

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50157911 (13a-hydroxy-10-(4-methoxyphenyl)-5,5,5a,7a,13b-pen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Binding affinity for Acetylcholinesterase | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Butyrylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50157914 (4a,12a-dihydroxy-9-(4-methoxyphenyl)-4,4,6a,12b-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Acetylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Acetylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50157915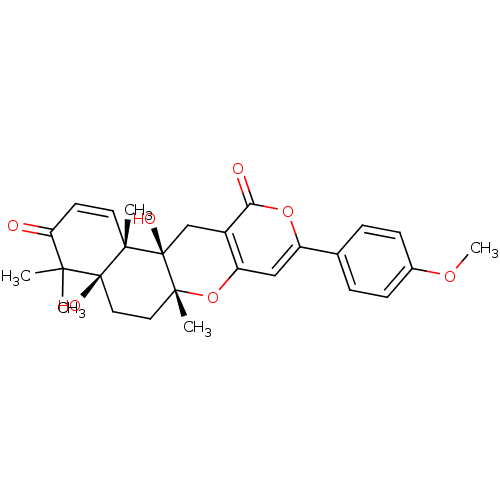 (4a,12a-dihydroxy-9-(4-methoxyphenyl)-4,4,6a,12b-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Acetylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50157912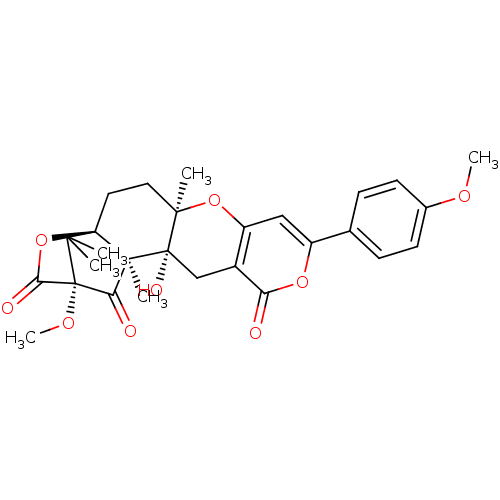 (13-hydroxy-16-methoxy-8-(4-methoxyphenyl)-4,14,19,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Acetylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50157913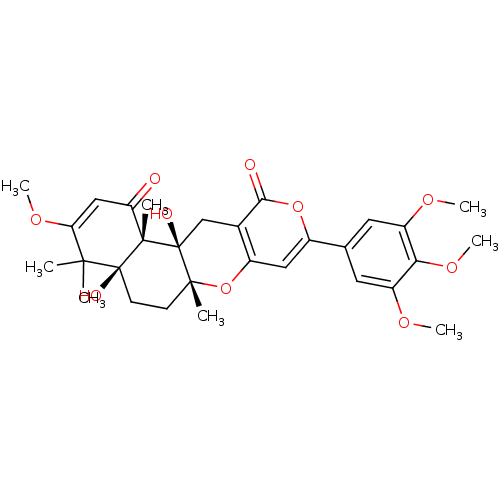 (4a,12a-dihydroxy-3-methoxy-4,4,6a,12b-tetramethyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Acetylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50157911 (13a-hydroxy-10-(4-methoxyphenyl)-5,5,5a,7a,13b-pen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Acetylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50157915 (4a,12a-dihydroxy-9-(4-methoxyphenyl)-4,4,6a,12b-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Butyrylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50157914 (4a,12a-dihydroxy-9-(4-methoxyphenyl)-4,4,6a,12b-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Butyrylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50157913 (4a,12a-dihydroxy-3-methoxy-4,4,6a,12b-tetramethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Butyrylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50157912 (13-hydroxy-16-methoxy-8-(4-methoxyphenyl)-4,14,19,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Butyrylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50157911 (13a-hydroxy-10-(4-methoxyphenyl)-5,5,5a,7a,13b-pen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Butyrylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase, mitochondrial (Homo sapiens) | BDBM50553183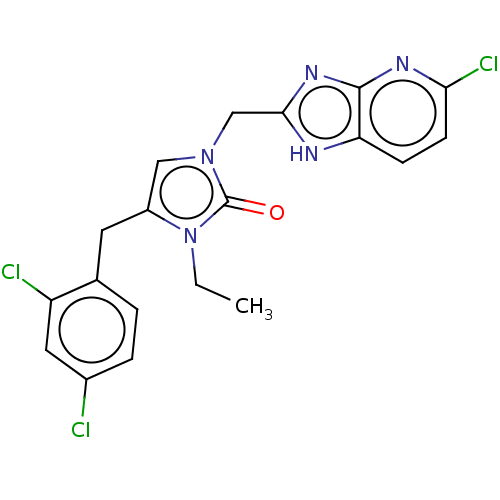 (CHEMBL4791458) | MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mitochondrial MetRS-mediated mitochondrial protein synthesis in human HepG2 cells measured after 6 days by ELISA | Citation and Details Article DOI: 10.1039/d0md00057d BindingDB Entry DOI: 10.7270/Q2SQ942Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase, mitochondrial (Homo sapiens) | BDBM50553184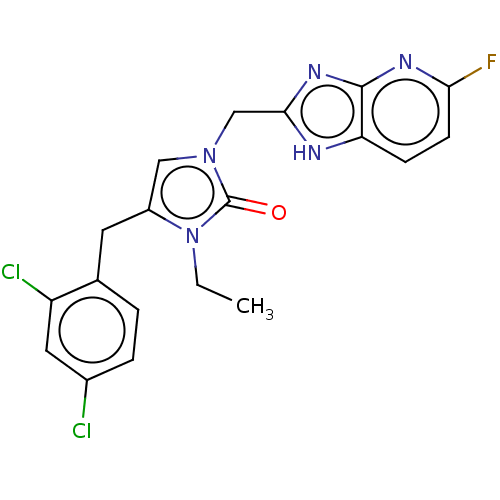 (CHEMBL4781504) | MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mitochondrial MetRS-mediated mitochondrial protein synthesis in human HepG2 cells measured after 6 days by ELISA | Citation and Details Article DOI: 10.1039/d0md00057d BindingDB Entry DOI: 10.7270/Q2SQ942Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase, mitochondrial (Homo sapiens) | BDBM50553185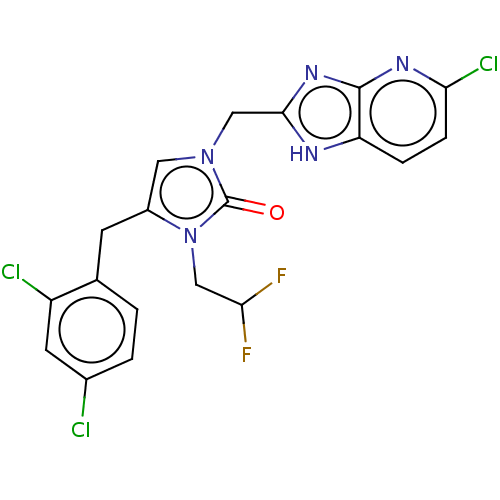 (CHEMBL4742903) | MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 430 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mitochondrial MetRS-mediated mitochondrial protein synthesis in human HepG2 cells measured after 6 days by ELISA | Citation and Details Article DOI: 10.1039/d0md00057d BindingDB Entry DOI: 10.7270/Q2SQ942Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase, mitochondrial (Homo sapiens) | BDBM50553186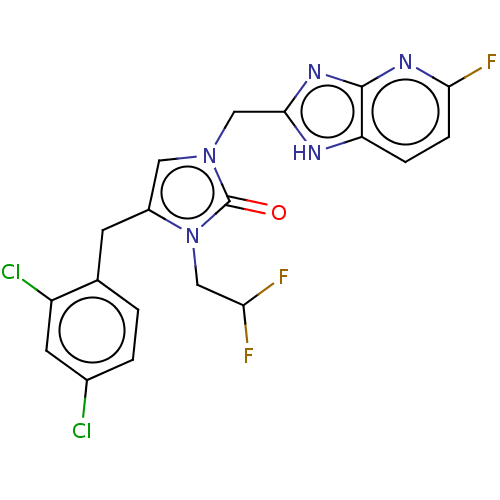 (CHEMBL4777699) | MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 175 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mitochondrial MetRS-mediated mitochondrial protein synthesis in human HepG2 cells measured after 6 days by ELISA | Citation and Details Article DOI: 10.1039/d0md00057d BindingDB Entry DOI: 10.7270/Q2SQ942Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase, mitochondrial (Homo sapiens) | BDBM50553187 (CHEMBL4761163) | MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 195 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mitochondrial MetRS-mediated mitochondrial protein synthesis in human HepG2 cells measured after 6 days by ELISA | Citation and Details Article DOI: 10.1039/d0md00057d BindingDB Entry DOI: 10.7270/Q2SQ942Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase, mitochondrial (Homo sapiens) | BDBM50553193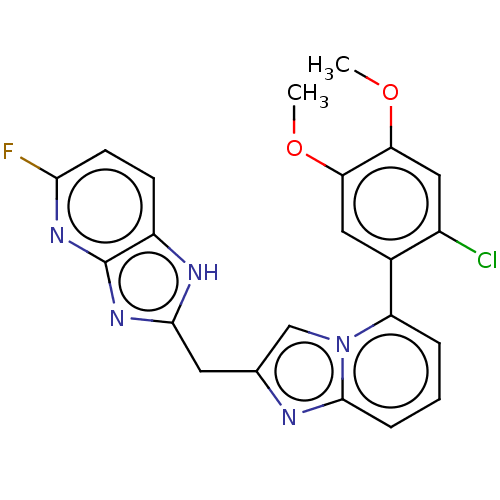 (CHEMBL4795266) | MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 316 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mitochondrial MetRS-mediated mitochondrial protein synthesis in human HepG2 cells measured after 6 days by ELISA | Citation and Details Article DOI: 10.1039/d0md00057d BindingDB Entry DOI: 10.7270/Q2SQ942Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase, mitochondrial (Homo sapiens) | BDBM50553189 (CHEMBL4762630) | MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 75 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mitochondrial MetRS-mediated mitochondrial protein synthesis in human HepG2 cells measured after 6 days by ELISA | Citation and Details Article DOI: 10.1039/d0md00057d BindingDB Entry DOI: 10.7270/Q2SQ942Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase, mitochondrial (Homo sapiens) | BDBM50553190 (CHEMBL4740481) | MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 507 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mitochondrial MetRS-mediated mitochondrial protein synthesis in human HepG2 cells measured after 6 days by ELISA | Citation and Details Article DOI: 10.1039/d0md00057d BindingDB Entry DOI: 10.7270/Q2SQ942Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase, mitochondrial (Homo sapiens) | BDBM50553191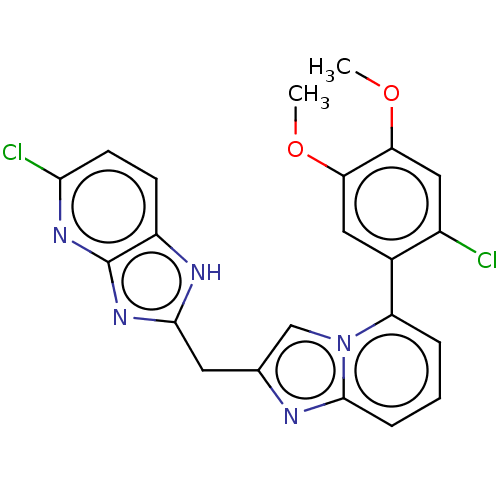 (CHEMBL4745092) | MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 478 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mitochondrial MetRS-mediated mitochondrial protein synthesis in human HepG2 cells measured after 6 days by ELISA | Citation and Details Article DOI: 10.1039/d0md00057d BindingDB Entry DOI: 10.7270/Q2SQ942Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase, mitochondrial (Homo sapiens) | BDBM50553192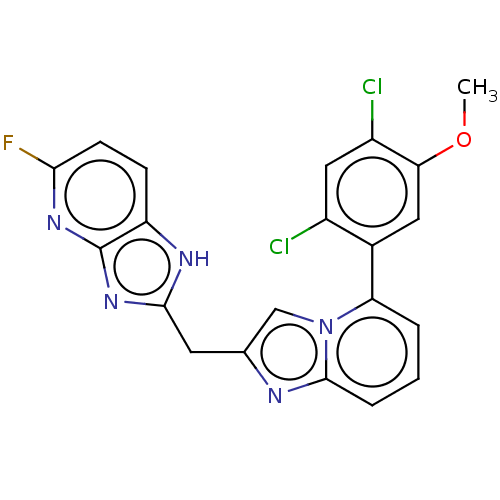 (CHEMBL4750041) | MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mitochondrial MetRS-mediated mitochondrial protein synthesis in human HepG2 cells measured after 6 days by ELISA | Citation and Details Article DOI: 10.1039/d0md00057d BindingDB Entry DOI: 10.7270/Q2SQ942Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase, mitochondrial (Homo sapiens) | BDBM23447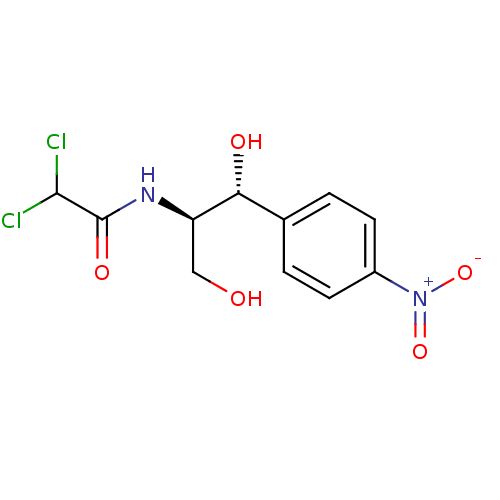 (2,2-dichloro-N-[(1R,2R)-1,3-dihydroxy-1-(4-nitroph...) | MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mitochondrial MetRS-mediated mitochondrial protein synthesis in human HepG2 cells measured after 6 days by ELISA | Citation and Details Article DOI: 10.1039/d0md00057d BindingDB Entry DOI: 10.7270/Q2SQ942Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase, mitochondrial (Homo sapiens) | BDBM50553188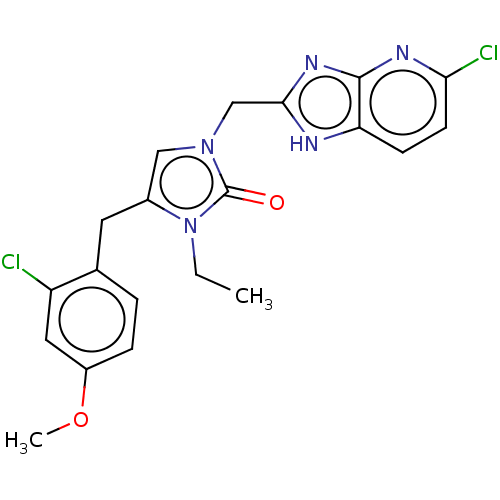 (CHEMBL4757779) | MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mitochondrial MetRS-mediated mitochondrial protein synthesis in human HepG2 cells measured after 6 days by ELISA | Citation and Details Article DOI: 10.1039/d0md00057d BindingDB Entry DOI: 10.7270/Q2SQ942Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||