5,6,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Binding affinity to recombinant human CK2alpha/beta expressed in Escherichia coli by surface plasmon resonance analysis | Bioorg Med Chem 26: 3953-3957 (2018) Article DOI: 10.1016/j.bmc.2018.06.019 BindingDB Entry DOI: 10.7270/Q2KH0R05 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50462627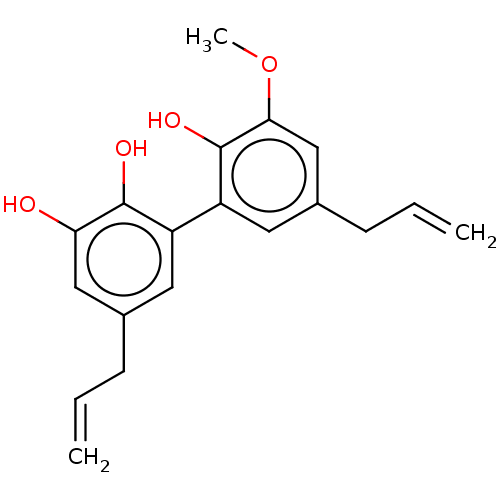 (CHEMBL1958308) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal GSt-tagged TNKS1 (1000 to 1328 residues) expressed in baculovirus infected Sf9 cells by surface plas... | Bioorg Med Chem 26: 3953-3957 (2018) Article DOI: 10.1016/j.bmc.2018.06.019 BindingDB Entry DOI: 10.7270/Q2KH0R05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50462627 (CHEMBL1958308) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal His-tagged TNKS2 (Ser959 to Gly1166 residues) expressed in Escherichia coli by surface plasmon reson... | Bioorg Med Chem 26: 3953-3957 (2018) Article DOI: 10.1016/j.bmc.2018.06.019 BindingDB Entry DOI: 10.7270/Q2KH0R05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50462626 (CHEMBL4127893) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 5.26E+3 | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal GSt-tagged TNKS1 (1000 to 1328 residues) expressed in baculovirus infected Sf9 cells by surface plas... | Bioorg Med Chem 26: 3953-3957 (2018) Article DOI: 10.1016/j.bmc.2018.06.019 BindingDB Entry DOI: 10.7270/Q2KH0R05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50188594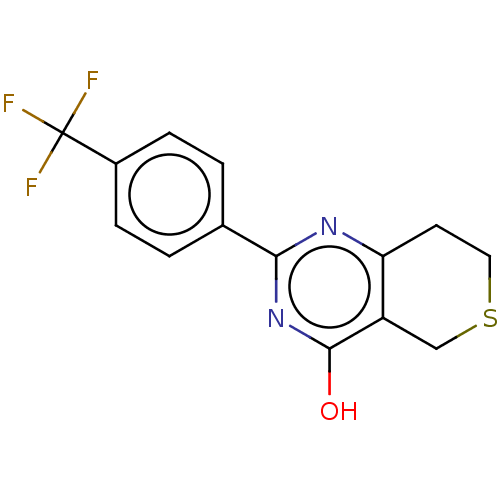 (CHEBI:62878 | CHEMBL1086580) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Binding affinity to recombinant human N-terminal His-tagged TNKS2 (Ser959 to Gly1166 residues) expressed in Escherichia coli by surface plasmon reson... | Bioorg Med Chem 26: 3953-3957 (2018) Article DOI: 10.1016/j.bmc.2018.06.019 BindingDB Entry DOI: 10.7270/Q2KH0R05 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||