

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110577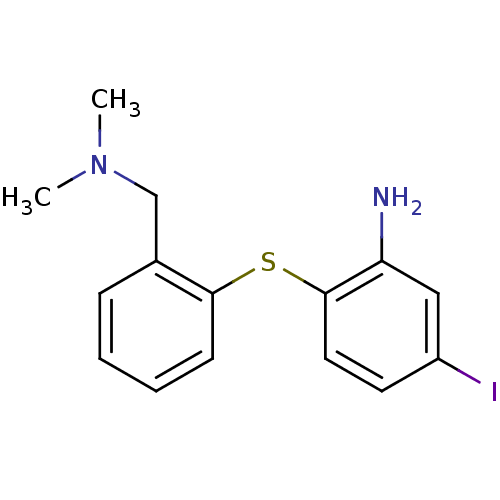 (2-(2-((dimethylamino)methyl)phenylthio)-5-iodoanil...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro binding affinity of the compound in LLC-PK1 cells by SERT binding assay by using [125I]-IDAM as a radioligand | J Med Chem 45: 4716-23 (2002) BindingDB Entry DOI: 10.7270/Q23N22RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Donesta Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9561238 (2017) BindingDB Entry DOI: 10.7270/Q2B56MR1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 0.0150 | -61.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
PANTARHEI BIOSCIENCE B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9034854 (2015) BindingDB Entry DOI: 10.7270/Q2W66JJT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 0.0150 | -61.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pantarhei Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osboum et al. (1993, Biochemistry, 32, 6229-6236). Recombin... | US Patent US9040509 (2015) BindingDB Entry DOI: 10.7270/Q2Z036WK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM158504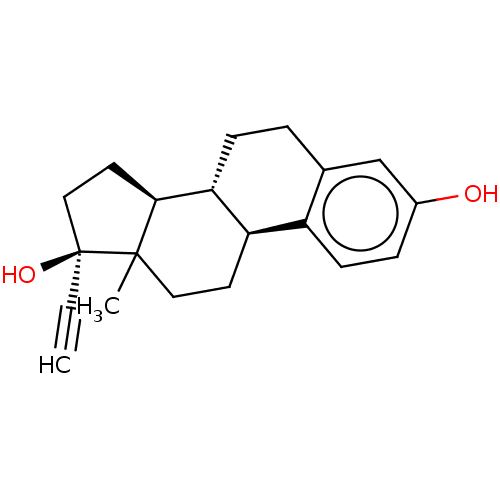 (US9034854, EE | US9040509, EE | US9561238, EE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Donesta Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9561238 (2017) BindingDB Entry DOI: 10.7270/Q2B56MR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM158504 (US9034854, EE | US9040509, EE | US9561238, EE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.0250 | -60.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
PANTARHEI BIOSCIENCE B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9034854 (2015) BindingDB Entry DOI: 10.7270/Q2W66JJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM158504 (US9034854, EE | US9040509, EE | US9561238, EE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.0250 | -60.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pantarhei Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osboum et al. (1993, Biochemistry, 32, 6229-6236). Recombin... | US Patent US9040509 (2015) BindingDB Entry DOI: 10.7270/Q2Z036WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50119550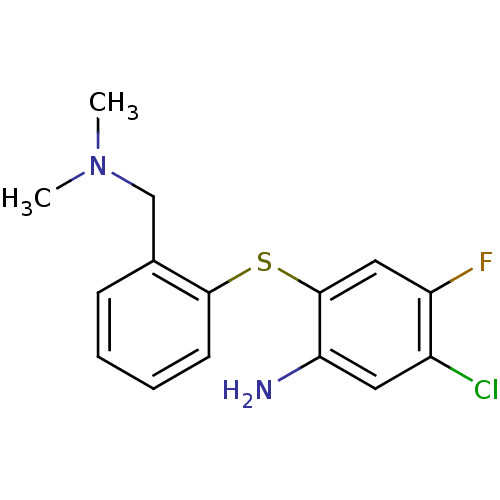 (5-Chloro-2-(2-dimethylaminomethyl-phenylsulfanyl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro binding affinity of the compound in LLC-PK1 cells by SERT binding assay by using [125I]-IDAM as a radioligand | J Med Chem 45: 4716-23 (2002) BindingDB Entry DOI: 10.7270/Q23N22RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50073436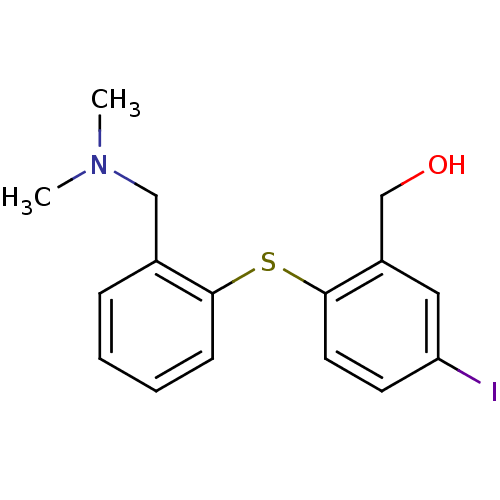 ((2-(2-((dimethylamino)methyl)phenylthio)-5-iodophe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro binding affinity of the compound in LLC-PK1 cells by SERT binding assay by using [125I]-IDAM as a radioligand | J Med Chem 45: 4716-23 (2002) BindingDB Entry DOI: 10.7270/Q23N22RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50062852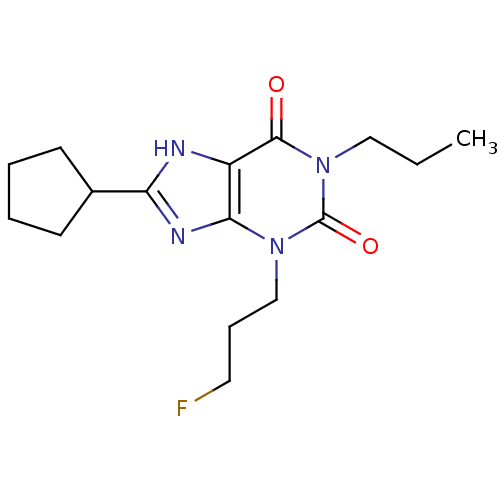 (8-Cyclopentyl-3-(3-fluoro-propyl)-1-propyl-3,7-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forschungszentrum Jülich GmbH Curated by ChEMBL | Assay Description In vitro binding affinity for Adenosine A1 receptor of bovine cortex | J Med Chem 45: 5150-6 (2002) BindingDB Entry DOI: 10.7270/Q21J993X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 0.210 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
PANTARHEI BIOSCIENCE B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9034854 (2015) BindingDB Entry DOI: 10.7270/Q2W66JJT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Donesta Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9561238 (2017) BindingDB Entry DOI: 10.7270/Q2B56MR1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 0.210 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pantarhei Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osboum et al. (1993, Biochemistry, 32, 6229-6236). Recombin... | US Patent US9040509 (2015) BindingDB Entry DOI: 10.7270/Q2Z036WK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM158504 (US9034854, EE | US9040509, EE | US9561238, EE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Donesta Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9561238 (2017) BindingDB Entry DOI: 10.7270/Q2B56MR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM158504 (US9034854, EE | US9040509, EE | US9561238, EE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.230 | -55.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pantarhei Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osboum et al. (1993, Biochemistry, 32, 6229-6236). Recombin... | US Patent US9040509 (2015) BindingDB Entry DOI: 10.7270/Q2Z036WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM158504 (US9034854, EE | US9040509, EE | US9561238, EE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.230 | -55.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
PANTARHEI BIOSCIENCE B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9034854 (2015) BindingDB Entry DOI: 10.7270/Q2W66JJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210579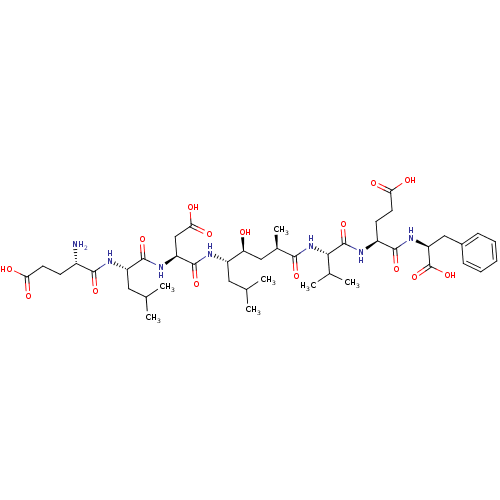 ((2S,5S,8S,11R,13S,14S,17S,20S,23S)-23-amino-2-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Inhibition of active BACE1 (unknown origin) | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50091095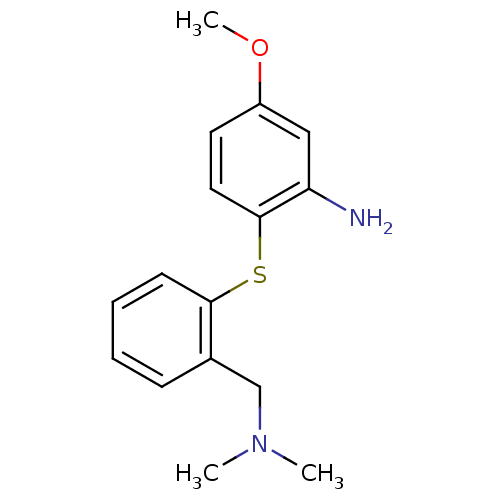 (2-(2-Dimethylaminomethyl-phenylsulfanyl)-5-methoxy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro binding affinity of the compound in LLC-PK1 cells by SERT binding assay by using [125I]-IDAM as a radioligand | J Med Chem 45: 4716-23 (2002) BindingDB Entry DOI: 10.7270/Q23N22RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50119548 (2-(2-Dimethylaminomethyl-phenylsulfanyl)-5-fluorom...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro binding affinity of the compound in LLC-PK1 cells by SERT binding assay by using [125I]-IDAM as a radioligand | J Med Chem 45: 4716-23 (2002) BindingDB Entry DOI: 10.7270/Q23N22RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110788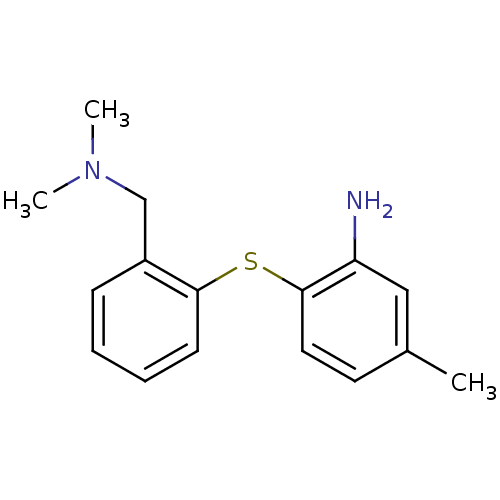 (2-(2-((dimethylamino)methyl)phenylthio)-5-methylan...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro binding affinity of the compound in LLC-PK1 cells by SERT binding assay by using [125I]-IDAM as a radioligand | J Med Chem 45: 4716-23 (2002) BindingDB Entry DOI: 10.7270/Q23N22RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50091097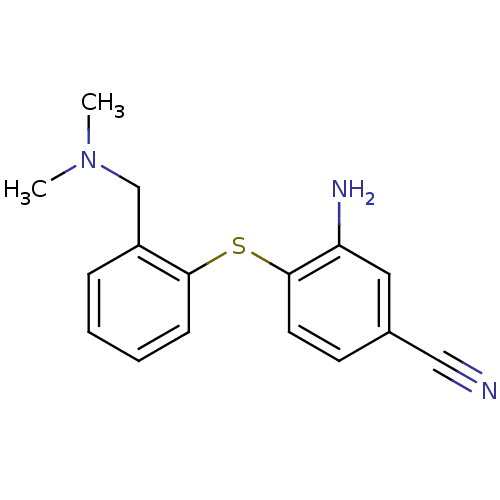 (3-Amino-4-(2-dimethylaminomethyl-phenylsulfanyl)-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro binding affinity of the compound in LLC-PK1 cells by SERT binding assay by using [125I]-IDAM as a radioligand | J Med Chem 45: 4716-23 (2002) BindingDB Entry DOI: 10.7270/Q23N22RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210579 ((2S,5S,8S,11R,13S,14S,17S,20S,23S)-23-amino-2-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 2 hrs by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Inhibition of active BACE1 (unknown origin) | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50094979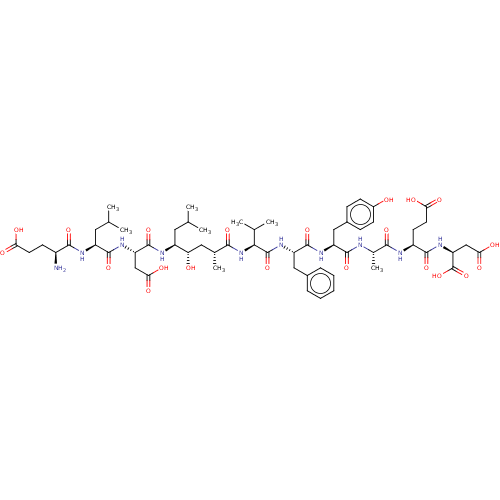 (CHEMBL3589002) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 2 hrs by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50094977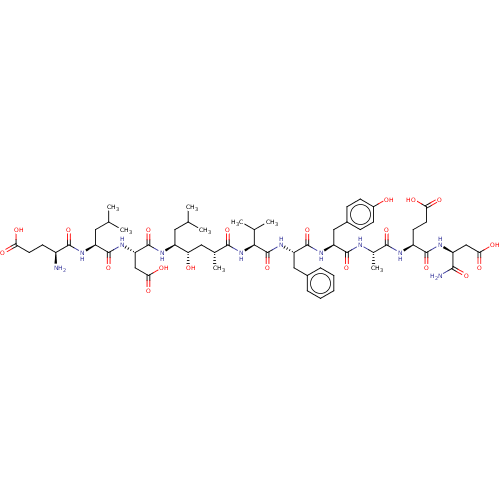 (CHEMBL3589000) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 2 hrs by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50094978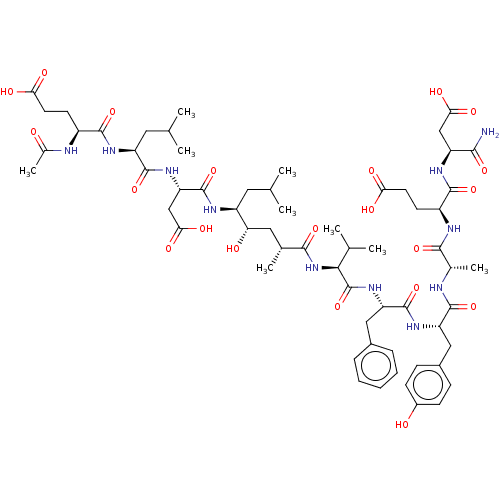 (CHEMBL3589001) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 2 hrs by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50094976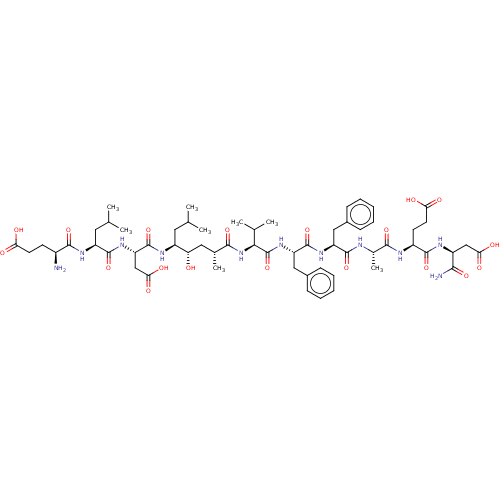 (CHEMBL3588999) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 2 hrs by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50073437 (CHEMBL113382 | [5-Chloro-2-(2-dimethylaminomethyl-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of 5-HT reuptake (SERT) in rat brain synaptosomes. | J Med Chem 45: 4716-23 (2002) BindingDB Entry DOI: 10.7270/Q23N22RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50119549 (2-(5-Amino-4-chloro-2-nitro-phenylsulfanyl)-N,N-di...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 2.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro binding affinity of the compound in LLC-PK1 cells by SERT binding assay by using [125I]-IDAM as a radioligand | J Med Chem 45: 4716-23 (2002) BindingDB Entry DOI: 10.7270/Q23N22RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (RAT) | BDBM50073805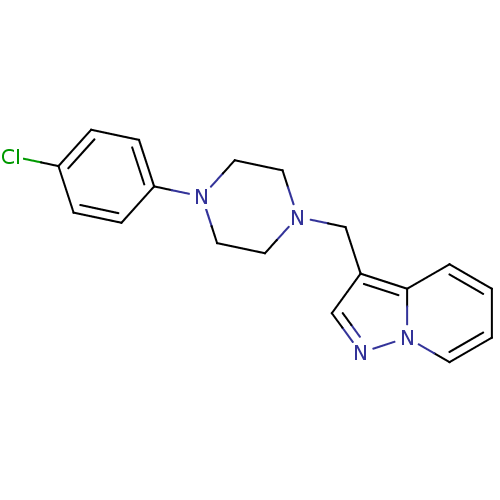 (3-((4-(4-chlorophenyl)piperazin-1-yl)methyl)H-pyra...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forschungszentrum J£lich GmbH Curated by ChEMBL | Assay Description Binding affinity to dopamine D4 receptor in Wistar rat brain slices | J Med Chem 54: 8343-52 (2011) Article DOI: 10.1021/jm200762g BindingDB Entry DOI: 10.7270/Q2W096B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50210579 ((2S,5S,8S,11R,13S,14S,17S,20S,23S)-23-amino-2-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Apparent inhibition of human recombinant BACE2 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 1 hr by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50119551 (CHEMBL142517 | [2-(4-Chloro-5-fluoro-2-nitro-pheny...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 4.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro binding affinity of the compound in LLC-PK1 cells by SERT binding assay by using [125I]-IDAM as a radioligand | J Med Chem 45: 4716-23 (2002) BindingDB Entry DOI: 10.7270/Q23N22RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM158505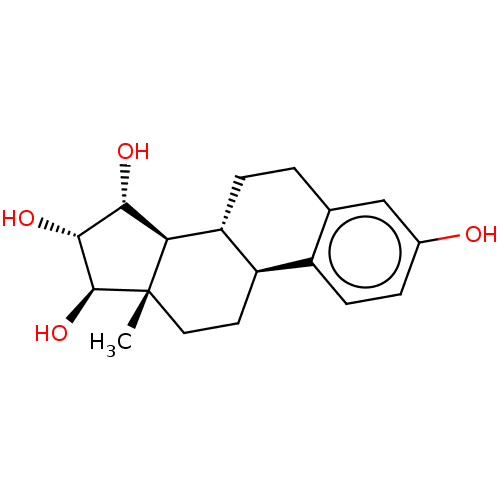 (US9034854, E4 | US9040509, E4 | US9561238, E4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Donesta Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9561238 (2017) BindingDB Entry DOI: 10.7270/Q2B56MR1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM158505 (US9034854, E4 | US9040509, E4 | US9561238, E4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | 4.90 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
PANTARHEI BIOSCIENCE B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9034854 (2015) BindingDB Entry DOI: 10.7270/Q2W66JJT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM158505 (US9034854, E4 | US9040509, E4 | US9561238, E4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | 4.90 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pantarhei Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osboum et al. (1993, Biochemistry, 32, 6229-6236). Recombin... | US Patent US9040509 (2015) BindingDB Entry DOI: 10.7270/Q2Z036WK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50094976 (CHEMBL3588999) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Apparent inhibition of human recombinant BACE2 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 1 hr by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Inhibition of pro-BACE1 (unknown origin) | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50094979 (CHEMBL3589002) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Apparent inhibition of human recombinant BACE2 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 1 hr by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50094977 (CHEMBL3589000) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Apparent inhibition of human recombinant BACE2 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 1 hr by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50094978 (CHEMBL3589001) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Apparent inhibition of human recombinant BACE2 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 1 hr by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM158505 (US9034854, E4 | US9040509, E4 | US9561238, E4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | 19 | -44.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pantarhei Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osboum et al. (1993, Biochemistry, 32, 6229-6236). Recombin... | US Patent US9040509 (2015) BindingDB Entry DOI: 10.7270/Q2Z036WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM158505 (US9034854, E4 | US9040509, E4 | US9561238, E4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | 19 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Donesta Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9561238 (2017) BindingDB Entry DOI: 10.7270/Q2B56MR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM158505 (US9034854, E4 | US9040509, E4 | US9561238, E4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | 19 | -44.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
PANTARHEI BIOSCIENCE B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9034854 (2015) BindingDB Entry DOI: 10.7270/Q2W66JJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50073437 (CHEMBL113382 | [5-Chloro-2-(2-dimethylaminomethyl-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Selectivity of the compound for norepinephrine transporter | J Med Chem 45: 4716-23 (2002) BindingDB Entry DOI: 10.7270/Q23N22RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50119550 (5-Chloro-2-(2-dimethylaminomethyl-phenylsulfanyl)-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro binding affinity of the compound in LLC-PK1 cells by Norepinephrin transpoter binding assay by using [125I]-IPT as a radioligand | J Med Chem 45: 4716-23 (2002) BindingDB Entry DOI: 10.7270/Q23N22RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50110577 (2-(2-((dimethylamino)methyl)phenylthio)-5-iodoanil...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 699 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro binding affinity of the compound in LLC-PK1 cells by Dopamine transporter binding assay by using [125I]-IPT as a radioligand | J Med Chem 45: 4716-23 (2002) BindingDB Entry DOI: 10.7270/Q23N22RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50110577 (2-(2-((dimethylamino)methyl)phenylthio)-5-iodoanil...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro binding affinity of the compound in LLC-PK1 cells by Norepinephrin transpoter binding assay by using [125I]-IPT as a radioligand | J Med Chem 45: 4716-23 (2002) BindingDB Entry DOI: 10.7270/Q23N22RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (PIG) | BDBM50358840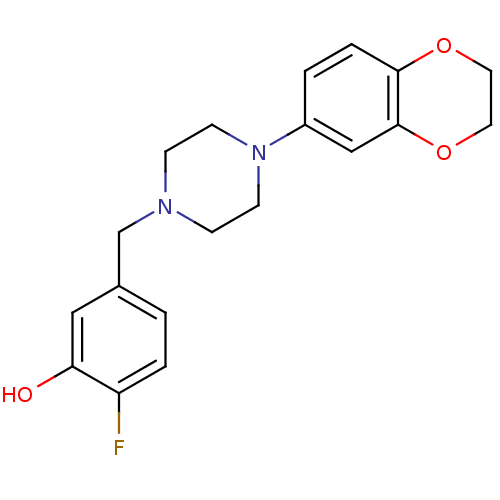 (CHEMBL1923415) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forschungszentrum J£lich GmbH Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5-HT2 receptor in porcine cortical membranes | J Med Chem 54: 8343-52 (2011) Article DOI: 10.1021/jm200762g BindingDB Entry DOI: 10.7270/Q2W096B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50119551 (CHEMBL142517 | [2-(4-Chloro-5-fluoro-2-nitro-pheny...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 2.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro binding affinity of the compound in LLC-PK1 cells by Norepinephrin transpoter binding assay by using [125I]-IPT as a radioligand | J Med Chem 45: 4716-23 (2002) BindingDB Entry DOI: 10.7270/Q23N22RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50119550 (5-Chloro-2-(2-dimethylaminomethyl-phenylsulfanyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro binding affinity of the compound in LLC-PK1 cells by Dopamine transporter binding assay by using [125I]-IPT as a radioligand | J Med Chem 45: 4716-23 (2002) BindingDB Entry DOI: 10.7270/Q23N22RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 144 total ) | Next | Last >> |