 Found 42 hits with Last Name = 'chong' and Initial = 'wk'
Found 42 hits with Last Name = 'chong' and Initial = 'wk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50303032
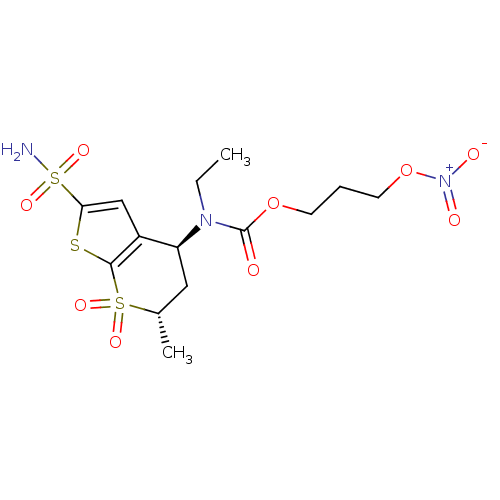
(CHEMBL584939 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)OCCCO[N+]([O-])=O |r| Show InChI InChI=1S/C14H21N3O9S3/c1-3-16(14(18)25-5-4-6-26-17(19)20)11-7-9(2)28(21,22)13-10(11)8-12(27-13)29(15,23)24/h8-9,11H,3-7H2,1-2H3,(H2,15,23,24)/t9-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50303030
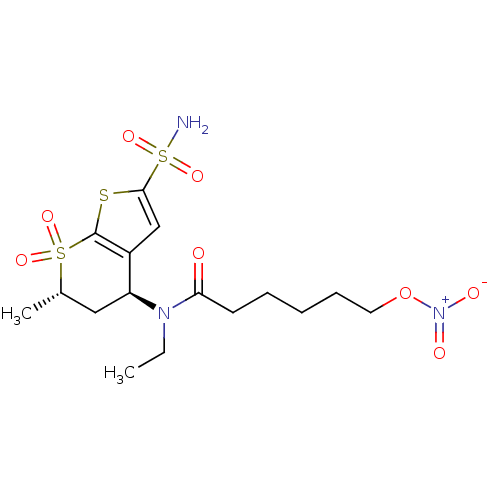
(6-Nitrooxy-hexanoic acid ethyl-((4S,6S)-6-methyl-7...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)CCCCCO[N+]([O-])=O |r| Show InChI InChI=1S/C16H25N3O8S3/c1-3-18(14(20)7-5-4-6-8-27-19(21)22)13-9-11(2)29(23,24)16-12(13)10-15(28-16)30(17,25)26/h10-11,13H,3-9H2,1-2H3,(H2,17,25,26)/t11-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 4 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50303031
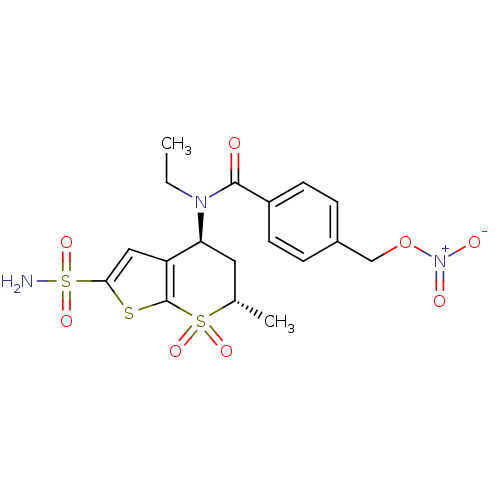
(CHEMBL570894 | N-Ethyl-N-((4S,6S)-6-methyl-7,7-dio...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)c1ccc(CO[N+]([O-])=O)cc1 |r| Show InChI InChI=1S/C18H21N3O8S3/c1-3-20(17(22)13-6-4-12(5-7-13)10-29-21(23)24)15-8-11(2)31(25,26)18-14(15)9-16(30-18)32(19,27)28/h4-7,9,11,15H,3,8,10H2,1-2H3,(H2,19,27,28)/t11-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 4 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50303034
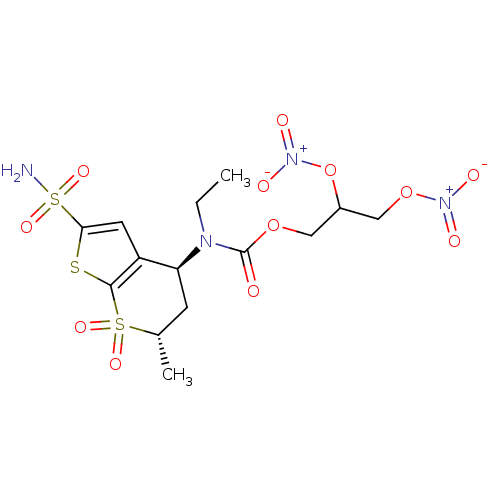
(CHEMBL571337 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)OCC(CO[N+]([O-])=O)O[N+]([O-])=O |r| Show InChI InChI=1S/C14H20N4O12S3/c1-3-16(14(19)28-6-9(30-18(22)23)7-29-17(20)21)11-4-8(2)32(24,25)13-10(11)5-12(31-13)33(15,26)27/h5,8-9,11H,3-4,6-7H2,1-2H3,(H2,15,26,27)/t8-,9?,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50303031

(CHEMBL570894 | N-Ethyl-N-((4S,6S)-6-methyl-7,7-dio...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)c1ccc(CO[N+]([O-])=O)cc1 |r| Show InChI InChI=1S/C18H21N3O8S3/c1-3-20(17(22)13-6-4-12(5-7-13)10-29-21(23)24)15-8-11(2)31(25,26)18-14(15)9-16(30-18)32(19,27)28/h4-7,9,11,15H,3,8,10H2,1-2H3,(H2,19,27,28)/t11-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50303033
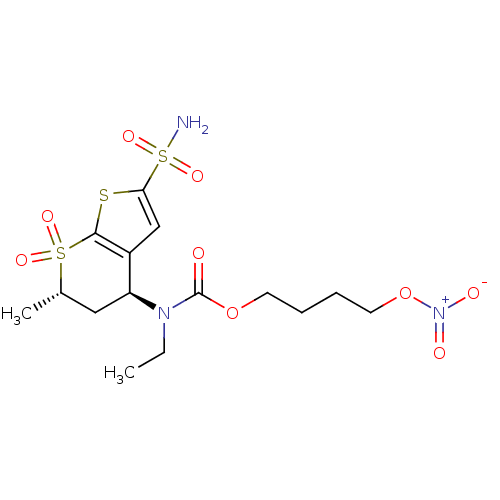
(CHEMBL585756 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)OCCCCO[N+]([O-])=O |r| Show InChI InChI=1S/C15H23N3O9S3/c1-3-17(15(19)26-6-4-5-7-27-18(20)21)12-8-10(2)29(22,23)14-11(12)9-13(28-14)30(16,24)25/h9-10,12H,3-8H2,1-2H3,(H2,16,24,25)/t10-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50303032

(CHEMBL584939 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)OCCCO[N+]([O-])=O |r| Show InChI InChI=1S/C14H21N3O9S3/c1-3-16(14(18)25-5-4-6-26-17(19)20)11-7-9(2)28(21,22)13-10(11)8-12(27-13)29(15,23)24/h8-9,11H,3-7H2,1-2H3,(H2,15,23,24)/t9-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 4 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50303033

(CHEMBL585756 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)OCCCCO[N+]([O-])=O |r| Show InChI InChI=1S/C15H23N3O9S3/c1-3-17(15(19)26-6-4-5-7-27-18(20)21)12-8-10(2)29(22,23)14-11(12)9-13(28-14)30(16,24)25/h9-10,12H,3-8H2,1-2H3,(H2,16,24,25)/t10-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 339 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 4 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50303032

(CHEMBL584939 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)OCCCO[N+]([O-])=O |r| Show InChI InChI=1S/C14H21N3O9S3/c1-3-16(14(18)25-5-4-6-26-17(19)20)11-7-9(2)28(21,22)13-10(11)8-12(27-13)29(15,23)24/h8-9,11H,3-7H2,1-2H3,(H2,15,23,24)/t9-,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50303031

(CHEMBL570894 | N-Ethyl-N-((4S,6S)-6-methyl-7,7-dio...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)c1ccc(CO[N+]([O-])=O)cc1 |r| Show InChI InChI=1S/C18H21N3O8S3/c1-3-20(17(22)13-6-4-12(5-7-13)10-29-21(23)24)15-8-11(2)31(25,26)18-14(15)9-16(30-18)32(19,27)28/h4-7,9,11,15H,3,8,10H2,1-2H3,(H2,19,27,28)/t11-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50303033

(CHEMBL585756 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)OCCCCO[N+]([O-])=O |r| Show InChI InChI=1S/C15H23N3O9S3/c1-3-17(15(19)26-6-4-5-7-27-18(20)21)12-8-10(2)29(22,23)14-11(12)9-13(28-14)30(16,24)25/h9-10,12H,3-8H2,1-2H3,(H2,16,24,25)/t10-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 705 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50303034

(CHEMBL571337 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)OCC(CO[N+]([O-])=O)O[N+]([O-])=O |r| Show InChI InChI=1S/C14H20N4O12S3/c1-3-16(14(19)28-6-9(30-18(22)23)7-29-17(20)21)11-4-8(2)32(24,25)13-10(11)5-12(31-13)33(15,26)27/h5,8-9,11H,3-4,6-7H2,1-2H3,(H2,15,26,27)/t8-,9?,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50303030

(6-Nitrooxy-hexanoic acid ethyl-((4S,6S)-6-methyl-7...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)CCCCCO[N+]([O-])=O |r| Show InChI InChI=1S/C16H25N3O8S3/c1-3-18(14(20)7-5-4-6-8-27-19(21)22)13-9-11(2)29(23,24)16-12(13)10-15(28-16)30(17,25)26/h10-11,13H,3-9H2,1-2H3,(H2,17,25,26)/t11-,13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50303034

(CHEMBL571337 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)OCC(CO[N+]([O-])=O)O[N+]([O-])=O |r| Show InChI InChI=1S/C14H20N4O12S3/c1-3-16(14(19)28-6-9(30-18(22)23)7-29-17(20)21)11-4-8(2)32(24,25)13-10(11)5-12(31-13)33(15,26)27/h5,8-9,11H,3-4,6-7H2,1-2H3,(H2,15,26,27)/t8-,9?,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 4 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50303030

(6-Nitrooxy-hexanoic acid ethyl-((4S,6S)-6-methyl-7...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)CCCCCO[N+]([O-])=O |r| Show InChI InChI=1S/C16H25N3O8S3/c1-3-18(14(20)7-5-4-6-8-27-19(21)22)13-9-11(2)29(23,24)16-12(13)10-15(28-16)30(17,25)26/h10-11,13H,3-9H2,1-2H3,(H2,17,25,26)/t11-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 4 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50228277
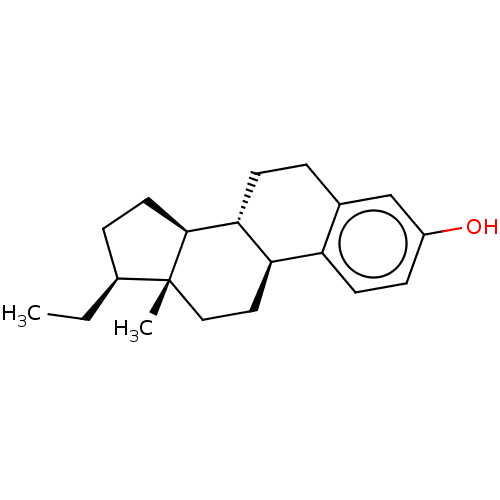
(CHEMBL1627386)Show SMILES [H][C@]1(CC)CC[C@@]2([H])[C@]3([H])CCc4cc(O)ccc4[C@@]3([H])CC[C@]12C Show InChI InChI=1S/C20H28O/c1-3-14-5-9-19-18-7-4-13-12-15(21)6-8-16(13)17(18)10-11-20(14,19)2/h6,8,12,14,17-19,21H,3-5,7,9-11H2,1-2H3/t14-,17+,18+,19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 3.86E+4 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in immature rabbit |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(RAT-Rattus norvegicus) | BDBM50228277

(CHEMBL1627386)Show SMILES [H][C@]1(CC)CC[C@@]2([H])[C@]3([H])CCc4cc(O)ccc4[C@@]3([H])CC[C@]12C Show InChI InChI=1S/C20H28O/c1-3-14-5-9-19-18-7-4-13-12-15(21)6-8-16(13)17(18)10-11-20(14,19)2/h6,8,12,14,17-19,21H,3-5,7,9-11H2,1-2H3/t14-,17+,18+,19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 7.03E+4 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]-estradiol from estrogen receptor in immature rat |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50187243

(17-ethinyl-3,17-estradiol | 17-ethinyl-3,17-oestra...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)C#C |r| Show InChI InChI=1S/C20H24O2/c1-3-20(22)11-9-18-17-6-4-13-12-14(21)5-7-15(13)16(17)8-10-19(18,20)2/h1,5,7,12,16-18,21-22H,4,6,8-11H2,2H3/t16-,17-,18+,19+,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.37E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in squirrel monkey |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(RAT-Rattus norvegicus) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]-estradiol from estrogen receptor in mature rat |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(RAT-Rattus norvegicus) | BDBM50187243

(17-ethinyl-3,17-estradiol | 17-ethinyl-3,17-oestra...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)C#C |r| Show InChI InChI=1S/C20H24O2/c1-3-20(22)11-9-18-17-6-4-13-12-14(21)5-7-15(13)16(17)8-10-19(18,20)2/h1,5,7,12,16-18,21-22H,4,6,8-11H2,2H3/t16-,17-,18+,19+,20+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >3.37E+4 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]-estradiol from estrogen receptor in immature rat |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50228277

(CHEMBL1627386)Show SMILES [H][C@]1(CC)CC[C@@]2([H])[C@]3([H])CCc4cc(O)ccc4[C@@]3([H])CC[C@]12C Show InChI InChI=1S/C20H28O/c1-3-14-5-9-19-18-7-4-13-12-15(21)6-8-16(13)17(18)10-11-20(14,19)2/h6,8,12,14,17-19,21H,3-5,7,9-11H2,1-2H3/t14-,17+,18+,19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 2.10E+5 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in squirrel monkey |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(RAT-Rattus norvegicus) | BDBM50228276
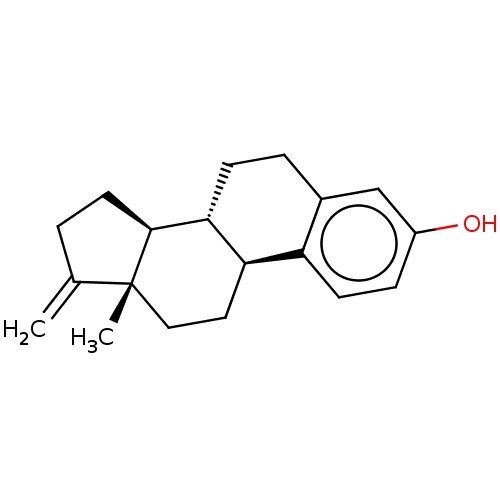
(CHEMBL1627365)Show SMILES [H][C@@]12CCC(=C)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C19H24O/c1-12-3-8-18-17-6-4-13-11-14(20)5-7-15(13)16(17)9-10-19(12,18)2/h5,7,11,16-18,20H,1,3-4,6,8-10H2,2H3/t16-,17-,18+,19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.98E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in mature rat |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(RAT-Rattus norvegicus) | BDBM50228273
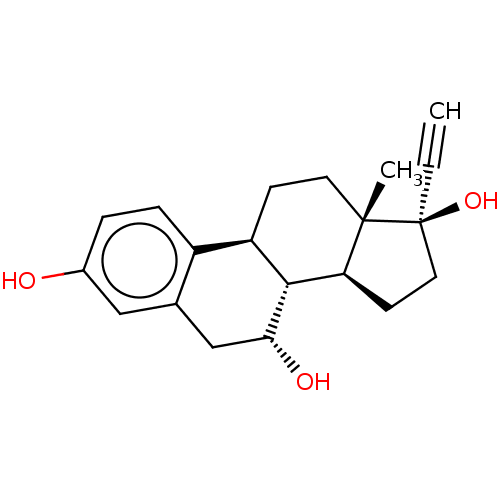
(CHEMBL1627375)Show SMILES [H][C@@]12CC[C@@](O)(C#C)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3C[C@@H](O)[C@@]21[H] Show InChI InChI=1S/C20H24O3/c1-3-20(23)9-7-16-18-15(6-8-19(16,20)2)14-5-4-13(21)10-12(14)11-17(18)22/h1,4-5,10,15-18,21-23H,6-9,11H2,2H3/t15-,16+,17-,18-,19+,20+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in mature rat |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50228273

(CHEMBL1627375)Show SMILES [H][C@@]12CC[C@@](O)(C#C)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3C[C@@H](O)[C@@]21[H] Show InChI InChI=1S/C20H24O3/c1-3-20(23)9-7-16-18-15(6-8-19(16,20)2)14-5-4-13(21)10-12(14)11-17(18)22/h1,4-5,10,15-18,21-23H,6-9,11H2,2H3/t15-,16+,17-,18-,19+,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.15E+5 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in immature rabbit |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(RAT-Rattus norvegicus) | BDBM50228275

(CHEMBL1627370)Show SMILES [H][C@@]12CC[C@@](O)(C#C)[C@@]1(C)C[C@H](O)[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C20H24O3/c1-3-20(23)9-8-16-15-6-4-12-10-13(21)5-7-14(12)18(15)17(22)11-19(16,20)2/h1,5,7,10,15-18,21-23H,4,6,8-9,11H2,2H3/t15-,16-,17-,18+,19-,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 5.12E+4 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]-estradiol from estrogen receptor in mature rat |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50228275

(CHEMBL1627370)Show SMILES [H][C@@]12CC[C@@](O)(C#C)[C@@]1(C)C[C@H](O)[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C20H24O3/c1-3-20(23)9-8-16-15-6-4-12-10-13(21)5-7-14(12)18(15)17(22)11-19(16,20)2/h1,5,7,10,15-18,21-23H,4,6,8-9,11H2,2H3/t15-,16-,17-,18+,19-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 4.80E+5 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in squirrel monkey |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(RAT-Rattus norvegicus) | BDBM50228274

(CHEMBL1627389)Show SMILES [H][C@]1(CC)CC[C@@]2([H])[C@]3([H])CCc4cc(O)ccc4[C@@]3([H])[C@@H](O)C[C@]12C Show InChI InChI=1S/C20H28O2/c1-3-13-5-9-17-16-7-4-12-10-14(21)6-8-15(12)19(16)18(22)11-20(13,17)2/h6,8,10,13,16-19,21-22H,3-5,7,9,11H2,1-2H3/t13-,16-,17-,18-,19+,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]-estradiol from estrogen receptor in immature rat |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(RAT-Rattus norvegicus) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 917 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in immature rat |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in squirrel monkey |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(RAT-Rattus norvegicus) | BDBM50228277

(CHEMBL1627386)Show SMILES [H][C@]1(CC)CC[C@@]2([H])[C@]3([H])CCc4cc(O)ccc4[C@@]3([H])CC[C@]12C Show InChI InChI=1S/C20H28O/c1-3-14-5-9-19-18-7-4-13-12-15(21)6-8-16(13)17(18)10-11-20(14,19)2/h6,8,12,14,17-19,21H,3-5,7,9-11H2,1-2H3/t14-,17+,18+,19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 2.81E+4 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]-estradiol from estrogen receptor in mature rat |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50228276

(CHEMBL1627365)Show SMILES [H][C@@]12CCC(=C)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C19H24O/c1-12-3-8-18-17-6-4-13-11-14(20)5-7-15(13)16(17)9-10-19(12,18)2/h5,7,11,16-18,20H,1,3-4,6,8-10H2,2H3/t16-,17-,18+,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 5.58E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in immature rabbit |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50228275

(CHEMBL1627370)Show SMILES [H][C@@]12CC[C@@](O)(C#C)[C@@]1(C)C[C@H](O)[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C20H24O3/c1-3-20(23)9-8-16-15-6-4-12-10-13(21)5-7-14(12)18(15)17(22)11-19(16,20)2/h1,5,7,10,15-18,21-23H,4,6,8-9,11H2,2H3/t15-,16-,17-,18+,19-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.56E+4 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in immature rabbit |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(RAT-Rattus norvegicus) | BDBM50228275

(CHEMBL1627370)Show SMILES [H][C@@]12CC[C@@](O)(C#C)[C@@]1(C)C[C@H](O)[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C20H24O3/c1-3-20(23)9-8-16-15-6-4-12-10-13(21)5-7-14(12)18(15)17(22)11-19(16,20)2/h1,5,7,10,15-18,21-23H,4,6,8-9,11H2,2H3/t15-,16-,17-,18+,19-,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]-estradiol from estrogen receptor in immature rat |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50228273

(CHEMBL1627375)Show SMILES [H][C@@]12CC[C@@](O)(C#C)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3C[C@@H](O)[C@@]21[H] Show InChI InChI=1S/C20H24O3/c1-3-20(23)9-7-16-18-15(6-8-19(16,20)2)14-5-4-13(21)10-12(14)11-17(18)22/h1,4-5,10,15-18,21-23H,6-9,11H2,2H3/t15-,16+,17-,18-,19+,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.66E+5 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in squirrel monkey |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50228274

(CHEMBL1627389)Show SMILES [H][C@]1(CC)CC[C@@]2([H])[C@]3([H])CCc4cc(O)ccc4[C@@]3([H])[C@@H](O)C[C@]12C Show InChI InChI=1S/C20H28O2/c1-3-13-5-9-17-16-7-4-12-10-14(21)6-8-15(12)19(16)18(22)11-20(13,17)2/h6,8,10,13,16-19,21-22H,3-5,7,9,11H2,1-2H3/t13-,16-,17-,18-,19+,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 7.32E+4 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in squirrel monkey |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50187243

(17-ethinyl-3,17-estradiol | 17-ethinyl-3,17-oestra...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)C#C |r| Show InChI InChI=1S/C20H24O2/c1-3-20(22)11-9-18-17-6-4-13-12-14(21)5-7-15(13)16(17)8-10-19(18,20)2/h1,5,7,12,16-18,21-22H,4,6,8-11H2,2H3/t16-,17-,18+,19+,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in immature rabbit |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in immature rabbit |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(RAT-Rattus norvegicus) | BDBM50228273

(CHEMBL1627375)Show SMILES [H][C@@]12CC[C@@](O)(C#C)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3C[C@@H](O)[C@@]21[H] Show InChI InChI=1S/C20H24O3/c1-3-20(23)9-7-16-18-15(6-8-19(16,20)2)14-5-4-13(21)10-12(14)11-17(18)22/h1,4-5,10,15-18,21-23H,6-9,11H2,2H3/t15-,16+,17-,18-,19+,20+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 5.76E+4 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in immature rat |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(RAT-Rattus norvegicus) | BDBM50187243

(17-ethinyl-3,17-estradiol | 17-ethinyl-3,17-oestra...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)C#C |r| Show InChI InChI=1S/C20H24O2/c1-3-20(22)11-9-18-17-6-4-13-12-14(21)5-7-15(13)16(17)8-10-19(18,20)2/h1,5,7,12,16-18,21-22H,4,6,8-11H2,2H3/t16-,17-,18+,19+,20+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.68E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]-estradiol from estrogen receptor in mature rat |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data