

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50078243 ((8-Phenyl-chroman-3-yl)-dipropyl-amine | CHEMBL411...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50078250 (CHEMBL39847 | Dipropyl-(8-thiophen-3-yl-chroman-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50078242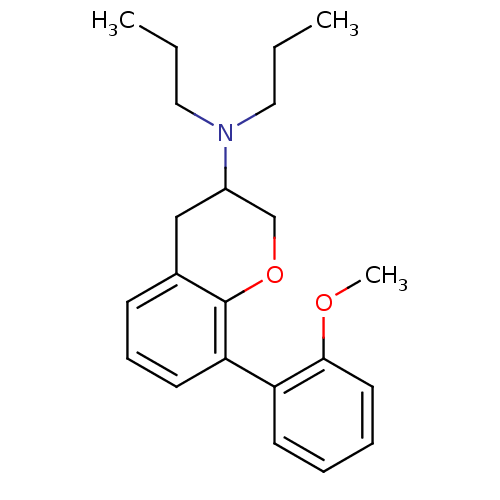 (CHEMBL41491 | [8-(2-Methoxy-phenyl)-chroman-3-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50078240 (CHEMBL41200 | [8-(4-Methoxy-phenyl)-chroman-3-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50052869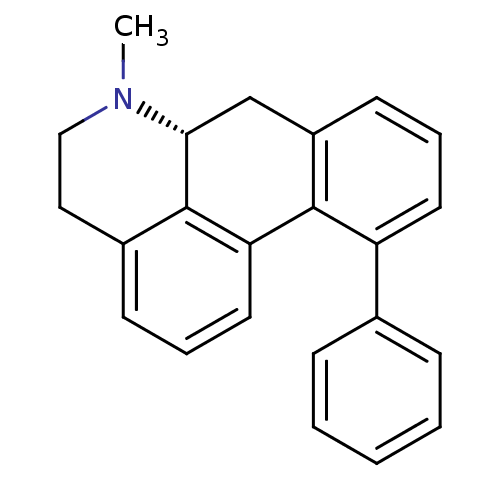 ((R)-6-Methyl-11-phenyl-5,6,6a,7-tetrahydro-4H-dibe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50078244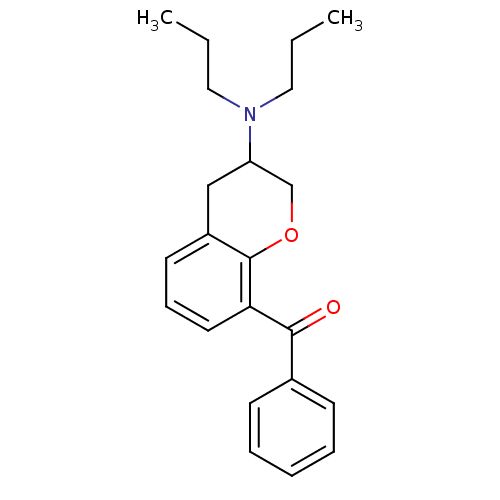 ((3-Dipropylamino-chroman-8-yl)-phenyl-methanone | ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50078241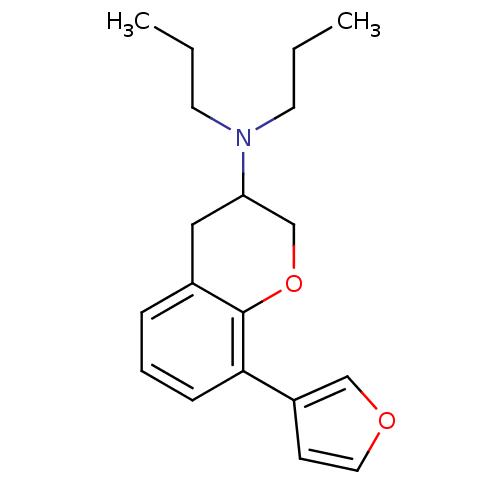 ((8-Furan-3-yl-chroman-3-yl)-dipropyl-amine | CHEMB...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50078245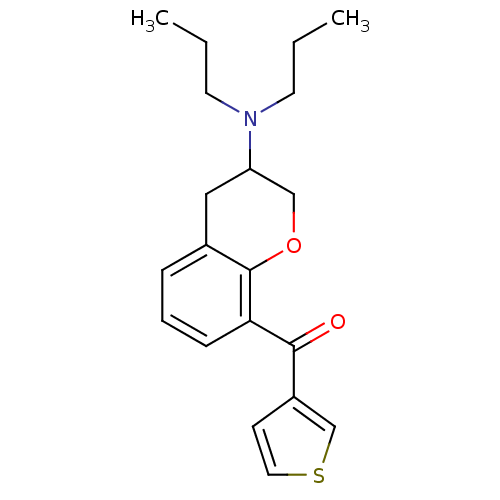 ((3-Dipropylamino-chroman-8-yl)-thiophen-3-yl-metha...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50078247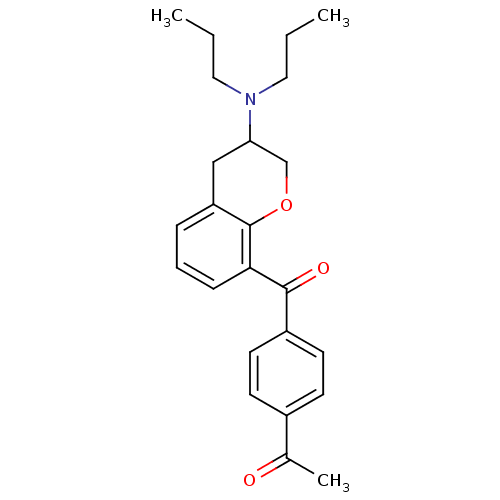 (1-[4-(3-Dipropylamino-chroman-8-carbonyl)-phenyl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50078249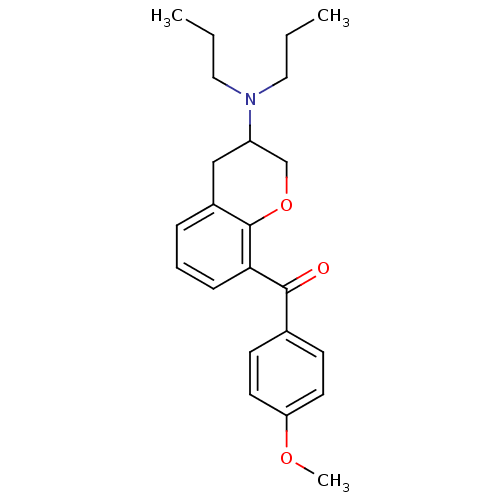 ((3-Dipropylamino-chroman-8-yl)-(4-methoxy-phenyl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50078246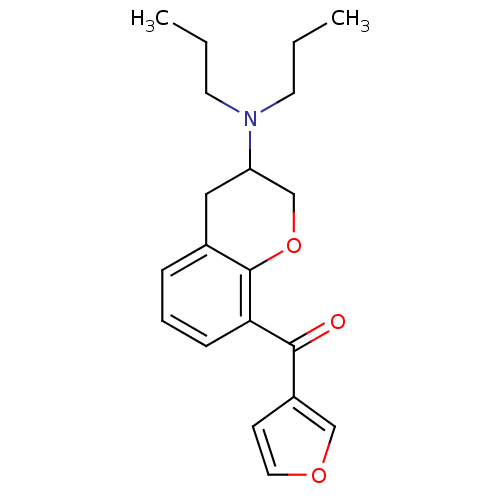 ((3-Dipropylamino-chroman-8-yl)-furan-3-yl-methanon...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50078251 ((3-Dipropylamino-chroman-8-yl)-(4-trifluoromethyl-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50078241 ((8-Furan-3-yl-chroman-3-yl)-dipropyl-amine | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-raclopride binding to cloned human D2A receptors expressed in mouse fibroblast (LtK-) cells | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50078252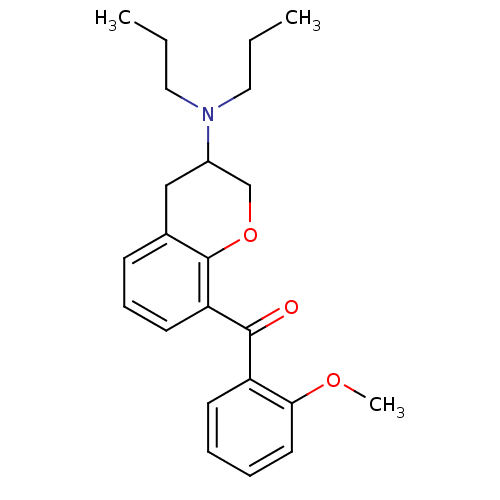 ((3-Dipropylamino-chroman-8-yl)-(2-methoxy-phenyl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50078248 (CHEMBL43073 | Dipropyl-(8-thiophen-3-yl-chroman-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50036864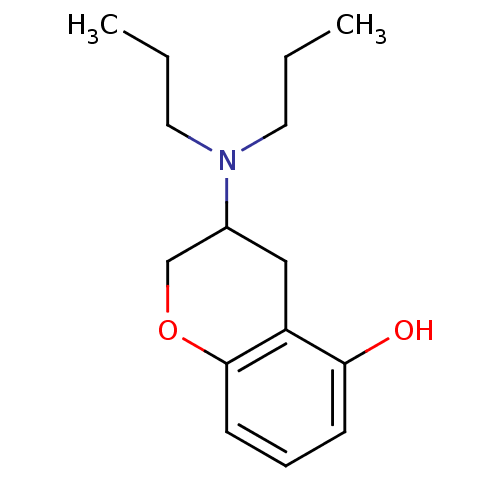 (3-Dipropylamino-chroman-5-ol | CHEMBL27995) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50020220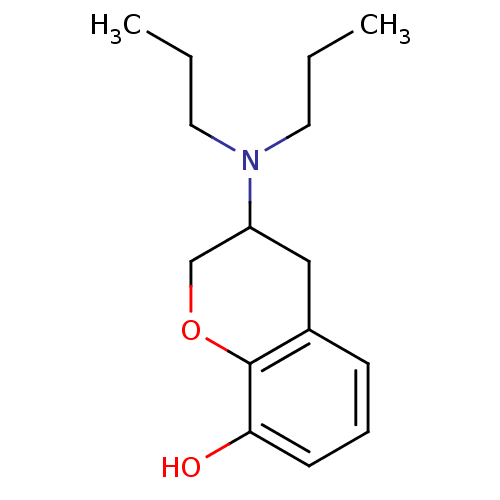 (3-Dipropylamino-chroman-8-ol | CHEMBL38428) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-raclopride binding to cloned human D2A receptors expressed in mouse fibroblast (LtK-) cells | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50503648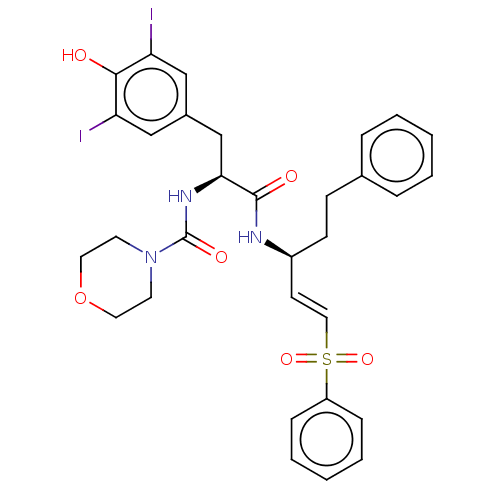 (CHEMBL4474444) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Houston Curated by ChEMBL | Assay Description Inhibition of cathepsin B (unknown origin) using Z-Arg-Arg-AMC as substrate after 5 mins by fluorimetric method | Bioorg Med Chem Lett 29: 36-39 (2019) Article DOI: 10.1016/j.bmcl.2018.11.019 BindingDB Entry DOI: 10.7270/Q2028VTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50078250 (CHEMBL39847 | Dipropyl-(8-thiophen-3-yl-chroman-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-raclopride binding to cloned human D2A receptors expressed in mouse fibroblast (LtK-) cells | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50078243 ((8-Phenyl-chroman-3-yl)-dipropyl-amine | CHEMBL411...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-raclopride binding to cloned human D2A receptors expressed in mouse fibroblast (LtK-) cells | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50503648 (CHEMBL4474444) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Houston Curated by ChEMBL | Assay Description Inhibition of cathepsin L (unknown origin) using Z-Phe-Arg-AMC as substrate after 5 mins by fluorimetric method | Bioorg Med Chem Lett 29: 36-39 (2019) Article DOI: 10.1016/j.bmcl.2018.11.019 BindingDB Entry DOI: 10.7270/Q2028VTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50078248 (CHEMBL43073 | Dipropyl-(8-thiophen-3-yl-chroman-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 763 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-raclopride binding to cloned human D2A receptors expressed in mouse fibroblast (LtK-) cells | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50078245 ((3-Dipropylamino-chroman-8-yl)-thiophen-3-yl-metha...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-raclopride binding to cloned human D2A receptors expressed in mouse fibroblast (LtK-) cells | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50503647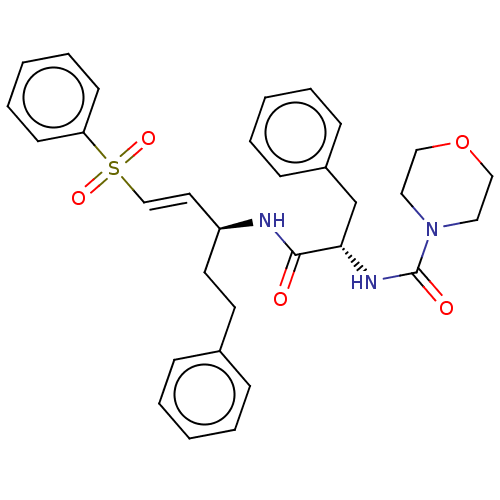 (CHEMBL327624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Houston Curated by ChEMBL | Assay Description Inhibition of cathepsin L (unknown origin) using Z-Phe-Arg-AMC as substrate after 5 mins by fluorimetric method | Bioorg Med Chem Lett 29: 36-39 (2019) Article DOI: 10.1016/j.bmcl.2018.11.019 BindingDB Entry DOI: 10.7270/Q2028VTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50078244 ((3-Dipropylamino-chroman-8-yl)-phenyl-methanone | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-raclopride binding to cloned human D2A receptors expressed in mouse fibroblast (LtK-) cells | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50078252 ((3-Dipropylamino-chroman-8-yl)-(2-methoxy-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-raclopride binding to cloned human D2A receptors expressed in mouse fibroblast (LtK-) cells | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50078247 (1-[4-(3-Dipropylamino-chroman-8-carbonyl)-phenyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-raclopride binding to cloned human D2A receptors expressed in mouse fibroblast (LtK-) cells | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50078251 ((3-Dipropylamino-chroman-8-yl)-(4-trifluoromethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-raclopride binding to cloned human D2A receptors expressed in mouse fibroblast (LtK-) cells | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50078249 ((3-Dipropylamino-chroman-8-yl)-(4-methoxy-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-raclopride binding to cloned human D2A receptors expressed in mouse fibroblast (LtK-) cells | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50078242 (CHEMBL41491 | [8-(2-Methoxy-phenyl)-chroman-3-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-raclopride binding to cloned human D2A receptors expressed in mouse fibroblast (LtK-) cells | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50078240 (CHEMBL41200 | [8-(4-Methoxy-phenyl)-chroman-3-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-raclopride binding to cloned human D2A receptors expressed in mouse fibroblast (LtK-) cells | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50078246 ((3-Dipropylamino-chroman-8-yl)-furan-3-yl-methanon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-raclopride binding to cloned human D2A receptors expressed in mouse fibroblast (LtK-) cells | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 2A (Homo sapiens (Human)) | BDBM50395076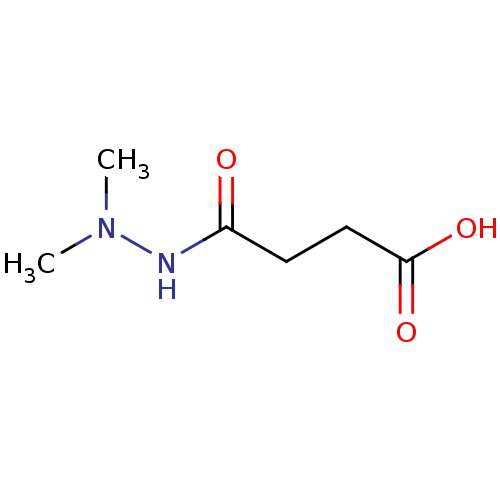 (CHEMBL2164243) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Competitive inhibition of human KDM2A expressed in Escherichia coli using 2-oxoglutarate by enzyme kinetic assay | J Med Chem 55: 6639-43 (2012) Article DOI: 10.1021/jm300677j BindingDB Entry DOI: 10.7270/Q2JH3N9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50020220 (3-Dipropylamino-chroman-8-ol | CHEMBL38428) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50036864 (3-Dipropylamino-chroman-5-ol | CHEMBL27995) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-raclopride binding to cloned human D2A receptors expressed in mouse fibroblast (LtK-) cells | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50503647 (CHEMBL327624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Houston Curated by ChEMBL | Assay Description Inhibition of cathepsin B (unknown origin) using Z-Arg-Arg-AMC as substrate after 5 mins by fluorimetric method | Bioorg Med Chem Lett 29: 36-39 (2019) Article DOI: 10.1016/j.bmcl.2018.11.019 BindingDB Entry DOI: 10.7270/Q2028VTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50503646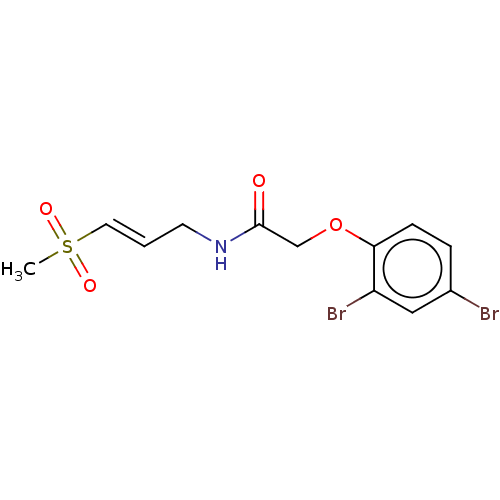 (CHEMBL4564457) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Houston Curated by ChEMBL | Assay Description Competitive inhibition of human liver cathepsin L using Z-FR-AMC as substrate measured at 1 min interval for 20 mins followed by every 15 mins for 2 ... | Bioorg Med Chem Lett 29: 36-39 (2019) Article DOI: 10.1016/j.bmcl.2018.11.019 BindingDB Entry DOI: 10.7270/Q2028VTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 2A (Homo sapiens (Human)) | BDBM50395076 (CHEMBL2164243) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Mixed type inhibition of human KDM2A expressed in Escherichia coli assessed inhibition constant for compound-enzyme-substrate complex using methyl ly... | J Med Chem 55: 6639-43 (2012) Article DOI: 10.1021/jm300677j BindingDB Entry DOI: 10.7270/Q2JH3N9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50503650 (CHEMBL4516952) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Houston Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin L using Z-FR-AMC as substrate measured at 1 min interval for 20 mins followed by every 15 mins for 2 hrs by fluor... | Bioorg Med Chem Lett 29: 36-39 (2019) Article DOI: 10.1016/j.bmcl.2018.11.019 BindingDB Entry DOI: 10.7270/Q2028VTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50503649 (CHEMBL4476113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Houston Curated by ChEMBL | Assay Description Inhibition of KRAS G12C mutant (unknown origin) | Bioorg Med Chem Lett 29: 36-39 (2019) Article DOI: 10.1016/j.bmcl.2018.11.019 BindingDB Entry DOI: 10.7270/Q2028VTF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate measured after 7 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113541 BindingDB Entry DOI: 10.7270/Q25D8WNM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50115732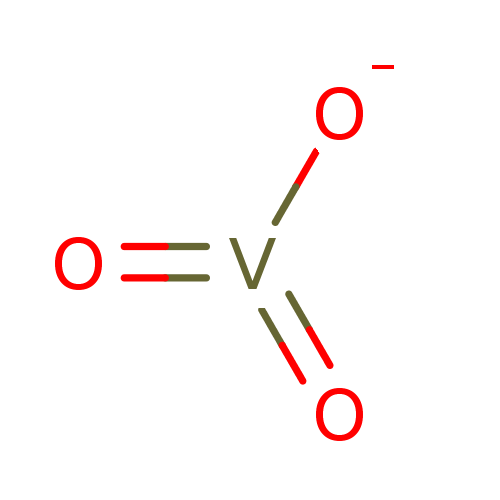 (CHEMBL294467 | Sodium vanadate) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TCPTP using pNPP as substrate measured after 30 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112508 BindingDB Entry DOI: 10.7270/Q2QJ7N1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50391109 (CHEMBL179166 | Sodium orthovanadate (SOV) | Vanada...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University (SJTU) Curated by ChEMBL | Assay Description Inhibition of TCPTP (unknown origin) using pNPP as substrate after 30 mins | Eur J Med Chem 164: 408-422 (2019) Article DOI: 10.1016/j.ejmech.2018.12.032 BindingDB Entry DOI: 10.7270/Q2KK9G90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115732 (CHEMBL294467 | Sodium vanadate) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human PTP1B using pNPP as substrate measured after 30 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112508 BindingDB Entry DOI: 10.7270/Q2QJ7N1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50391109 (CHEMBL179166 | Sodium orthovanadate (SOV) | Vanada...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University (SJTU) Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate after 30 mins | Eur J Med Chem 164: 408-422 (2019) Article DOI: 10.1016/j.ejmech.2018.12.032 BindingDB Entry DOI: 10.7270/Q2KK9G90 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50391109 (CHEMBL179166 | Sodium orthovanadate (SOV) | Vanada...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University (SJTU) Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using pNPP as substrate after 30 mins | Eur J Med Chem 164: 408-422 (2019) Article DOI: 10.1016/j.ejmech.2018.12.032 BindingDB Entry DOI: 10.7270/Q2KK9G90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BuChe using S-butyrylthiocholine iodide as substrate measured after 7 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113541 BindingDB Entry DOI: 10.7270/Q25D8WNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531695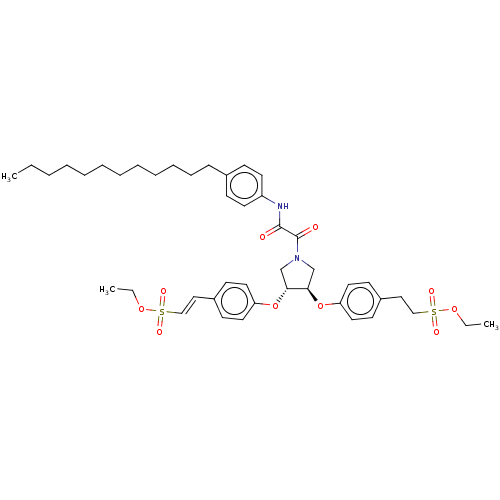 (CHEMBL4473987) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University (SJTU) Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate after 30 mins | Eur J Med Chem 164: 408-422 (2019) Article DOI: 10.1016/j.ejmech.2018.12.032 BindingDB Entry DOI: 10.7270/Q2KK9G90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4E (Homo sapiens (Human)) | BDBM50395083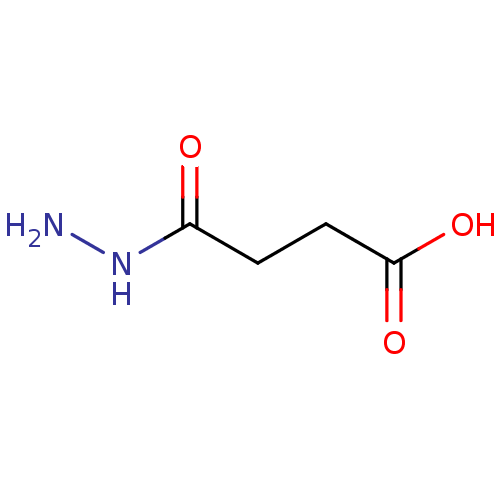 (CHEMBL2164246) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human KDM4E expressed in Escherichia coli using methyl lysine peptide substrate by AlphaScreen assay | J Med Chem 55: 6639-43 (2012) Article DOI: 10.1021/jm300677j BindingDB Entry DOI: 10.7270/Q2JH3N9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531709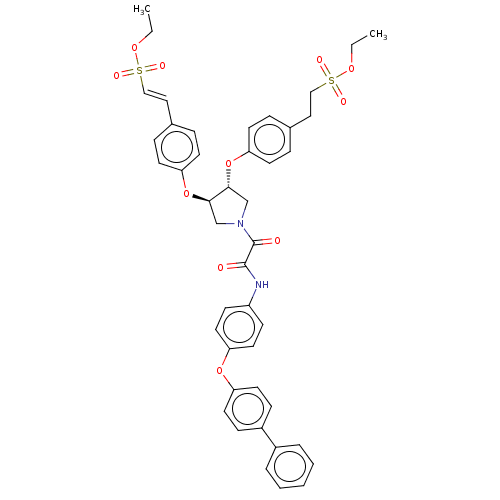 (CHEMBL4528006) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University (SJTU) Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate after 30 mins | Eur J Med Chem 164: 408-422 (2019) Article DOI: 10.1016/j.ejmech.2018.12.032 BindingDB Entry DOI: 10.7270/Q2KK9G90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 327 total ) | Next | Last >> |