 Found 82 hits with Last Name = 'ceccarini' and Initial = 'l'
Found 82 hits with Last Name = 'ceccarini' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308280
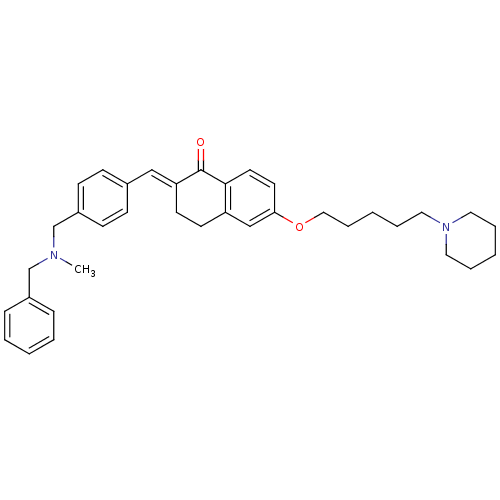
(2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(5-...)Show SMILES CN(Cc1ccccc1)Cc1ccc(\C=C2/CCc3cc(OCCCCCN4CCCCC4)ccc3C2=O)cc1 Show InChI InChI=1S/C36H44N2O2/c1-37(27-30-11-5-2-6-12-30)28-31-15-13-29(14-16-31)25-33-18-17-32-26-34(19-20-35(32)36(33)39)40-24-10-4-9-23-38-21-7-3-8-22-38/h2,5-6,11-16,19-20,25-26H,3-4,7-10,17-18,21-24,27-28H2,1H3/b33-25+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE-mediated hydrolysis of acetylcholine by Lineweaver-Burk plot analysis |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50263215
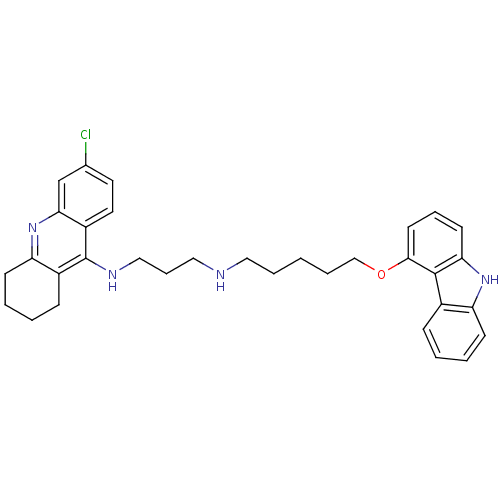
(CHEMBL473866 | N-[5-(9H-Carbazol-4-yloxy)pentyl]-N...)Show SMILES Clc1ccc2c(NCCCNCCCCCOc3cccc4[nH]c5ccccc5c34)c3CCCCc3nc2c1 Show InChI InChI=1S/C33H37ClN4O/c34-23-16-17-26-30(22-23)38-28-13-5-3-11-25(28)33(26)36-20-9-19-35-18-6-1-7-21-39-31-15-8-14-29-32(31)24-10-2-4-12-27(24)37-29/h2,4,8,10,12,14-17,22,35,37H,1,3,5-7,9,11,13,18-21H2,(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's method |
J Med Chem 51: 4381-4 (2008)
Article DOI: 10.1021/jm800577j
BindingDB Entry DOI: 10.7270/Q21G0M23 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50263214
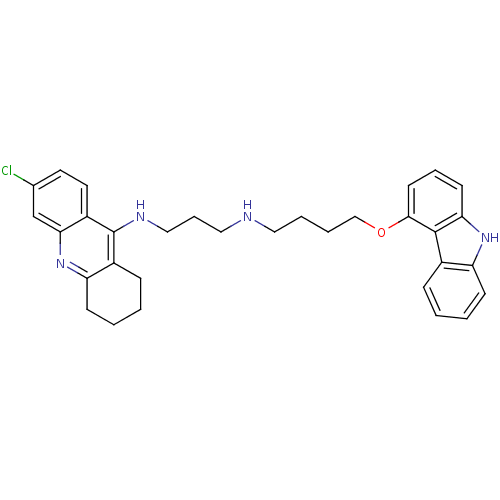
(CHEMBL478667 | N-[4-(9H-Carbazol-4-yloxy)butyl]-N'...)Show SMILES Clc1ccc2c(NCCCNCCCCOc3cccc4[nH]c5ccccc5c34)c3CCCCc3nc2c1 Show InChI InChI=1S/C32H35ClN4O/c33-22-15-16-25-29(21-22)37-27-12-4-2-10-24(27)32(25)35-19-8-18-34-17-5-6-20-38-30-14-7-13-28-31(30)23-9-1-3-11-26(23)36-28/h1,3,7,9,11,13-16,21,34,36H,2,4-6,8,10,12,17-20H2,(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.65 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's method |
J Med Chem 51: 4381-4 (2008)
Article DOI: 10.1021/jm800577j
BindingDB Entry DOI: 10.7270/Q21G0M23 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50263213
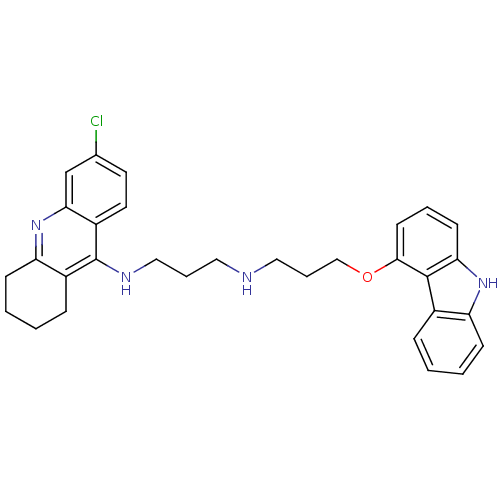
(CHEMBL478666 | N-[3-(9H-Carbazol-4-yloxy)propyl]-N...)Show SMILES Clc1ccc2c(NCCCNCCCOc3cccc4[nH]c5ccccc5c34)c3CCCCc3nc2c1 Show InChI InChI=1S/C31H33ClN4O/c32-21-14-15-24-28(20-21)36-26-11-4-2-9-23(26)31(24)34-18-6-16-33-17-7-19-37-29-13-5-12-27-30(29)22-8-1-3-10-25(22)35-27/h1,3,5,8,10,12-15,20,33,35H,2,4,6-7,9,11,16-19H2,(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.15 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's method |
J Med Chem 51: 4381-4 (2008)
Article DOI: 10.1021/jm800577j
BindingDB Entry DOI: 10.7270/Q21G0M23 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50386758

(CHEMBL2046893)Show InChI InChI=1S/C23H24F2N2/c24-20-8-12-22(13-9-20)27(23-14-10-21(25)11-15-23)18-17-26-16-4-7-19-5-2-1-3-6-19/h1-3,5-6,8-15,26H,4,7,16-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by patch clamp assay |
J Med Chem 55: 4010-4 (2012)
Article DOI: 10.1021/jm201194q
BindingDB Entry DOI: 10.7270/Q2BR8T7D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50386758

(CHEMBL2046893)Show InChI InChI=1S/C23H24F2N2/c24-20-8-12-22(13-9-20)27(23-14-10-21(25)11-15-23)18-17-26-16-4-7-19-5-2-1-3-6-19/h1-3,5-6,8-15,26H,4,7,16-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by patch clamp assay |
J Med Chem 55: 4010-4 (2012)
Article DOI: 10.1021/jm201194q
BindingDB Entry DOI: 10.7270/Q2BR8T7D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50263262
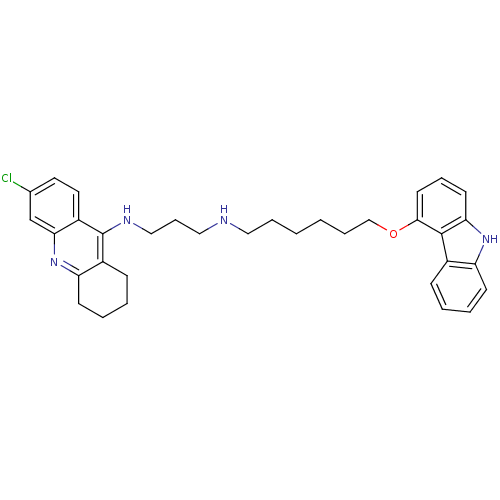
(CHEMBL474268 | N-[6-(9H-Carbazol-4-yloxy)hexyl]-N'...)Show SMILES Clc1ccc2c(NCCCNCCCCCCOc3cccc4[nH]c5ccccc5c34)c3CCCCc3nc2c1 Show InChI InChI=1S/C34H39ClN4O/c35-24-17-18-27-31(23-24)39-29-14-6-4-12-26(29)34(27)37-21-10-20-36-19-7-1-2-8-22-40-32-16-9-15-30-33(32)25-11-3-5-13-28(25)38-30/h3,5,9,11,13,15-18,23,36,38H,1-2,4,6-8,10,12,14,19-22H2,(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.57 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's method |
J Med Chem 51: 4381-4 (2008)
Article DOI: 10.1021/jm800577j
BindingDB Entry DOI: 10.7270/Q21G0M23 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50386755
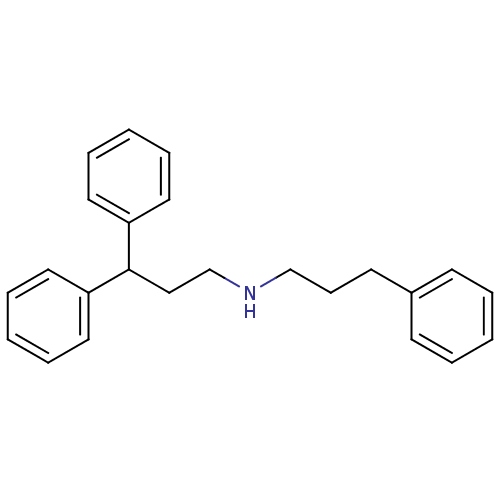
(CHEMBL2046889)Show InChI InChI=1S/C24H27N/c1-4-11-21(12-5-1)13-10-19-25-20-18-24(22-14-6-2-7-15-22)23-16-8-3-9-17-23/h1-9,11-12,14-17,24-25H,10,13,18-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by patch clamp assay |
J Med Chem 55: 4010-4 (2012)
Article DOI: 10.1021/jm201194q
BindingDB Entry DOI: 10.7270/Q2BR8T7D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50386755

(CHEMBL2046889)Show InChI InChI=1S/C24H27N/c1-4-11-21(12-5-1)13-10-19-25-20-18-24(22-14-6-2-7-15-22)23-16-8-3-9-17-23/h1-9,11-12,14-17,24-25H,10,13,18-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by patch clamp assay |
J Med Chem 55: 4010-4 (2012)
Article DOI: 10.1021/jm201194q
BindingDB Entry DOI: 10.7270/Q2BR8T7D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10514
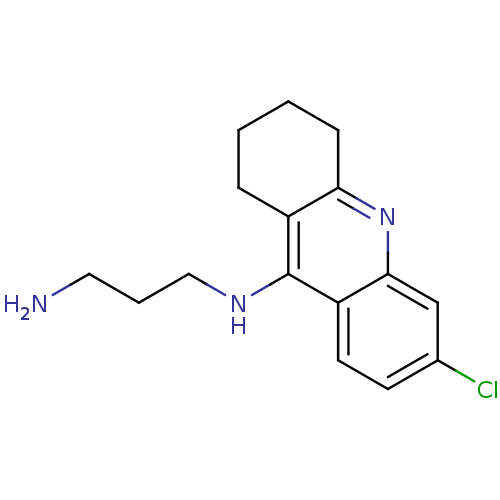
(CHEMBL191758 | N-(3-aminopropyl)-6-chloro-1,2,3,4-...)Show InChI InChI=1S/C16H20ClN3/c17-11-6-7-13-15(10-11)20-14-5-2-1-4-12(14)16(13)19-9-3-8-18/h6-7,10H,1-5,8-9,18H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21.5 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's method |
J Med Chem 51: 4381-4 (2008)
Article DOI: 10.1021/jm800577j
BindingDB Entry DOI: 10.7270/Q21G0M23 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50386757
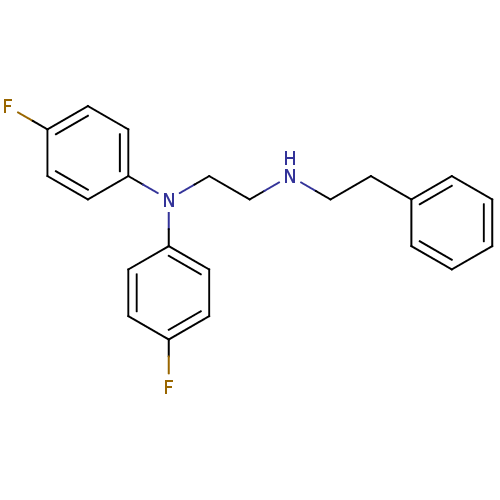
(CHEMBL2046892)Show InChI InChI=1S/C22H22F2N2/c23-19-6-10-21(11-7-19)26(22-12-8-20(24)9-13-22)17-16-25-15-14-18-4-2-1-3-5-18/h1-13,25H,14-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by patch clamp assay |
J Med Chem 55: 4010-4 (2012)
Article DOI: 10.1021/jm201194q
BindingDB Entry DOI: 10.7270/Q2BR8T7D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50386757

(CHEMBL2046892)Show InChI InChI=1S/C22H22F2N2/c23-19-6-10-21(11-7-19)26(22-12-8-20(24)9-13-22)17-16-25-15-14-18-4-2-1-3-5-18/h1-13,25H,14-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by patch clamp assay |
J Med Chem 55: 4010-4 (2012)
Article DOI: 10.1021/jm201194q
BindingDB Entry DOI: 10.7270/Q2BR8T7D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10949
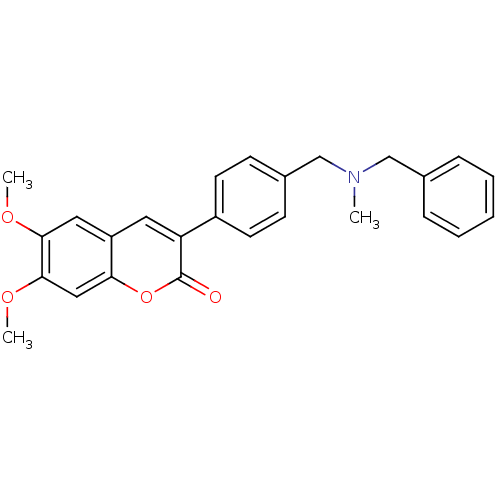
(3-(4-{[Benzyl(methyl)amino]methyl}-phenyl)-6,7-dim...)Show SMILES COc1cc2cc(-c3ccc(CN(C)Cc4ccccc4)cc3)c(=O)oc2cc1OC Show InChI InChI=1S/C26H25NO4/c1-27(16-18-7-5-4-6-8-18)17-19-9-11-20(12-10-19)22-13-21-14-24(29-2)25(30-3)15-23(21)31-26(22)28/h4-15H,16-17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 45.8 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 51: 4381-4 (2008)
Article DOI: 10.1021/jm800577j
BindingDB Entry DOI: 10.7270/Q21G0M23 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308280

(2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(5-...)Show SMILES CN(Cc1ccccc1)Cc1ccc(\C=C2/CCc3cc(OCCCCCN4CCCCC4)ccc3C2=O)cc1 Show InChI InChI=1S/C36H44N2O2/c1-37(27-30-11-5-2-6-12-30)28-31-15-13-29(14-16-31)25-33-18-17-32-26-34(19-20-35(32)36(33)39)40-24-10-4-9-23-38-21-7-3-8-22-38/h2,5-6,11-16,19-20,25-26H,3-4,7-10,17-18,21-24,27-28H2,1H3/b33-25+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308281
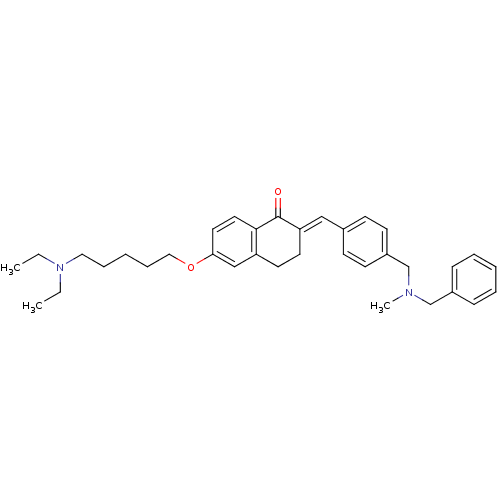
(2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(5-...)Show SMILES CCN(CC)CCCCCOc1ccc2C(=O)\C(CCc2c1)=C\c1ccc(CN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C35H44N2O2/c1-4-37(5-2)22-10-7-11-23-39-33-20-21-34-31(25-33)18-19-32(35(34)38)24-28-14-16-30(17-15-28)27-36(3)26-29-12-8-6-9-13-29/h6,8-9,12-17,20-21,24-25H,4-5,7,10-11,18-19,22-23,26-27H2,1-3H3/b32-24+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50386756
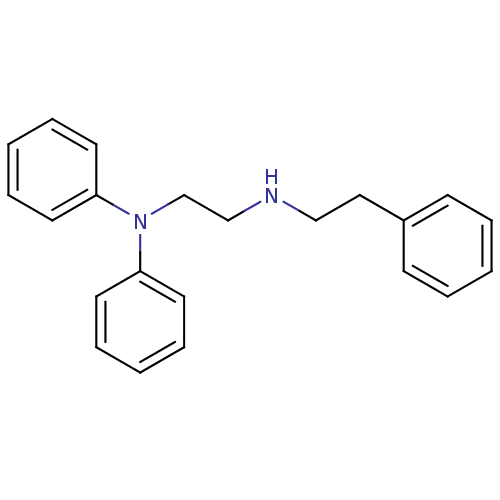
(CHEMBL2046890)Show InChI InChI=1S/C22H24N2/c1-4-10-20(11-5-1)16-17-23-18-19-24(21-12-6-2-7-13-21)22-14-8-3-9-15-22/h1-15,23H,16-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by patch clamp assay |
J Med Chem 55: 4010-4 (2012)
Article DOI: 10.1021/jm201194q
BindingDB Entry DOI: 10.7270/Q2BR8T7D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50386756

(CHEMBL2046890)Show InChI InChI=1S/C22H24N2/c1-4-10-20(11-5-1)16-17-23-18-19-24(21-12-6-2-7-13-21)22-14-8-3-9-15-22/h1-15,23H,16-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by patch clamp assay |
J Med Chem 55: 4010-4 (2012)
Article DOI: 10.1021/jm201194q
BindingDB Entry DOI: 10.7270/Q2BR8T7D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308262
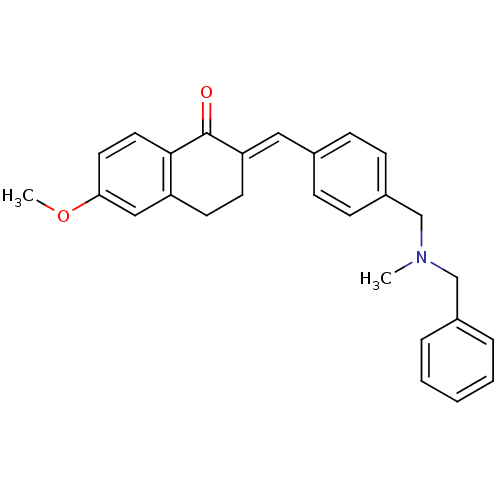
(2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-met...)Show SMILES COc1ccc2C(=O)\C(CCc2c1)=C\c1ccc(CN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C27H27NO2/c1-28(18-21-6-4-3-5-7-21)19-22-10-8-20(9-11-22)16-24-13-12-23-17-25(30-2)14-15-26(23)27(24)29/h3-11,14-17H,12-13,18-19H2,1-2H3/b24-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50263262

(CHEMBL474268 | N-[6-(9H-Carbazol-4-yloxy)hexyl]-N'...)Show SMILES Clc1ccc2c(NCCCNCCCCCCOc3cccc4[nH]c5ccccc5c34)c3CCCCc3nc2c1 Show InChI InChI=1S/C34H39ClN4O/c35-24-17-18-27-31(23-24)39-29-14-6-4-12-26(29)34(27)37-21-10-20-36-19-7-1-2-8-22-40-32-16-9-15-30-33(32)25-11-3-5-13-28(25)38-30/h3,5,9,11,13,15-18,23,36,38H,1-2,4,6-8,10,12,14,19-22H2,(H,37,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 51: 4381-4 (2008)
Article DOI: 10.1021/jm800577j
BindingDB Entry DOI: 10.7270/Q21G0M23 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308282
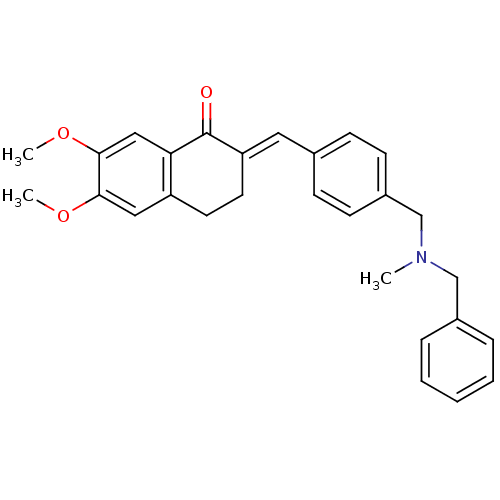
(2-{4-[(Benzylmethylamino)methyl]benzylidene}-6,7-d...)Show SMILES COc1cc2CC\C(=C/c3ccc(CN(C)Cc4ccccc4)cc3)C(=O)c2cc1OC Show InChI InChI=1S/C28H29NO3/c1-29(18-21-7-5-4-6-8-21)19-22-11-9-20(10-12-22)15-24-14-13-23-16-26(31-2)27(32-3)17-25(23)28(24)30/h4-12,15-17H,13-14,18-19H2,1-3H3/b24-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308267
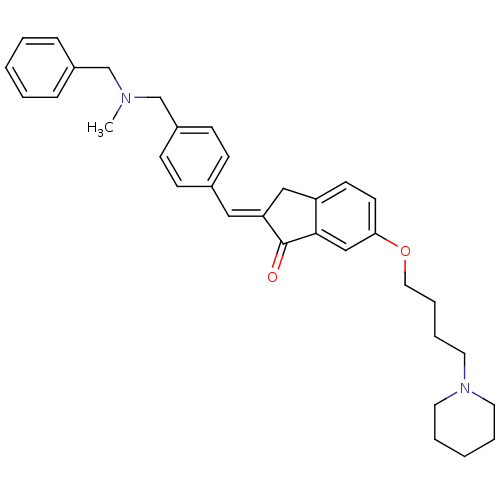
(2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(4-...)Show SMILES CN(Cc1ccccc1)Cc1ccc(\C=C2/Cc3ccc(OCCCCN4CCCCC4)cc3C2=O)cc1 Show InChI InChI=1S/C34H40N2O2/c1-35(25-28-10-4-2-5-11-28)26-29-14-12-27(13-15-29)22-31-23-30-16-17-32(24-33(30)34(31)37)38-21-9-8-20-36-18-6-3-7-19-36/h2,4-5,10-17,22,24H,3,6-9,18-21,23,25-26H2,1H3/b31-22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50263215

(CHEMBL473866 | N-[5-(9H-Carbazol-4-yloxy)pentyl]-N...)Show SMILES Clc1ccc2c(NCCCNCCCCCOc3cccc4[nH]c5ccccc5c34)c3CCCCc3nc2c1 Show InChI InChI=1S/C33H37ClN4O/c34-23-16-17-26-30(22-23)38-28-13-5-3-11-25(28)33(26)36-20-9-19-35-18-6-1-7-21-39-31-15-8-14-29-32(31)24-10-2-4-12-27(24)37-29/h2,4,8,10,12,14-17,22,35,37H,1,3,5-7,9,11,13,18-21H2,(H,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 51: 4381-4 (2008)
Article DOI: 10.1021/jm800577j
BindingDB Entry DOI: 10.7270/Q21G0M23 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308266
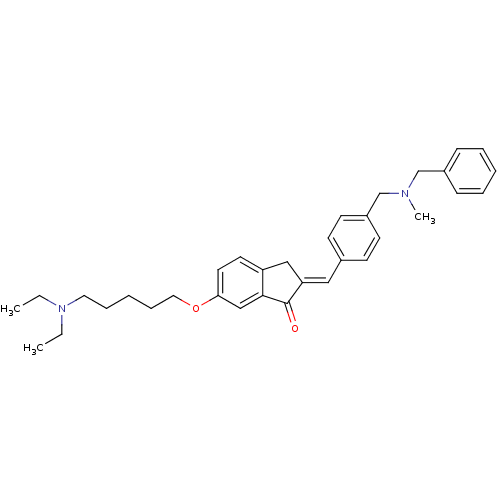
(2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(5-...)Show SMILES CCN(CC)CCCCCOc1ccc2C\C(=C/c3ccc(CN(C)Cc4ccccc4)cc3)C(=O)c2c1 Show InChI InChI=1S/C34H42N2O2/c1-4-36(5-2)20-10-7-11-21-38-32-19-18-30-23-31(34(37)33(30)24-32)22-27-14-16-29(17-15-27)26-35(3)25-28-12-8-6-9-13-28/h6,8-9,12-19,22,24H,4-5,7,10-11,20-21,23,25-26H2,1-3H3/b31-22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50386760
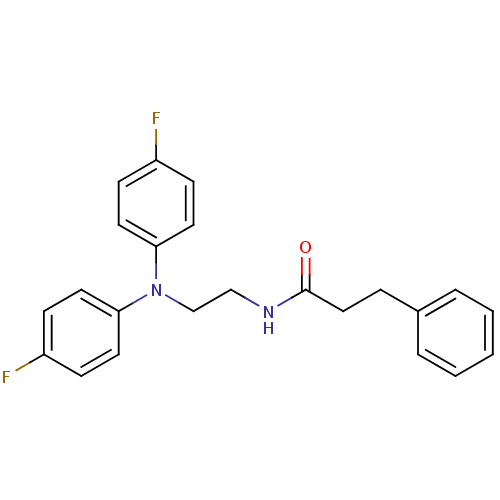
(CHEMBL2046895)Show SMILES Fc1ccc(cc1)N(CCNC(=O)CCc1ccccc1)c1ccc(F)cc1 Show InChI InChI=1S/C23H22F2N2O/c24-19-7-11-21(12-8-19)27(22-13-9-20(25)10-14-22)17-16-26-23(28)15-6-18-4-2-1-3-5-18/h1-5,7-14H,6,15-17H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 192 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by patch clamp assay |
J Med Chem 55: 4010-4 (2012)
Article DOI: 10.1021/jm201194q
BindingDB Entry DOI: 10.7270/Q2BR8T7D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50386760

(CHEMBL2046895)Show SMILES Fc1ccc(cc1)N(CCNC(=O)CCc1ccccc1)c1ccc(F)cc1 Show InChI InChI=1S/C23H22F2N2O/c24-19-7-11-21(12-8-19)27(22-13-9-20(25)10-14-22)17-16-26-23(28)15-6-18-4-2-1-3-5-18/h1-5,7-14H,6,15-17H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by patch clamp assay |
J Med Chem 55: 4010-4 (2012)
Article DOI: 10.1021/jm201194q
BindingDB Entry DOI: 10.7270/Q2BR8T7D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308265

(2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(5-...)Show SMILES CN(Cc1ccccc1)Cc1ccc(\C=C2/Cc3ccc(OCCCCCN4CCCCC4)cc3C2=O)cc1 Show InChI InChI=1S/C35H42N2O2/c1-36(26-29-11-5-2-6-12-29)27-30-15-13-28(14-16-30)23-32-24-31-17-18-33(25-34(31)35(32)38)39-22-10-4-9-21-37-19-7-3-8-20-37/h2,5-6,11-18,23,25H,3-4,7-10,19-22,24,26-27H2,1H3/b32-23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50263214

(CHEMBL478667 | N-[4-(9H-Carbazol-4-yloxy)butyl]-N'...)Show SMILES Clc1ccc2c(NCCCNCCCCOc3cccc4[nH]c5ccccc5c34)c3CCCCc3nc2c1 Show InChI InChI=1S/C32H35ClN4O/c33-22-15-16-25-29(21-22)37-27-12-4-2-10-24(27)32(25)35-19-8-18-34-17-5-6-20-38-30-14-7-13-28-31(30)23-9-1-3-11-26(23)36-28/h1,3,7,9,11,13-16,21,34,36H,2,4-6,8,10,12,17-20H2,(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 211 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 51: 4381-4 (2008)
Article DOI: 10.1021/jm800577j
BindingDB Entry DOI: 10.7270/Q21G0M23 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50263213

(CHEMBL478666 | N-[3-(9H-Carbazol-4-yloxy)propyl]-N...)Show SMILES Clc1ccc2c(NCCCNCCCOc3cccc4[nH]c5ccccc5c34)c3CCCCc3nc2c1 Show InChI InChI=1S/C31H33ClN4O/c32-21-14-15-24-28(20-21)36-26-11-4-2-9-23(26)31(24)34-18-6-16-33-17-7-19-37-29-13-5-12-27-30(29)22-8-1-3-10-25(22)35-27/h1,3,5,8,10,12-15,20,33,35H,2,4,6-7,9,11,16-19H2,(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 296 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 51: 4381-4 (2008)
Article DOI: 10.1021/jm800577j
BindingDB Entry DOI: 10.7270/Q21G0M23 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308264
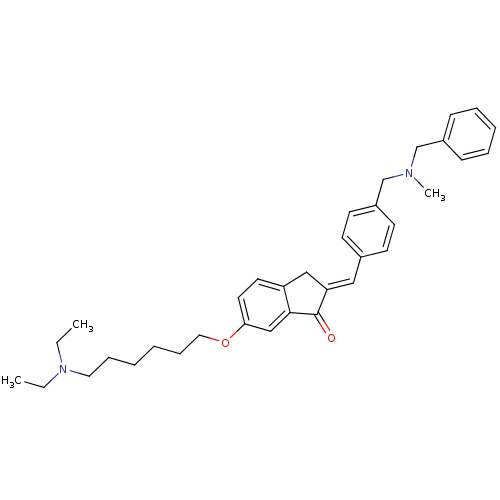
(2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(6-...)Show SMILES CCN(CC)CCCCCCOc1ccc2C\C(=C/c3ccc(CN(C)Cc4ccccc4)cc3)C(=O)c2c1 Show InChI InChI=1S/C35H44N2O2/c1-4-37(5-2)21-11-6-7-12-22-39-33-20-19-31-24-32(35(38)34(31)25-33)23-28-15-17-30(18-16-28)27-36(3)26-29-13-9-8-10-14-29/h8-10,13-20,23,25H,4-7,11-12,21-22,24,26-27H2,1-3H3/b32-23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 424 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's method |
J Med Chem 51: 4381-4 (2008)
Article DOI: 10.1021/jm800577j
BindingDB Entry DOI: 10.7270/Q21G0M23 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308263
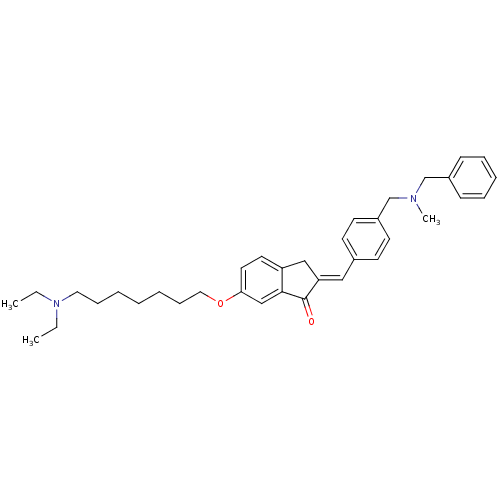
(2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(7-...)Show SMILES CCN(CC)CCCCCCCOc1ccc2C\C(=C/c3ccc(CN(C)Cc4ccccc4)cc3)C(=O)c2c1 Show InChI InChI=1S/C36H46N2O2/c1-4-38(5-2)22-12-7-6-8-13-23-40-34-21-20-32-25-33(36(39)35(32)26-34)24-29-16-18-31(19-17-29)28-37(3)27-30-14-10-9-11-15-30/h9-11,14-21,24,26H,4-8,12-13,22-23,25,27-28H2,1-3H3/b33-24+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50386753
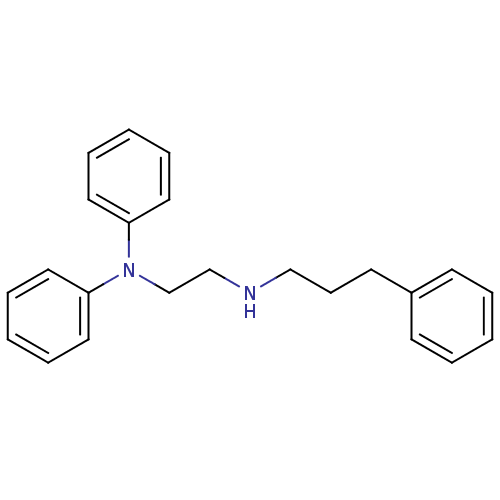
(CHEMBL2046891)Show InChI InChI=1S/C23H26N2/c1-4-11-21(12-5-1)13-10-18-24-19-20-25(22-14-6-2-7-15-22)23-16-8-3-9-17-23/h1-9,11-12,14-17,24H,10,13,18-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by patch clamp assay |
J Med Chem 55: 4010-4 (2012)
Article DOI: 10.1021/jm201194q
BindingDB Entry DOI: 10.7270/Q2BR8T7D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308277
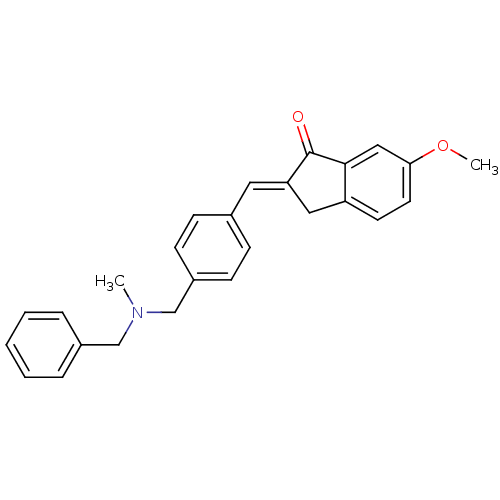
(2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-met...)Show SMILES COc1ccc2C\C(=C/c3ccc(CN(C)Cc4ccccc4)cc3)C(=O)c2c1 Show InChI InChI=1S/C26H25NO2/c1-27(17-20-6-4-3-5-7-20)18-21-10-8-19(9-11-21)14-23-15-22-12-13-24(29-2)16-25(22)26(23)28/h3-14,16H,15,17-18H2,1-2H3/b23-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50386753

(CHEMBL2046891)Show InChI InChI=1S/C23H26N2/c1-4-11-21(12-5-1)13-10-18-24-19-20-25(22-14-6-2-7-15-22)23-16-8-3-9-17-23/h1-9,11-12,14-17,24H,10,13,18-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by patch clamp assay |
J Med Chem 55: 4010-4 (2012)
Article DOI: 10.1021/jm201194q
BindingDB Entry DOI: 10.7270/Q2BR8T7D |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50263213

(CHEMBL478666 | N-[3-(9H-Carbazol-4-yloxy)propyl]-N...)Show SMILES Clc1ccc2c(NCCCNCCCOc3cccc4[nH]c5ccccc5c34)c3CCCCc3nc2c1 Show InChI InChI=1S/C31H33ClN4O/c32-21-14-15-24-28(20-21)36-26-11-4-2-9-23(26)31(24)34-18-6-16-33-17-7-19-37-29-13-5-12-27-30(29)22-8-1-3-10-25(22)35-27/h1,3,5,8,10,12-15,20,33,35H,2,4,6-7,9,11,16-19H2,(H,34,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of NMDA NR1/NR2B receptor (unknown origin) expressed in xenopus oocytes assessed as inhibition of NMDA and glycine-induced current respons... |
J Med Chem 51: 4381-4 (2008)
Article DOI: 10.1021/jm800577j
BindingDB Entry DOI: 10.7270/Q21G0M23 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308268
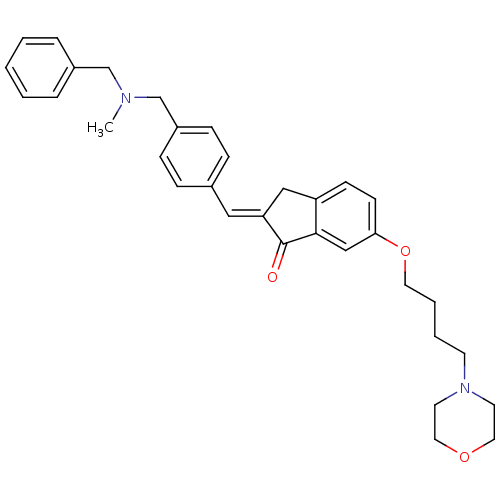
(2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(4-...)Show SMILES CN(Cc1ccccc1)Cc1ccc(\C=C2/Cc3ccc(OCCCCN4CCOCC4)cc3C2=O)cc1 Show InChI InChI=1S/C33H38N2O3/c1-34(24-27-7-3-2-4-8-27)25-28-11-9-26(10-12-28)21-30-22-29-13-14-31(23-32(29)33(30)36)38-18-6-5-15-35-16-19-37-20-17-35/h2-4,7-14,21,23H,5-6,15-20,22,24-25H2,1H3/b30-21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308269
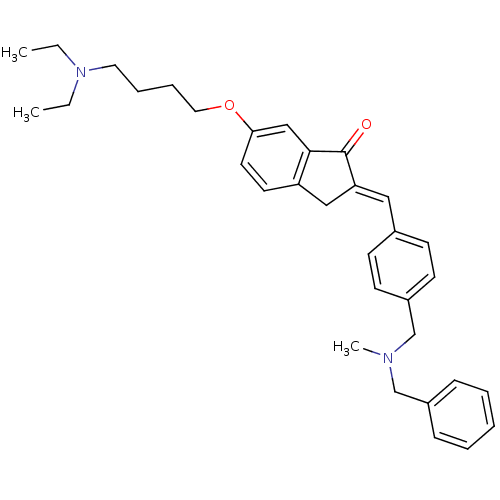
(2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(4-...)Show SMILES CCN(CC)CCCCOc1ccc2C\C(=C/c3ccc(CN(C)Cc4ccccc4)cc3)C(=O)c2c1 Show InChI InChI=1S/C33H40N2O2/c1-4-35(5-2)19-9-10-20-37-31-18-17-29-22-30(33(36)32(29)23-31)21-26-13-15-28(16-14-26)25-34(3)24-27-11-7-6-8-12-27/h6-8,11-18,21,23H,4-5,9-10,19-20,22,24-25H2,1-3H3/b30-21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308279
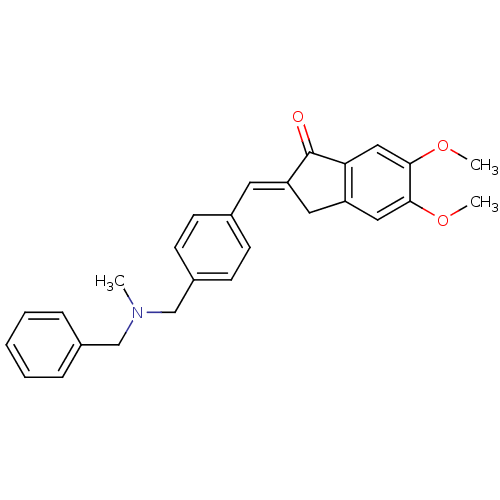
(CHEMBL598632 | N-(2-chloro-5-(S-2-fluoroethyl)thio...)Show SMILES COc1cc2C\C(=C/c3ccc(CN(C)Cc4ccccc4)cc3)C(=O)c2cc1OC Show InChI InChI=1S/C27H27NO3/c1-28(17-20-7-5-4-6-8-20)18-21-11-9-19(10-12-21)13-23-14-22-15-25(30-2)26(31-3)16-24(22)27(23)29/h4-13,15-16H,14,17-18H2,1-3H3/b23-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308270
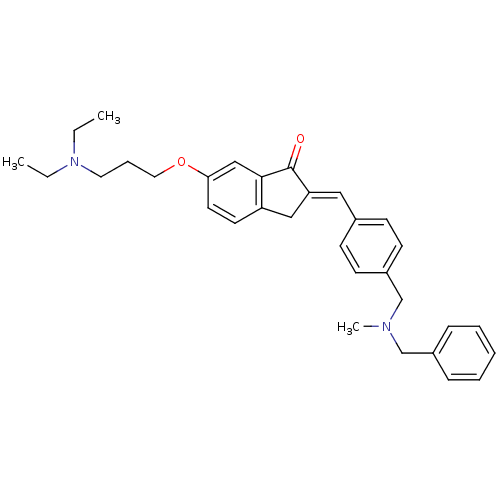
(2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(3-...)Show SMILES CCN(CC)CCCOc1ccc2C\C(=C/c3ccc(CN(C)Cc4ccccc4)cc3)C(=O)c2c1 Show InChI InChI=1S/C32H38N2O2/c1-4-34(5-2)18-9-19-36-30-17-16-28-21-29(32(35)31(28)22-30)20-25-12-14-27(15-13-25)24-33(3)23-26-10-7-6-8-11-26/h6-8,10-17,20,22H,4-5,9,18-19,21,23-24H2,1-3H3/b29-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
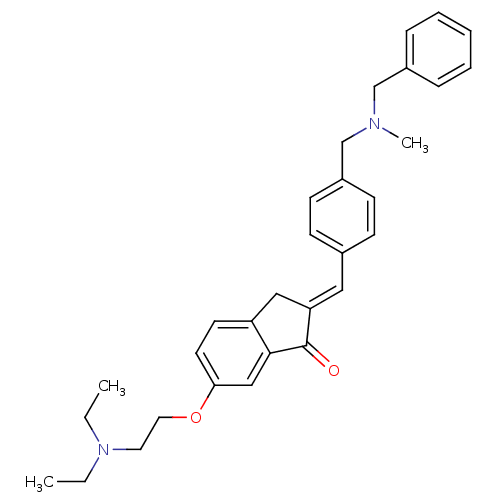
(Homo sapiens (Human)) | BDBM50308271
(2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(2-...)Show SMILES CCN(CC)CCOc1ccc2C\C(=C/c3ccc(CN(C)Cc4ccccc4)cc3)C(=O)c2c1 Show InChI InChI=1S/C31H36N2O2/c1-4-33(5-2)17-18-35-29-16-15-27-20-28(31(34)30(27)21-29)19-24-11-13-26(14-12-24)23-32(3)22-25-9-7-6-8-10-25/h6-16,19,21H,4-5,17-18,20,22-23H2,1-3H3/b28-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
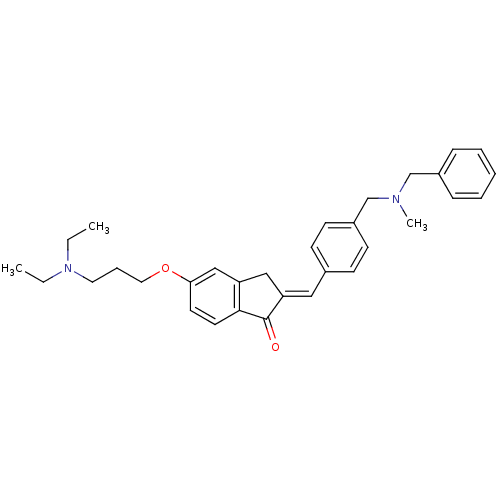
(Homo sapiens (Human)) | BDBM50308273
(2-{4-[(Benzylmethylamino)methyl]benzylidene}-5-(3-...)Show SMILES CCN(CC)CCCOc1ccc2C(=O)\C(Cc2c1)=C\c1ccc(CN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C32H38N2O2/c1-4-34(5-2)18-9-19-36-30-16-17-31-28(22-30)21-29(32(31)35)20-25-12-14-27(15-13-25)24-33(3)23-26-10-7-6-8-11-26/h6-8,10-17,20,22H,4-5,9,18-19,21,23-24H2,1-3H3/b29-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10514

(CHEMBL191758 | N-(3-aminopropyl)-6-chloro-1,2,3,4-...)Show InChI InChI=1S/C16H20ClN3/c17-11-6-7-13-15(10-11)20-14-5-2-1-4-12(14)16(13)19-9-3-8-18/h6-7,10H,1-5,8-9,18H2,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 51: 4381-4 (2008)
Article DOI: 10.1021/jm800577j
BindingDB Entry DOI: 10.7270/Q21G0M23 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308266

(2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(5-...)Show SMILES CCN(CC)CCCCCOc1ccc2C\C(=C/c3ccc(CN(C)Cc4ccccc4)cc3)C(=O)c2c1 Show InChI InChI=1S/C34H42N2O2/c1-4-36(5-2)20-10-7-11-21-38-32-19-18-30-23-31(34(37)33(30)24-32)22-27-14-16-29(17-15-27)26-35(3)25-28-12-8-6-9-13-28/h6,8-9,12-19,22,24H,4-5,7,10-11,20-21,23,25-26H2,1-3H3/b31-22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50386754
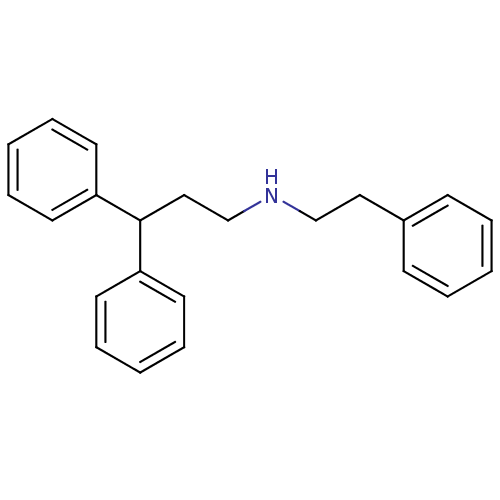
(CHEMBL2046888)Show InChI InChI=1S/C23H25N/c1-4-10-20(11-5-1)16-18-24-19-17-23(21-12-6-2-7-13-21)22-14-8-3-9-15-22/h1-15,23-24H,16-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by patch clamp assay |
J Med Chem 55: 4010-4 (2012)
Article DOI: 10.1021/jm201194q
BindingDB Entry DOI: 10.7270/Q2BR8T7D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50386754

(CHEMBL2046888)Show InChI InChI=1S/C23H25N/c1-4-10-20(11-5-1)16-18-24-19-17-23(21-12-6-2-7-13-21)22-14-8-3-9-15-22/h1-15,23-24H,16-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by patch clamp assay |
J Med Chem 55: 4010-4 (2012)
Article DOI: 10.1021/jm201194q
BindingDB Entry DOI: 10.7270/Q2BR8T7D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
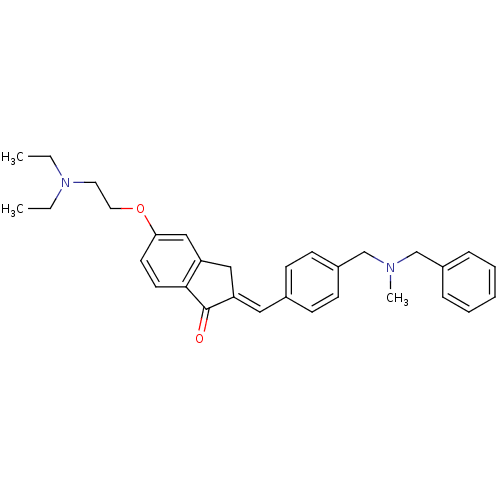
(Homo sapiens (Human)) | BDBM50308274
(2-{4-[(Benzylmethylamino)methyl]benzylidene}-5-(2-...)Show SMILES CCN(CC)CCOc1ccc2C(=O)\C(Cc2c1)=C\c1ccc(CN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C31H36N2O2/c1-4-33(5-2)17-18-35-29-15-16-30-27(21-29)20-28(31(30)34)19-24-11-13-26(14-12-24)23-32(3)22-25-9-7-6-8-10-25/h6-16,19,21H,4-5,17-18,20,22-23H2,1-3H3/b28-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308269

(2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(4-...)Show SMILES CCN(CC)CCCCOc1ccc2C\C(=C/c3ccc(CN(C)Cc4ccccc4)cc3)C(=O)c2c1 Show InChI InChI=1S/C33H40N2O2/c1-4-35(5-2)19-9-10-20-37-31-18-17-29-22-30(33(36)32(29)23-31)21-26-13-15-28(16-14-26)25-34(3)24-27-11-7-6-8-12-27/h6-8,11-18,21,23H,4-5,9-10,19-20,22,24-25H2,1-3H3/b30-21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308272
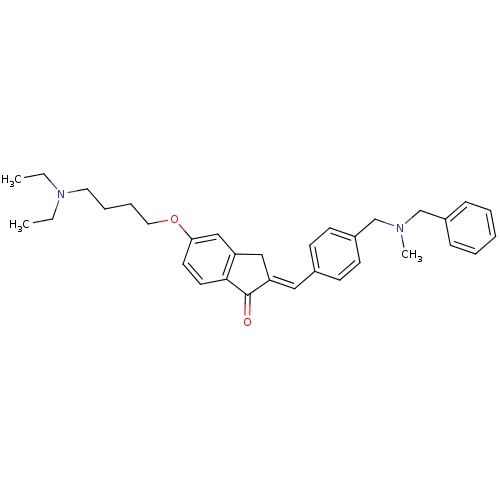
(2-{4-[(Benzylmethylamino)methyl]benzylidene}-5-(4-...)Show SMILES CCN(CC)CCCCOc1ccc2C(=O)\C(Cc2c1)=C\c1ccc(CN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C33H40N2O2/c1-4-35(5-2)19-9-10-20-37-31-17-18-32-29(23-31)22-30(33(32)36)21-26-13-15-28(16-14-26)25-34(3)24-27-11-7-6-8-12-27/h6-8,11-18,21,23H,4-5,9-10,19-20,22,24-25H2,1-3H3/b30-21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method |
Bioorg Med Chem 18: 1749-60 (2010)
Article DOI: 10.1016/j.bmc.2010.01.071
BindingDB Entry DOI: 10.7270/Q2P26Z7H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data