 Found 9514 hits with Last Name = 'chen' and Initial = 'n'
Found 9514 hits with Last Name = 'chen' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068810
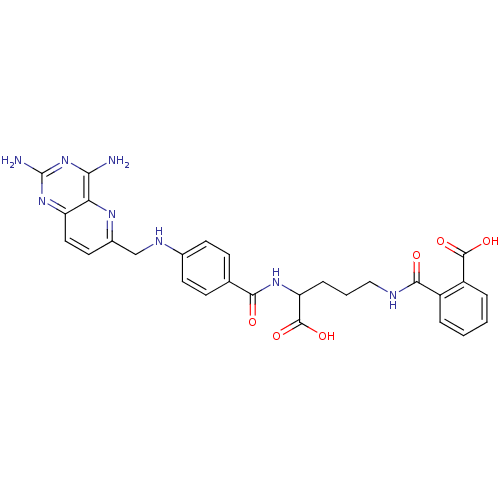
(CHEMBL149164 | N-(4-Carboxy-4-{4-[(2,4-diamino-pyr...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C28H28N8O6/c29-23-22-20(35-28(30)36-23)12-11-17(33-22)14-32-16-9-7-15(8-10-16)24(37)34-21(27(41)42)6-3-13-31-25(38)18-4-1-2-5-19(18)26(39)40/h1-2,4-5,7-12,21,32H,3,6,13-14H2,(H,31,38)(H,34,37)(H,39,40)(H,41,42)(H4,29,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068808
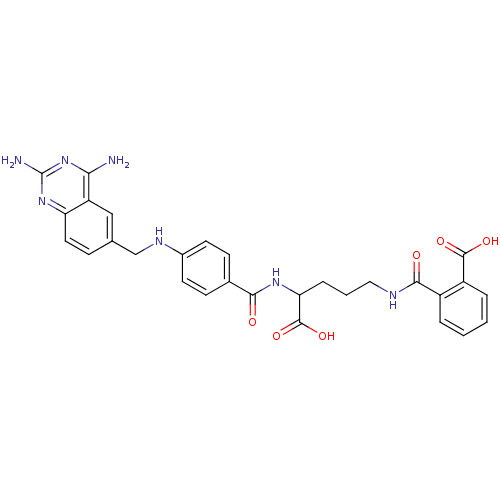
(CHEMBL297088 | N-(4-Carboxy-4-{4-[(2,4-diamino-qui...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C29H29N7O6/c30-24-21-14-16(7-12-22(21)35-29(31)36-24)15-33-18-10-8-17(9-11-18)25(37)34-23(28(41)42)6-3-13-32-26(38)19-4-1-2-5-20(19)27(39)40/h1-2,4-5,7-12,14,23,33H,3,6,13,15H2,(H,32,38)(H,34,37)(H,39,40)(H,41,42)(H4,30,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068812
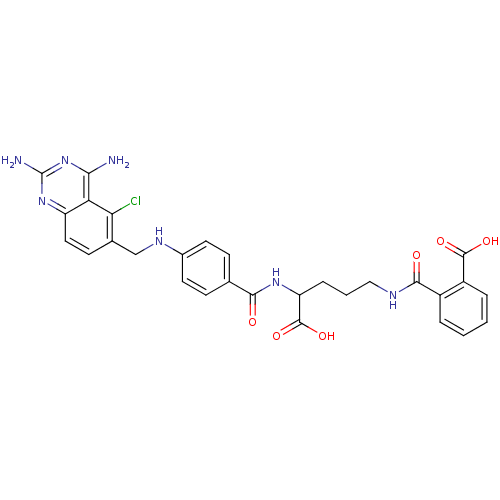
(CHEMBL146917 | N-(4-Carboxy-4-{4-[(2,4-diamino-5-c...)Show SMILES Nc1nc(N)c2c(Cl)c(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C29H28ClN7O6/c30-23-16(9-12-20-22(23)24(31)37-29(32)36-20)14-34-17-10-7-15(8-11-17)25(38)35-21(28(42)43)6-3-13-33-26(39)18-4-1-2-5-19(18)27(40)41/h1-2,4-5,7-12,21,34H,3,6,13-14H2,(H,33,39)(H,35,38)(H,40,41)(H,42,43)(H4,31,32,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50011320
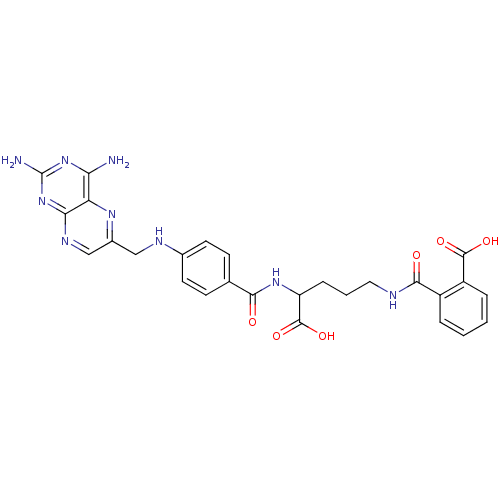
(CHEMBL18155 | N-(4-Carboxy-4-{4-[(2,4-diamino-pter...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C27H27N9O6/c28-21-20-22(36-27(29)35-21)32-13-16(33-20)12-31-15-9-7-14(8-10-15)23(37)34-19(26(41)42)6-3-11-30-24(38)17-4-1-2-5-18(17)25(39)40/h1-2,4-5,7-10,13,19,31H,3,6,11-12H2,(H,30,38)(H,34,37)(H,39,40)(H,41,42)(H4,28,29,32,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068813
(CHEMBL149962 | N-(4-Carboxy-4-{4-[(2,4-diamino-pyr...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C28H28N8O6/c29-22-20-12-15(14-33-23(20)36-28(30)35-22)13-32-17-9-7-16(8-10-17)24(37)34-21(27(41)42)6-3-11-31-25(38)18-4-1-2-5-19(18)26(39)40/h1-2,4-5,7-10,12,14,21,32H,3,6,11,13H2,(H,31,38)(H,34,37)(H,39,40)(H,41,42)(H4,29,30,33,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068809
(CHEMBL150607 | N-(4-Carboxy-4-{4-[(2,4-diamino-5-m...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCCNC(=O)c2ccccc2C(O)=O)C(O)=O)cnc2nc(N)nc(N)c12 Show InChI InChI=1S/C29H30N8O6/c1-15-17(14-34-24-22(15)23(30)36-29(31)37-24)13-33-18-10-8-16(9-11-18)25(38)35-21(28(42)43)7-4-12-32-26(39)19-5-2-3-6-20(19)27(40)41/h2-3,5-6,8-11,14,21,33H,4,7,12-13H2,1H3,(H,32,39)(H,35,38)(H,40,41)(H,42,43)(H4,30,31,34,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068811
(CHEMBL149218 | N-(4-Carboxy-4-{4-[(2,4-diamino-5-m...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCCNC(=O)c2ccccc2C(O)=O)C(O)=O)ccc2nc(N)nc(N)c12 Show InChI InChI=1S/C30H31N7O6/c1-16-18(10-13-22-24(16)25(31)37-30(32)36-22)15-34-19-11-8-17(9-12-19)26(38)35-23(29(42)43)7-4-14-33-27(39)20-5-2-3-6-21(20)28(40)41/h2-3,5-6,8-13,23,34H,4,7,14-15H2,1H3,(H,33,39)(H,35,38)(H,40,41)(H,42,43)(H4,31,32,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50367055

(4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM591249
(2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7-fl...)Show SMILES CC(C)N1CCc2cc(Nc3cc4cc(c(F)c(N)c4cn3)-c3cncc(N)c3C)nn2CC1=O | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00172
BindingDB Entry DOI: 10.7270/Q29G5RT0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519706
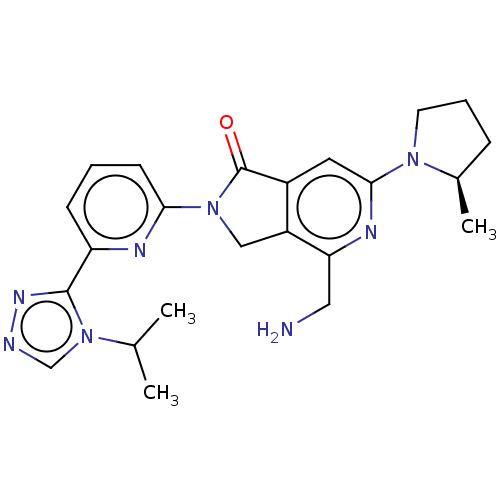
(US11142525, Example 107)Show SMILES CC(C)n1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CN)N2CCC[C@H]2C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00172
BindingDB Entry DOI: 10.7270/Q29G5RT0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50598490
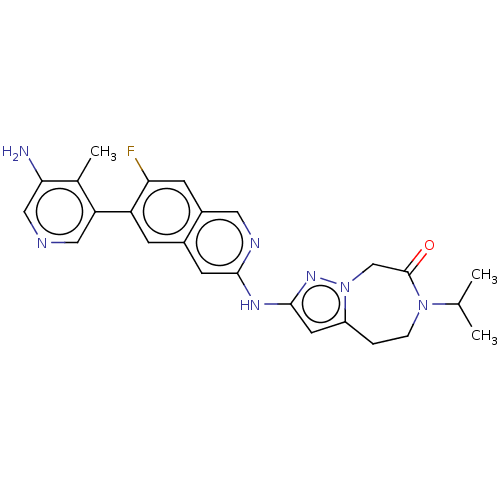
(CHEMBL5202032)Show SMILES CC(C)N1CCc2cc(Nc3cc4cc(c(F)cc4cn3)-c3cncc(N)c3C)nn2CC1=O | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00172
BindingDB Entry DOI: 10.7270/Q29G5RT0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM590938

(N-(8-amino-7-cyano-6-(4- methylpyridin-3-yl)isoqui...)Show SMILES Cc1ccncc1-c1cc2cc(NC(=O)C3CC3)ncc2c(N)c1C#N | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00172
BindingDB Entry DOI: 10.7270/Q29G5RT0 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University College of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma1 in human MDA-MB-468 cell membranes |
Bioorg Med Chem Lett 27: 2216-2220 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.030
BindingDB Entry DOI: 10.7270/Q2P84F1X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50598504
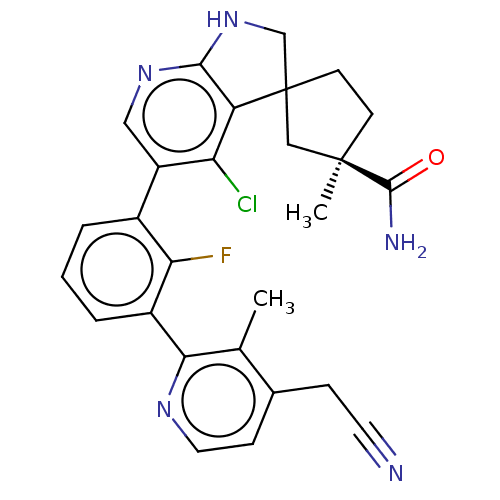
(CHEMBL5183178)Show SMILES Cc1c(CC#N)ccnc1-c1cccc(c1F)-c1cnc2NCC3(CC[C@](C)(C3)C(N)=O)c2c1Cl |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00172
BindingDB Entry DOI: 10.7270/Q29G5RT0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM590935
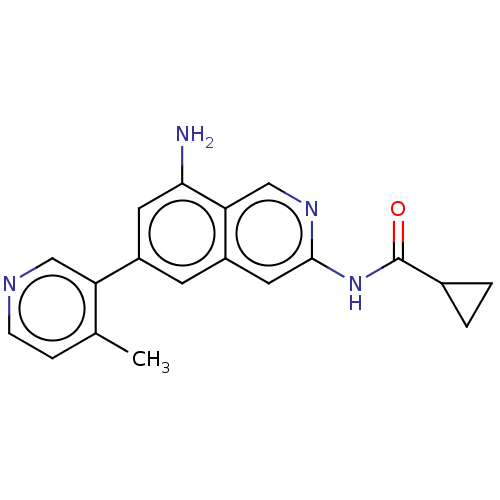
(N-(8-amino-6-(4-methylpyridin-3- yl)isoquinolin-3-...) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.391 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00172
BindingDB Entry DOI: 10.7270/Q29G5RT0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM503741
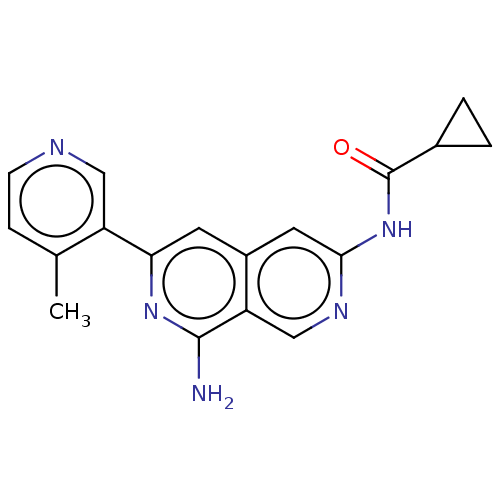
(N-(8-amino-6-(4-methylpyridin-3-yl)-2,7- naphthyri...) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00172
BindingDB Entry DOI: 10.7270/Q29G5RT0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50598501
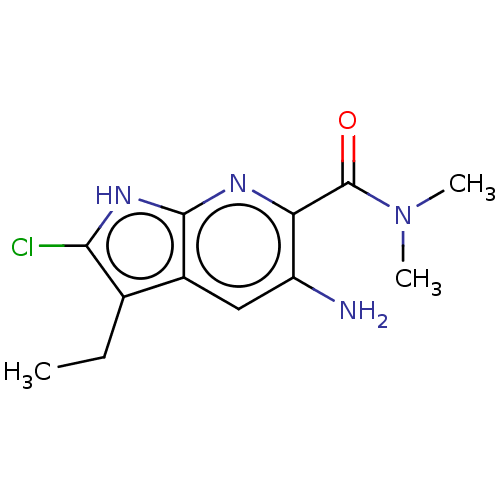
(CHEMBL5177877) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00172
BindingDB Entry DOI: 10.7270/Q29G5RT0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50598505
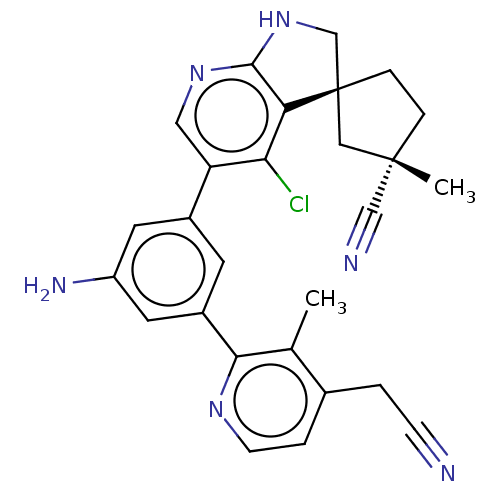
(CHEMBL5187417)Show SMILES Cc1c(CC#N)ccnc1-c1cc(N)cc(c1)-c1cnc2NC[C@@]3(CC[C@@](C)(C3)C#N)c2c1Cl |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00172
BindingDB Entry DOI: 10.7270/Q29G5RT0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519648
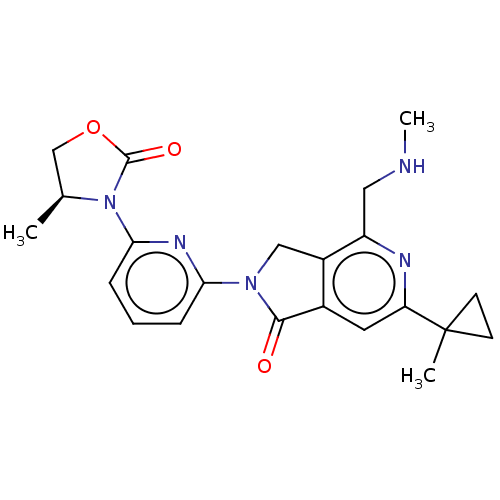
(US11142525, Example 49)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)N1[C@@H](C)COC1=O)C1(C)CC1 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00172
BindingDB Entry DOI: 10.7270/Q29G5RT0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50598500

(CHEMBL5196939) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00172
BindingDB Entry DOI: 10.7270/Q29G5RT0 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50006990
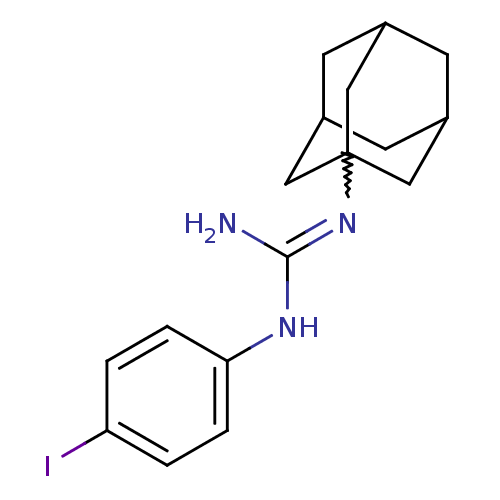
(CHEMBL299499 | N-Adamantan-1-yl-N'-(4-iodo-phenyl)...)Show SMILES NC(Nc1ccc(I)cc1)=NC12CC3CC(CC(C3)C1)C2 |w:10.11,TLB:18:17:20:13.12.14,18:13:20:17.19.16,THB:16:17:12:15.20.14,16:15:12:17.19.18| Show InChI InChI=1S/C17H22IN3/c18-14-1-3-15(4-2-14)20-16(19)21-17-8-11-5-12(9-17)7-13(6-11)10-17/h1-4,11-13H,5-10H2,(H3,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University College of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma1 in human MDA-MB-468 cell membranes |
Bioorg Med Chem Lett 27: 2216-2220 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.030
BindingDB Entry DOI: 10.7270/Q2P84F1X |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50240232
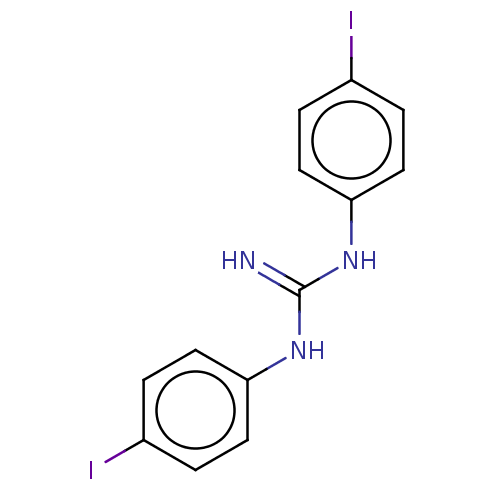
(CHEMBL4080735)Show InChI InChI=1S/C13H11I2N3/c14-9-1-5-11(6-2-9)17-13(16)18-12-7-3-10(15)4-8-12/h1-8H,(H3,16,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University College of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IPAG from sigma1 in human MDA-MB-468 cell membranes |
Bioorg Med Chem Lett 27: 2216-2220 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.030
BindingDB Entry DOI: 10.7270/Q2P84F1X |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496902

(CVD-0018409 | PF-07321332 | US11351149, Example 13...)Show SMILES CC(C)(C)[C@H](NC(=O)C(F)(F)F)C(=O)N1C[C@H]2[C@@H]([C@H]1C(=O)N[C@@H](C[C@@H]1CCNC1=O)C#N)C2(C)C Show InChI InChI=1S/C23H32F3N5O4/c1-21(2,3)16(30-20(35)23(24,25)26)19(34)31-10-13-14(22(13,4)5)15(31)18(33)29-12(9-27)8-11-6-7-28-17(11)32/h11-16H,6-8,10H2,1-5H3,(H,28,32)(H,29,33)(H,30,35)/t11-,12-,13-,14-,15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00954
BindingDB Entry DOI: 10.7270/Q2R49VTB |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50240230
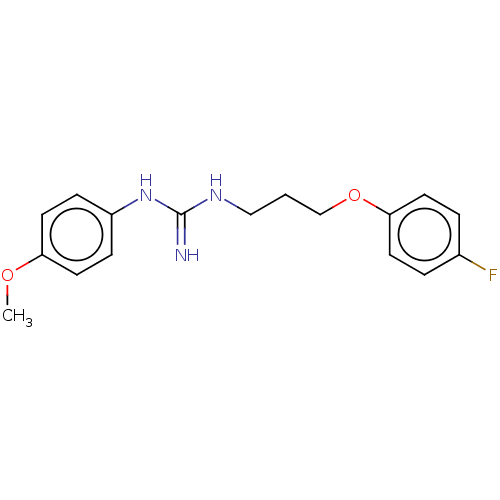
(CHEMBL4092687)Show InChI InChI=1S/C17H20FN3O2/c1-22-15-9-5-14(6-10-15)21-17(19)20-11-2-12-23-16-7-3-13(18)4-8-16/h3-10H,2,11-12H2,1H3,(H3,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University College of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma1 in human MDA-MB-468 cell membranes |
Bioorg Med Chem Lett 27: 2216-2220 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.030
BindingDB Entry DOI: 10.7270/Q2P84F1X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50598502
(CHEMBL5201995) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00172
BindingDB Entry DOI: 10.7270/Q29G5RT0 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM476960

(peptidomimetic coronavirus 3CLpro inhibitors 2i)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C33H41N5O6S/c1-19(2)16-25(36-31(42)27(20(3)4)38-33(43)44-18-21-10-6-5-7-11-21)30(41)35-24(17-22-14-15-34-29(22)40)28(39)32-37-23-12-8-9-13-26(23)45-32/h5-13,19-20,22,24-25,27H,14-18H2,1-4H3,(H,34,40)(H,35,41)(H,36,42)(H,38,43)/t22-,24-,25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00954
BindingDB Entry DOI: 10.7270/Q2R49VTB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM590941
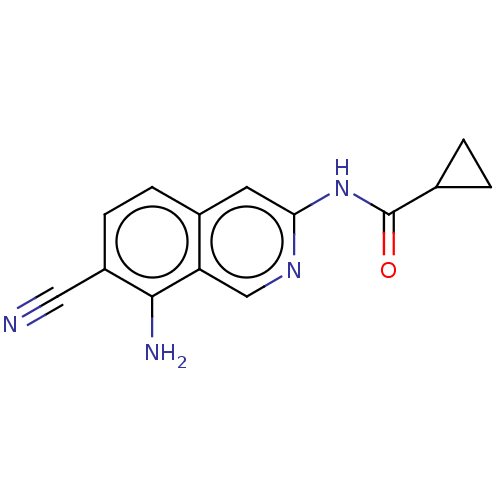
(N-(8-amino-7-cyanoisoquinolin-3- yl)cyclopropaneca...) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00172
BindingDB Entry DOI: 10.7270/Q29G5RT0 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50101563
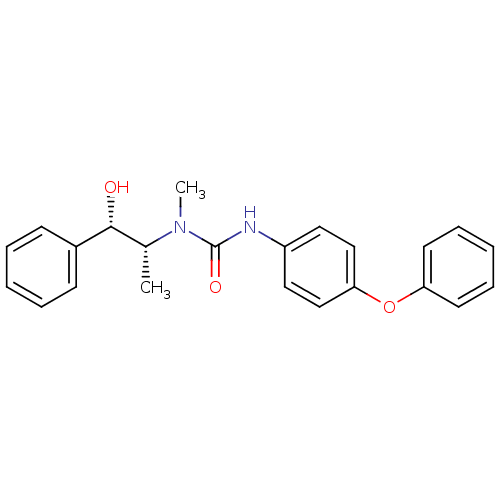
(1-(2-Hydroxy-1-methyl-2-phenyl-ethyl)-1-methyl-3-(...)Show SMILES C[C@H]([C@@H](O)c1ccccc1)N(C)C(=O)Nc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C23H24N2O3/c1-17(22(26)18-9-5-3-6-10-18)25(2)23(27)24-19-13-15-21(16-14-19)28-20-11-7-4-8-12-20/h3-17,22,26H,1-2H3,(H,24,27)/t17-,22-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Tested in a cellular assay measuring forskolin-induced cyclic AMP accumulation in 293 cells transfected with the human NPY5 receptor |
J Med Chem 44: 2344-56 (2001)
BindingDB Entry DOI: 10.7270/Q2DV1J56 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cells incubated for 1 hr by liquid scintillation spectrometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50240229

(CHEMBL4084182)Show InChI InChI=1S/C16H17ClFN3O/c17-12-2-6-14(7-3-12)21-16(19)20-10-1-11-22-15-8-4-13(18)5-9-15/h2-9H,1,10-11H2,(H3,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University College of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma1 in human MDA-MB-468 cell membranes |
Bioorg Med Chem Lett 27: 2216-2220 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.030
BindingDB Entry DOI: 10.7270/Q2P84F1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cells incubated for 1 hr by liquid scintillation spectrometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50240234
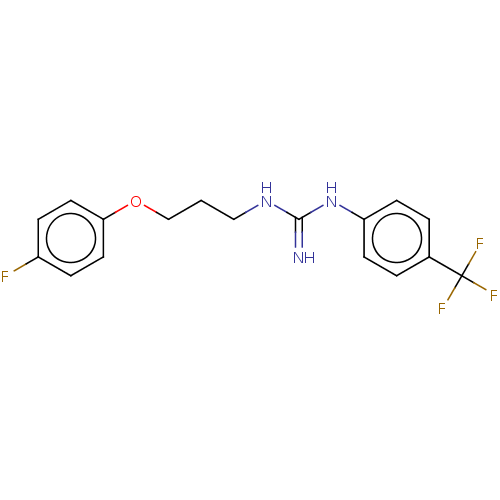
(CHEMBL4065001)Show InChI InChI=1S/C17H17F4N3O/c18-13-4-8-15(9-5-13)25-11-1-10-23-16(22)24-14-6-2-12(3-7-14)17(19,20)21/h2-9H,1,10-11H2,(H3,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University College of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma1 in human MDA-MB-468 cell membranes |
Bioorg Med Chem Lett 27: 2216-2220 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.030
BindingDB Entry DOI: 10.7270/Q2P84F1X |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50240231
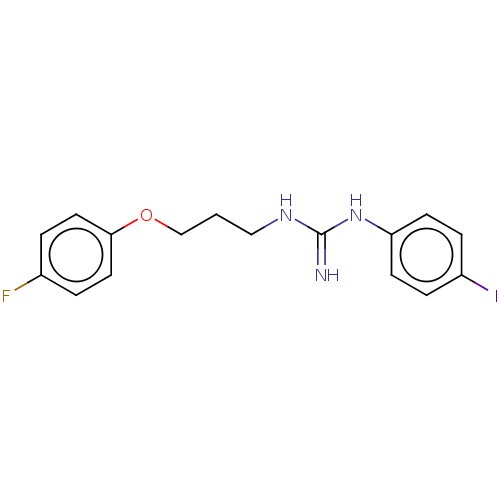
(CHEMBL4098778)Show InChI InChI=1S/C16H17FIN3O/c17-12-2-8-15(9-3-12)22-11-1-10-20-16(19)21-14-6-4-13(18)5-7-14/h2-9H,1,10-11H2,(H3,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University College of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma1 in human MDA-MB-468 cell membranes |
Bioorg Med Chem Lett 27: 2216-2220 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.030
BindingDB Entry DOI: 10.7270/Q2P84F1X |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50101548
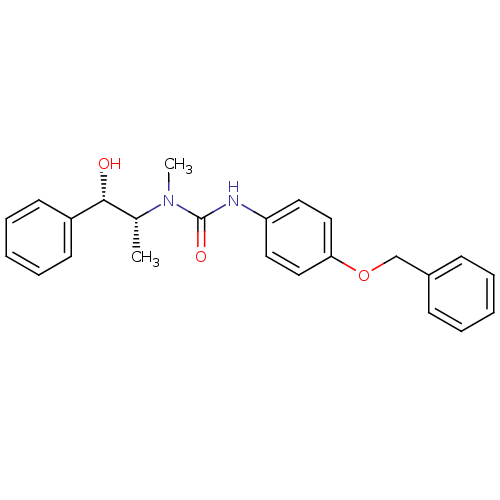
(3-(4-Benzyloxy-phenyl)-1-(2-hydroxy-1-methyl-2-phe...)Show SMILES C[C@H]([C@@H](O)c1ccccc1)N(C)C(=O)Nc1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C24H26N2O3/c1-18(23(27)20-11-7-4-8-12-20)26(2)24(28)25-21-13-15-22(16-14-21)29-17-19-9-5-3-6-10-19/h3-16,18,23,27H,17H2,1-2H3,(H,25,28)/t18-,23-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Tested in a cellular assay measuring forskolin-induced cyclic AMP accumulation in 293 cells transfected with the human NPY5 receptor |
J Med Chem 44: 2344-56 (2001)
BindingDB Entry DOI: 10.7270/Q2DV1J56 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A1
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of human recombinant CYP1A1 using 7-Ethoxyresorufin as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50602412

(CHEMBL5184473)Show SMILES O=C(N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC1CCNC1=O)C(=O)C(=O)NCc1ccccc1)\C=C\c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00954
BindingDB Entry DOI: 10.7270/Q2R49VTB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A5
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of human recombinant CYP3A5 expressed in baculovirus-infected insect cells using diltiazem as substrate incubated for 15 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50174315
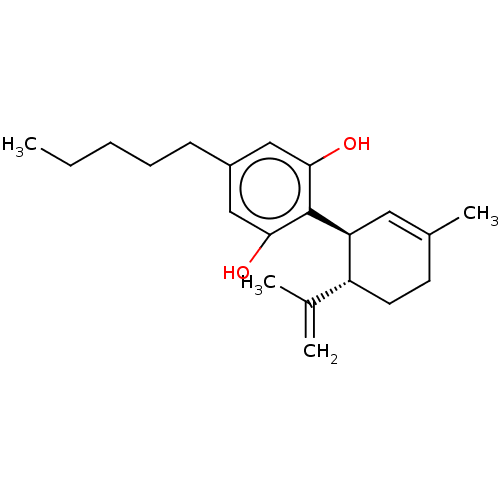
(CHEMBL3810140)Show SMILES CCCCCc1cc(O)c([C@H]2C=C(C)CC[C@@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-HU-243 from CB2 receptor (unknown origin) expressed in African green monkey Cos7 cell membranes incubated for 90 mins by radioli... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50004708
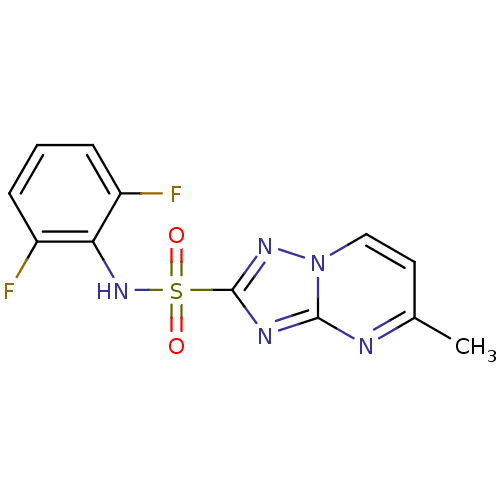
(FLUMETSULAM)Show InChI InChI=1S/C12H9F2N5O2S/c1-7-5-6-19-11(15-7)16-12(17-19)22(20,21)18-10-8(13)3-2-4-9(10)14/h2-6,18H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50101536
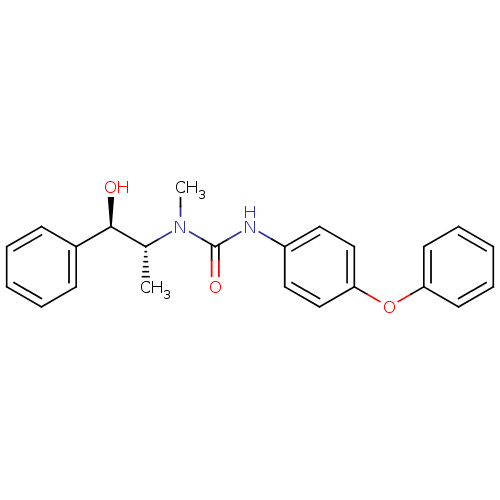
(1-(2-Hydroxy-1-methyl-2-phenyl-ethyl)-1-methyl-3-(...)Show SMILES C[C@H]([C@H](O)c1ccccc1)N(C)C(=O)Nc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C23H24N2O3/c1-17(22(26)18-9-5-3-6-10-18)25(2)23(27)24-19-13-15-21(16-14-19)28-20-11-7-4-8-12-20/h3-17,22,26H,1-2H3,(H,24,27)/t17-,22+/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 477 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Tested in a cellular assay measuring forskolin-induced cyclic AMP accumulation in 293 cells transfected with the human NPY5 receptor |
J Med Chem 44: 2344-56 (2001)
BindingDB Entry DOI: 10.7270/Q2DV1J56 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM414624

(US10435405, Example 41 | US10934288, Example 41)Show SMILES COc1cccc(F)c1-c1cc2c(NCc3ccc(cc3)N3CCN(C)CC3)n[nH]c2cn1 Show InChI InChI=1S/C25H27FN6O/c1-31-10-12-32(13-11-31)18-8-6-17(7-9-18)15-28-25-19-14-21(27-16-22(19)29-30-25)24-20(26)4-3-5-23(24)33-2/h3-9,14,16H,10-13,15H2,1-2H3,(H2,28,29,30) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00172
BindingDB Entry DOI: 10.7270/Q29G5RT0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50598499

(CHEMBL5199200) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00172
BindingDB Entry DOI: 10.7270/Q29G5RT0 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50101543

(1-(2-Hydroxy-1-methyl-2-phenyl-ethyl)-1-methyl-3-(...)Show SMILES C[C@@H]([C@H](O)c1ccccc1)N(C)C(=O)Nc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C23H24N2O3/c1-17(22(26)18-9-5-3-6-10-18)25(2)23(27)24-19-13-15-21(16-14-19)28-20-11-7-4-8-12-20/h3-17,22,26H,1-2H3,(H,24,27)/t17-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 515 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Tested in a cellular assay measuring forskolin-induced cyclic AMP accumulation in 293 cells transfected with the human NPY5 receptor |
J Med Chem 44: 2344-56 (2001)
BindingDB Entry DOI: 10.7270/Q2DV1J56 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed type inhibition of human recombinant CYP2B6 expressed in baculovirus-infected insect cells using coumarin as substrate preincubated for 5 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed type inhibition of human recombinant CYP2C19 using (S)-mephenytoin as substrate preincubated for 5 mins followed by NADPH-generating system add... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50174315

(CHEMBL3810140)Show SMILES CCCCCc1cc(O)c([C@H]2C=C(C)CC[C@@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 842 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-HU-243 from CB1 receptor in Sabra rat brain synaptosomes incubated for 90 mins by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50240233
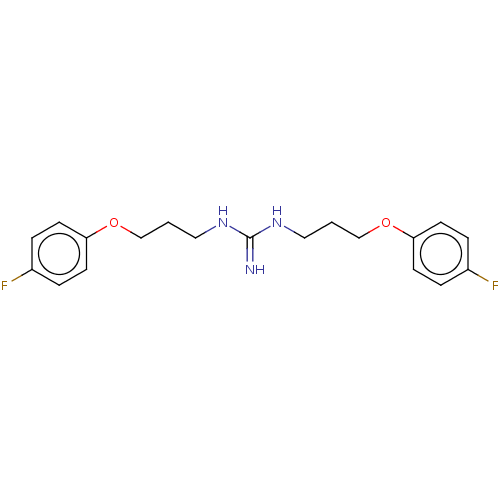
(CHEMBL4102152)Show InChI InChI=1S/C19H23F2N3O2/c20-15-3-7-17(8-4-15)25-13-1-11-23-19(22)24-12-2-14-26-18-9-5-16(21)6-10-18/h3-10H,1-2,11-14H2,(H3,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University College of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IPAG from sigma1 in human MDA-MB-468 cell membranes |
Bioorg Med Chem Lett 27: 2216-2220 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.030
BindingDB Entry DOI: 10.7270/Q2P84F1X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of human recombinant CYP3A4 expressed in baculovirus-infected insect cells using diltiazem as substrate incubated for 15 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 5-HT2C (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data