

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50241727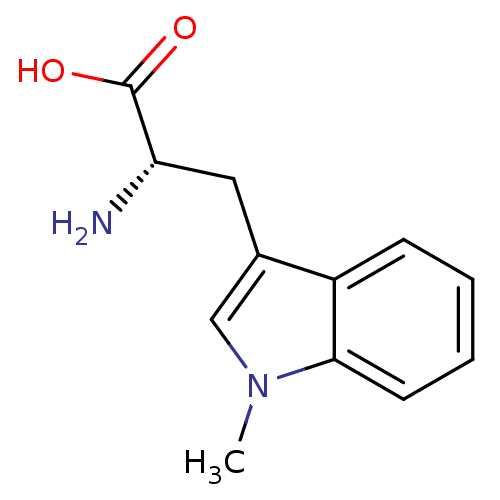 ((S)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of IDO by cell-free assay | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303906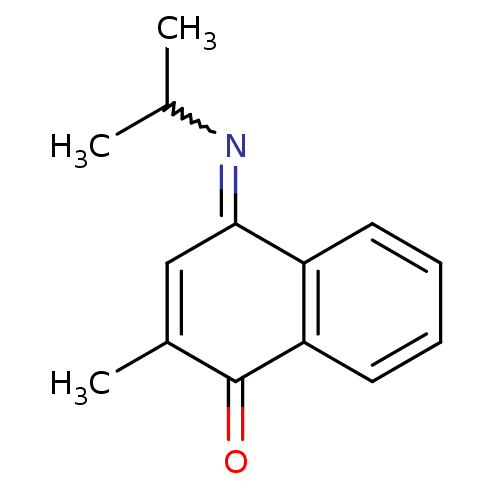 ((E)-4-(isopropylimino)-2-methylnaphthalen-1(4H)-on...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303904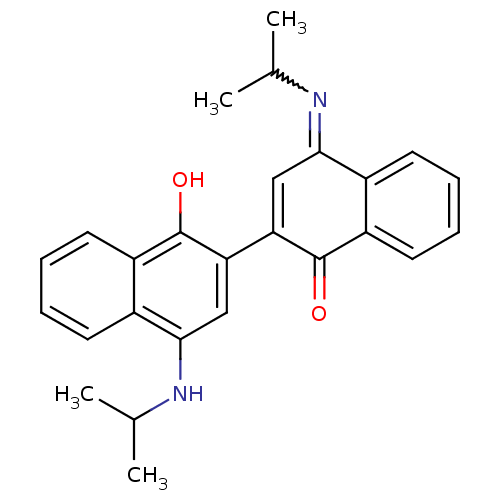 ((4E,4'E)-4,4'-bis(isopropylimino)-4',4'a-dihydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303905 ((4E,4'E)-4,4'-bis(pentan-3-ylimino)-4',4'a-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM32142 (5-amino-8-quinolinol | 5-aminoquinolin-8-ol | 5-az...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of mouse recombinant IDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303901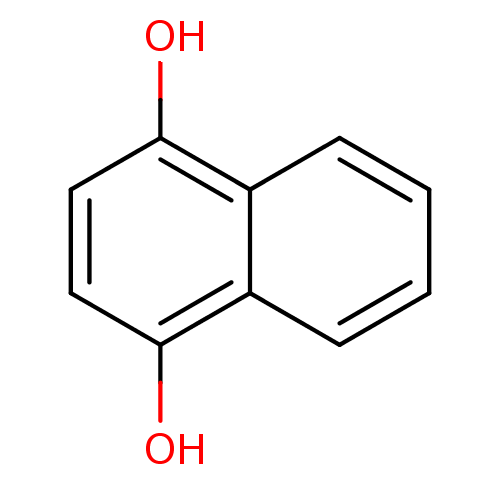 (1,4-Dihydroxynaphthalene | 1,4-Naphthohydroquinone...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303927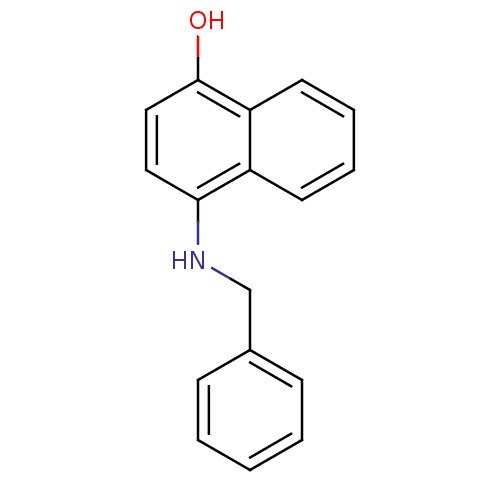 (4-(Benzylamino)-1-naphthol | CHEMBL569096) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303903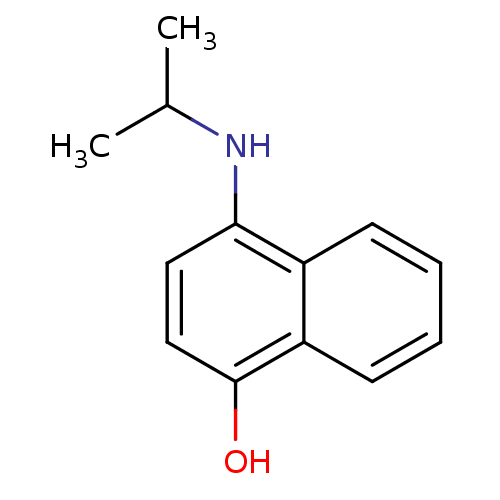 (4-(Isopropylamino)-1-naphthol | CHEMBL578212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303930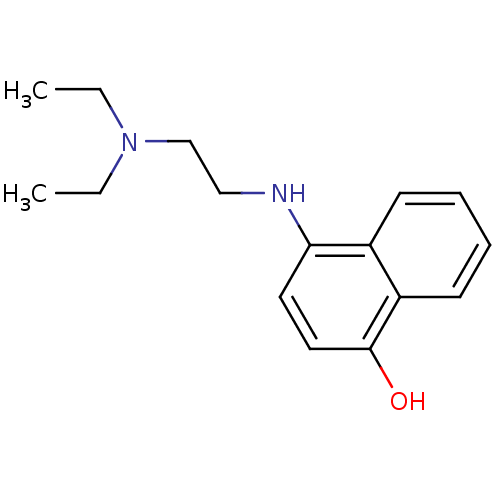 (4-(2-(diethylamino)ethylamino)-1-naphthol | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM32142 (5-amino-8-quinolinol | 5-aminoquinolin-8-ol | 5-az...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303925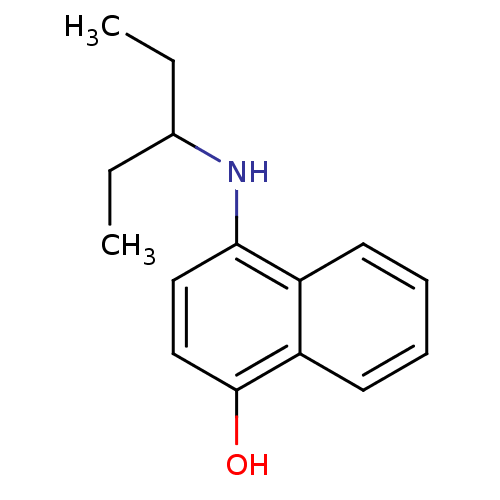 (4-(Pent-3-ylamino)-1-naphthol | CHEMBL571980) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303910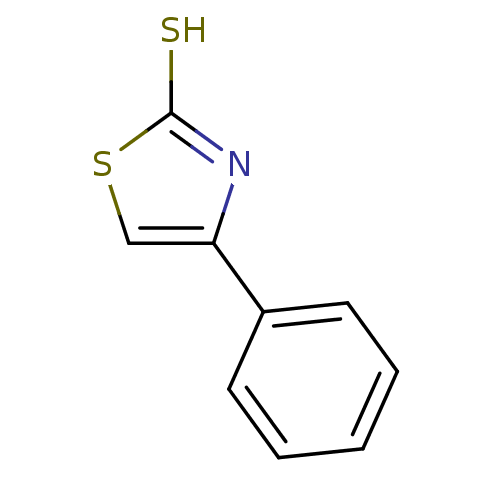 (4-phenylthiazole-2-thiol | CHEMBL571436) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in HEK293 cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50303906 ((E)-4-(isopropylimino)-2-methylnaphthalen-1(4H)-on...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of mouse recombinant IDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303923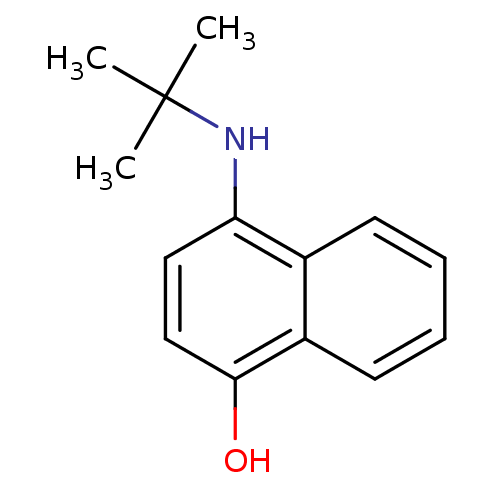 (4-(tert-butylamino)naphthalen-1-ol | CHEMBL568767) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303922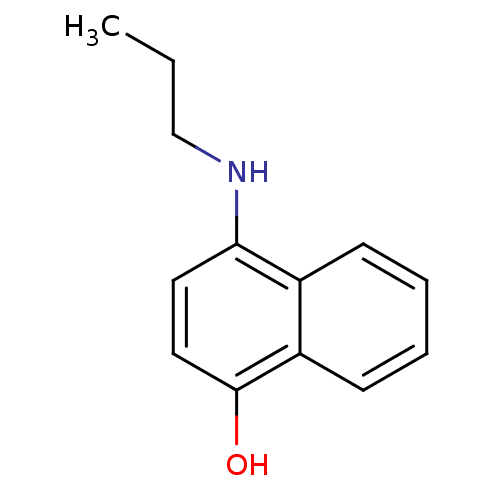 (4-(propylamino)naphthalen-1-ol | CHEMBL572064) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Mus musculus) | BDBM32142 (5-amino-8-quinolinol | 5-aminoquinolin-8-ol | 5-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of mouse recombinant TDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303929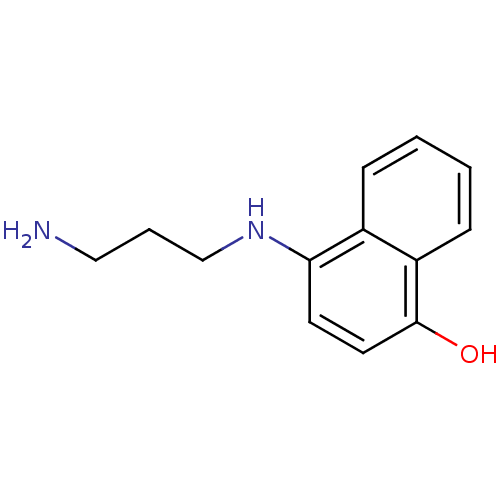 (CHEMBL576124 | N-(4-Hydroxy-1-naphthyl)propane-1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM17448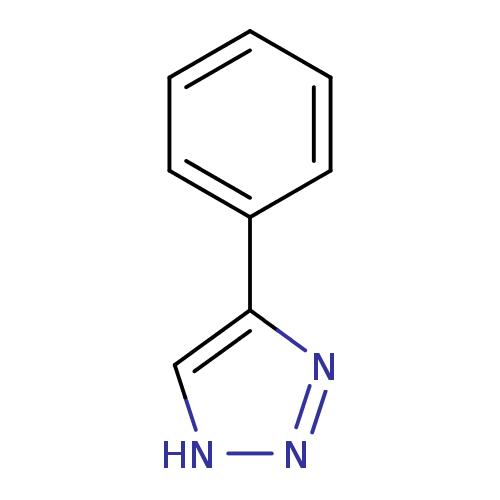 (1,2,3-triazole analogue, 4 | 5-phenyl-1H-1,2,3-tri...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of mouse recombinant IDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM17472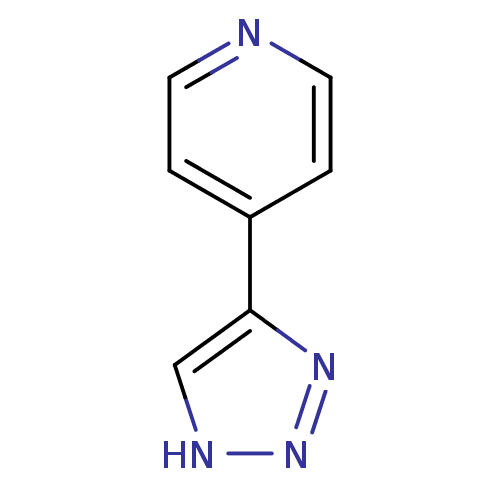 (1,2,3-triazole analogue, 28 | 4-(1H-1,2,3-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303938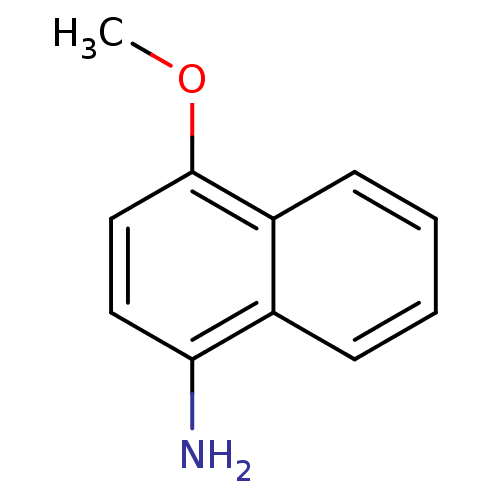 (4-methoxynaphthalen-1-amine | CHEMBL572058) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM43339 (4-amino-1-naphthalenol;hydrochloride | 4-amino-1-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Mus musculus) | BDBM50303906 ((E)-4-(isopropylimino)-2-methylnaphthalen-1(4H)-on...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of mouse recombinant TDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303907 (2,3-dihydrobenzo[d]thiazole-2-thiol | CHEMBL570797) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in HEK293 cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50303901 (1,4-Dihydroxynaphthalene | 1,4-Naphthohydroquinone...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of mouse recombinant IDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303901 (1,4-Dihydroxynaphthalene | 1,4-Naphthohydroquinone...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in HEK293 cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Mus musculus) | BDBM50303901 (1,4-Dihydroxynaphthalene | 1,4-Naphthohydroquinone...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of mouse recombinant TDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303921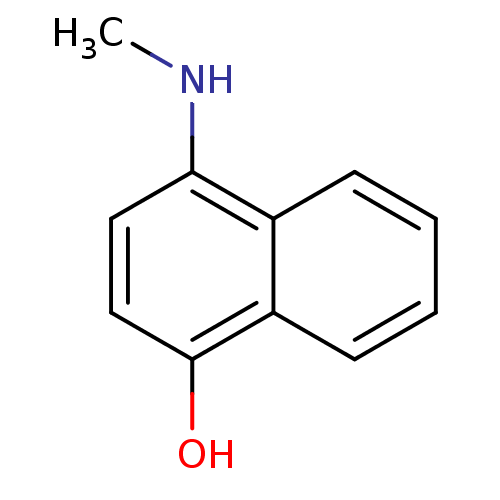 (4-(methylamino)naphthalen-1-ol | CHEMBL571240) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303903 (4-(Isopropylamino)-1-naphthol | CHEMBL578212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in HEK293 cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303937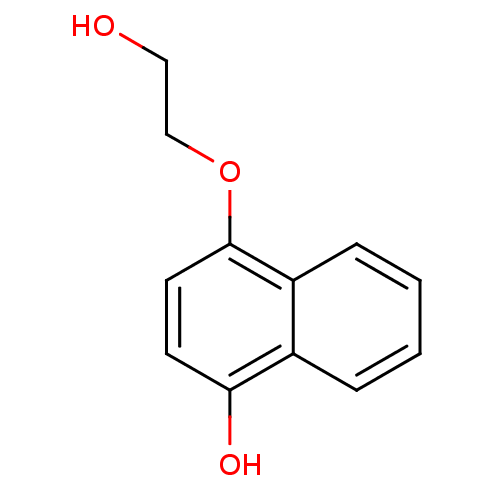 (4-(2-Hydroxyethoxy)-1-naphthol | CHEMBL570066) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303926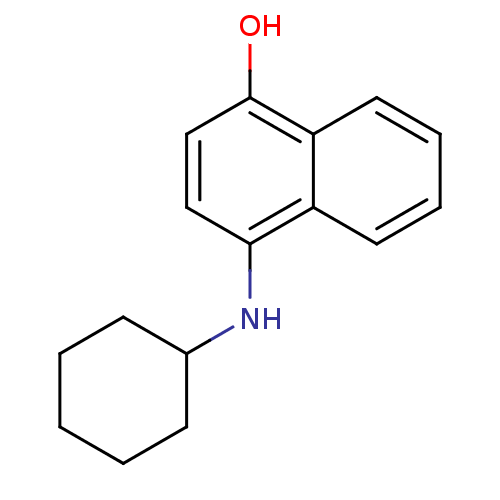 (4-(Cyclohexylamino)-1-naphthol | CHEMBL571437) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303920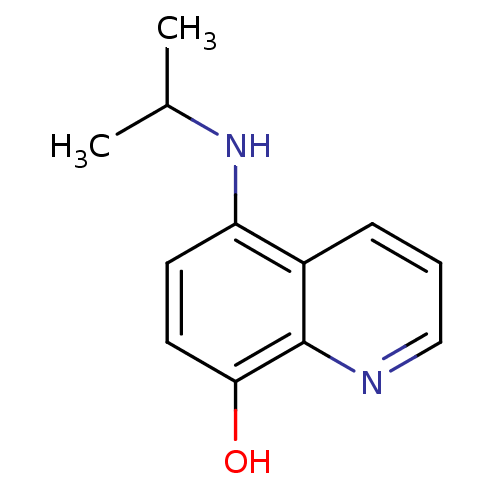 (5-(isopropylamino)quinolin-8-ol | CHEMBL571661) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Mus musculus) | BDBM50303903 (4-(Isopropylamino)-1-naphthol | CHEMBL578212) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of mouse recombinant TDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303902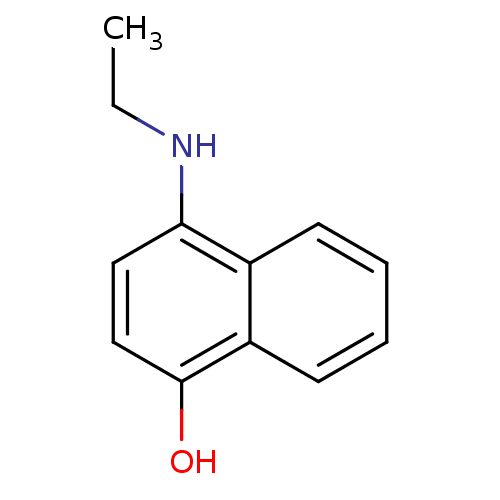 (4-(ethylamino)naphthalen-1-ol | CHEMBL577335) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50303907 (2,3-dihydrobenzo[d]thiazole-2-thiol | CHEMBL570797) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of mouse recombinant IDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50303910 (4-phenylthiazole-2-thiol | CHEMBL571436) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of mouse recombinant IDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303904 ((4E,4'E)-4,4'-bis(isopropylimino)-4',4'a-dihydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in HEK293 cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303924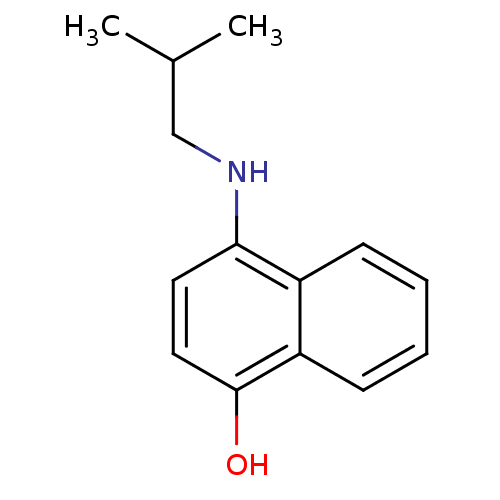 (4-(isobutylamino)naphthalen-1-ol | CHEMBL571865) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50303903 (4-(Isopropylamino)-1-naphthol | CHEMBL578212) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of mouse recombinant IDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50241727 ((S)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic ac...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of mouse recombinant IDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303919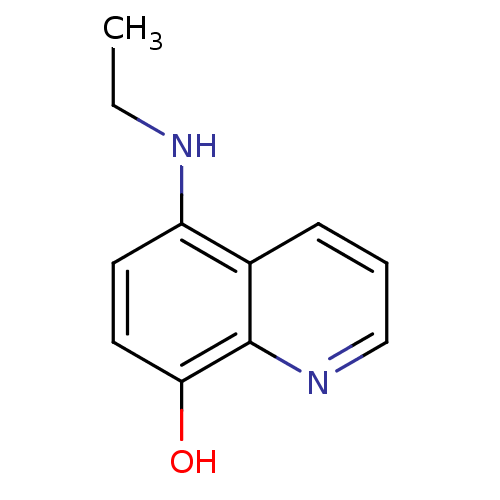 (5-(ethylamino)quinolin-8-ol | CHEMBL571660) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303928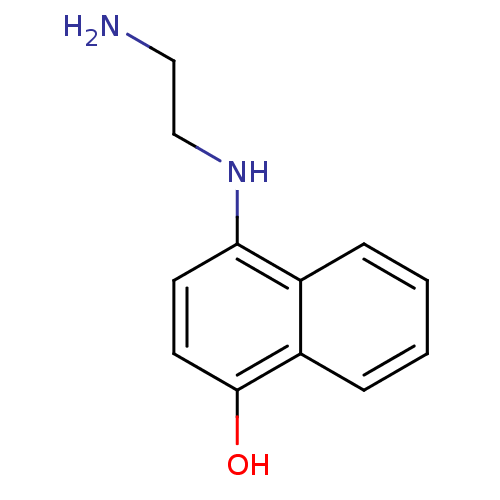 (CHEMBL569381 | N-(4-Hydroxy-1-naphthyl)ethane-1,2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303908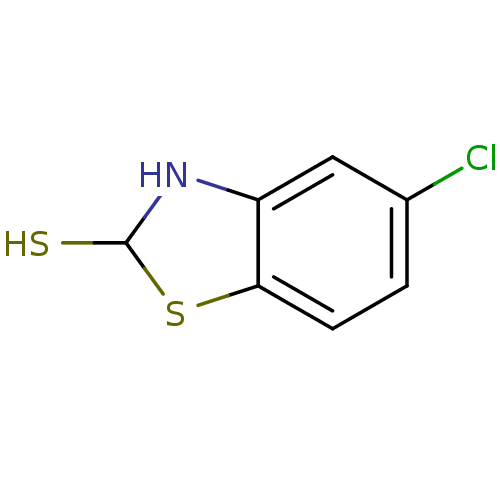 (5-chloro-2,3-dihydrobenzo[d]thiazole-2-thiol | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303907 (2,3-dihydrobenzo[d]thiazole-2-thiol | CHEMBL570797) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50303904 ((4E,4'E)-4,4'-bis(isopropylimino)-4',4'a-dihydro-2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of mouse recombinant IDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303906 ((E)-4-(isopropylimino)-2-methylnaphthalen-1(4H)-on...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in HEK293 cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Mus musculus) | BDBM50303904 ((4E,4'E)-4,4'-bis(isopropylimino)-4',4'a-dihydro-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of mouse recombinant TDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Mus musculus) | BDBM50303902 (4-(ethylamino)naphthalen-1-ol | CHEMBL577335) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of mouse recombinant TDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50303902 (4-(ethylamino)naphthalen-1-ol | CHEMBL577335) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of mouse recombinant IDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303909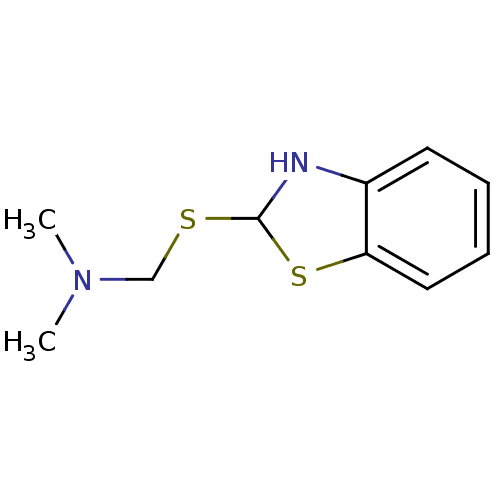 (1-(2,3-dihydrobenzo[d]thiazol-2-ylthio)-N,N-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303910 (4-phenylthiazole-2-thiol | CHEMBL571436) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 80 total ) | Next | Last >> |