 Found 2284 hits with Last Name = 'collin' and Initial = 'l'
Found 2284 hits with Last Name = 'collin' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50275902

(CHEMBL525577 | D-Phe-Gln-Trp-Ala-Val-b-Ala-His-Phe...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](C)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc1ccccc1)C(C)C)C(N)=O |r| Show InChI InChI=1S/C57H76N14O10/c1-6-7-21-42(49(60)73)66-55(79)44(26-36-18-12-9-13-19-36)69-56(80)46(28-38-30-61-31-63-38)68-50(74)33(4)65-57(81)48(32(2)3)71-51(75)34(5)64-54(78)45(27-37-29-62-41-22-15-14-20-39(37)41)70-53(77)43(23-24-47(59)72)67-52(76)40(58)25-35-16-10-8-11-17-35/h8-20,22,29-34,40,42-46,48,62H,6-7,21,23-28,58H2,1-5H3,(H2,59,72)(H2,60,73)(H,61,63)(H,64,78)(H,65,81)(H,66,79)(H,67,76)(H,68,74)(H,69,80)(H,70,77)(H,71,75)/t33-,34+,40-,42+,43+,44+,45+,46+,48+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to BRS-3 receptor (unknown origin) |
Bioorg Med Chem Lett 18: 5451-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.033
BindingDB Entry DOI: 10.7270/Q2H9952R |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471981

(CHEMBL446629)Show SMILES Cn1cc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H31N3O4/c1-37-23-31(25-10-4-2-5-11-25)36-32(37)20-21-41-27-18-16-24(17-19-27)22-30(34(39)40)35-29-15-9-8-14-28(29)33(38)26-12-6-3-7-13-26/h2-19,23,30,35H,20-22H2,1H3,(H,39,40)/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471983
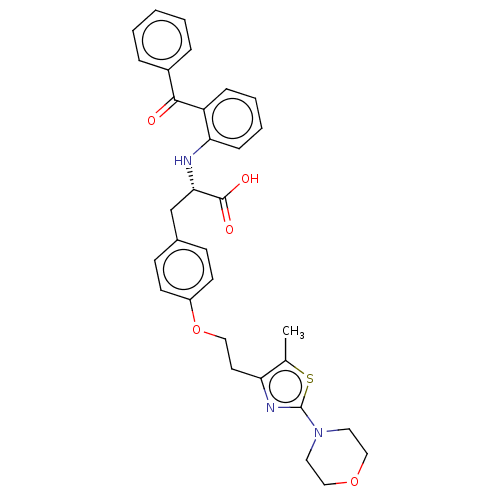
(CHEMBL149876)Show SMILES Cc1sc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)N1CCOCC1 Show InChI InChI=1S/C32H33N3O5S/c1-22-27(34-32(41-22)35-16-19-39-20-17-35)15-18-40-25-13-11-23(12-14-25)21-29(31(37)38)33-28-10-6-5-9-26(28)30(36)24-7-3-2-4-8-24/h2-14,29,33H,15-21H2,1H3,(H,37,38)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human D2 dopamine receptor in CHO cells by [3H]-spiperone displacement. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471980
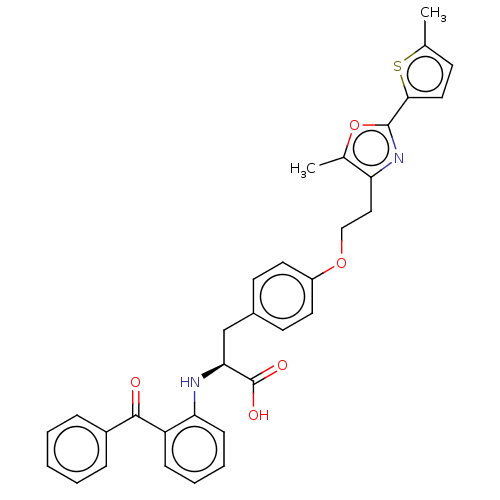
(CHEMBL147095)Show SMILES Cc1ccc(s1)-c1nc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)c(C)o1 Show InChI InChI=1S/C33H30N2O5S/c1-21-12-17-30(41-21)32-35-27(22(2)40-32)18-19-39-25-15-13-23(14-16-25)20-29(33(37)38)34-28-11-7-6-10-26(28)31(36)24-8-4-3-5-9-24/h3-17,29,34H,18-20H2,1-2H3,(H,37,38)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471978

(CHEMBL147090)Show SMILES Cc1sc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccncc1 Show InChI InChI=1S/C33H29N3O4S/c1-22-28(36-32(41-22)25-15-18-34-19-16-25)17-20-40-26-13-11-23(12-14-26)21-30(33(38)39)35-29-10-6-5-9-27(29)31(37)24-7-3-2-4-8-24/h2-16,18-19,30,35H,17,20-21H2,1H3,(H,38,39)/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471951

(CHEMBL343210)Show SMILES Cc1cc(no1)-c1nc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)c(C)s1 Show InChI InChI=1S/C32H29N3O5S/c1-20-18-28(35-40-20)31-34-26(21(2)41-31)16-17-39-24-14-12-22(13-15-24)19-29(32(37)38)33-27-11-7-6-10-25(27)30(36)23-8-4-3-5-9-23/h3-15,18,29,33H,16-17,19H2,1-2H3,(H,37,38)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 125: 202-8 (1998)
Article DOI: 10.1038/sj.bjp.0702059
BindingDB Entry DOI: 10.7270/Q2PC30XV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472023
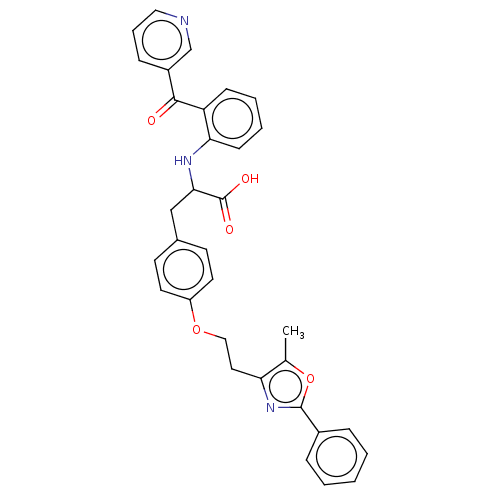
(CHEMBL148384)Show SMILES Cc1oc(nc1CCOc1ccc(CC(Nc2ccccc2C(=O)c2cccnc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C33H29N3O5/c1-22-28(36-32(41-22)24-8-3-2-4-9-24)17-19-40-26-15-13-23(14-16-26)20-30(33(38)39)35-29-12-6-5-11-27(29)31(37)25-10-7-18-34-21-25/h2-16,18,21,30,35H,17,19-20H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.933 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471959
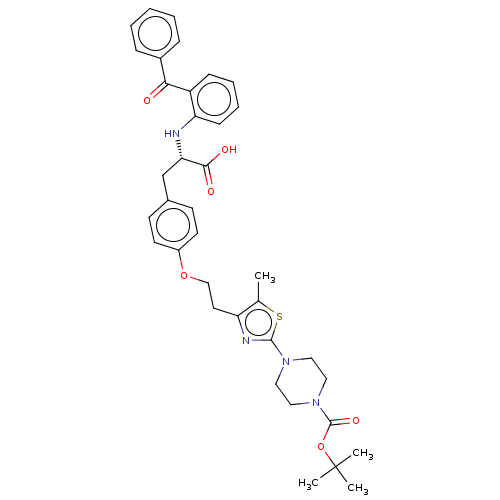
(CHEMBL146301)Show SMILES Cc1sc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)N1CCN(CC1)C(=O)OC(C)(C)C Show InChI InChI=1S/C37H42N4O6S/c1-25-30(39-35(48-25)40-19-21-41(22-20-40)36(45)47-37(2,3)4)18-23-46-28-16-14-26(15-17-28)24-32(34(43)44)38-31-13-9-8-12-29(31)33(42)27-10-6-5-7-11-27/h5-17,32,38H,18-24H2,1-4H3,(H,43,44)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.977 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472019
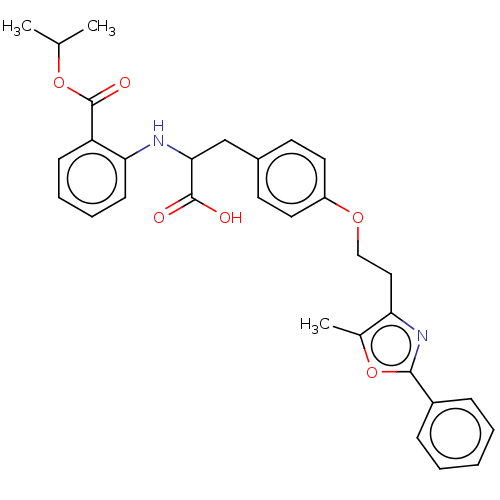
(CHEMBL148668)Show SMILES CC(C)OC(=O)c1ccccc1NC(Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C31H32N2O6/c1-20(2)38-31(36)25-11-7-8-12-27(25)32-28(30(34)35)19-22-13-15-24(16-14-22)37-18-17-26-21(3)39-29(33-26)23-9-5-4-6-10-23/h4-16,20,28,32H,17-19H2,1-3H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.977 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM50275902

(CHEMBL525577 | D-Phe-Gln-Trp-Ala-Val-b-Ala-His-Phe...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](C)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc1ccccc1)C(C)C)C(N)=O |r| Show InChI InChI=1S/C57H76N14O10/c1-6-7-21-42(49(60)73)66-55(79)44(26-36-18-12-9-13-19-36)69-56(80)46(28-38-30-61-31-63-38)68-50(74)33(4)65-57(81)48(32(2)3)71-51(75)34(5)64-54(78)45(27-37-29-62-41-22-15-14-20-39(37)41)70-53(77)43(23-24-47(59)72)67-52(76)40(58)25-35-16-10-8-11-17-35/h8-20,22,29-34,40,42-46,48,62H,6-7,21,23-28,58H2,1-5H3,(H2,59,72)(H2,60,73)(H,61,63)(H,64,78)(H,65,81)(H,66,79)(H,67,76)(H,68,74)(H,69,80)(H,70,77)(H,71,75)/t33-,34+,40-,42+,43+,44+,45+,46+,48+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to gastrin releasing peptide receptor (unknown origin) |
Bioorg Med Chem Lett 18: 5451-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.033
BindingDB Entry DOI: 10.7270/Q2H9952R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM85166
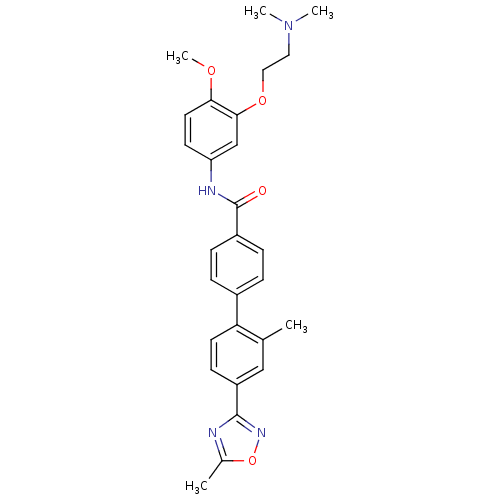
(CAS_3292447 | NSC_3292447 | SB 216641)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1OCCN(C)C Show InChI InChI=1S/C28H30N4O4/c1-18-16-22(27-29-19(2)36-31-27)10-12-24(18)20-6-8-21(9-7-20)28(33)30-23-11-13-25(34-5)26(17-23)35-15-14-32(3)4/h6-13,16-17H,14-15H2,1-5H3,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 356: 312-20 (1997)
Article DOI: 10.1007/pl00005056
BindingDB Entry DOI: 10.7270/Q2KW5DKB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 356: 312-20 (1997)
Article DOI: 10.1007/pl00005056
BindingDB Entry DOI: 10.7270/Q2KW5DKB |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471968
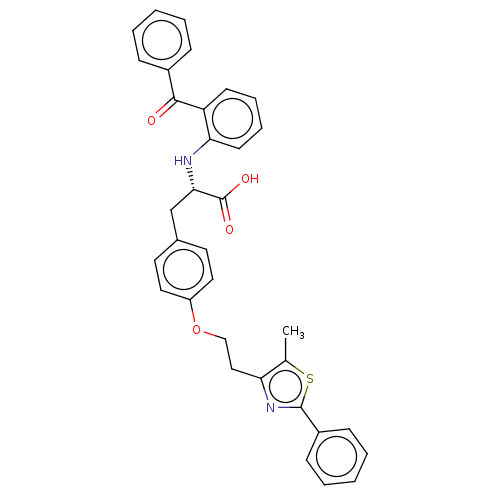
(CHEMBL358379)Show SMILES Cc1sc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O4S/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471953
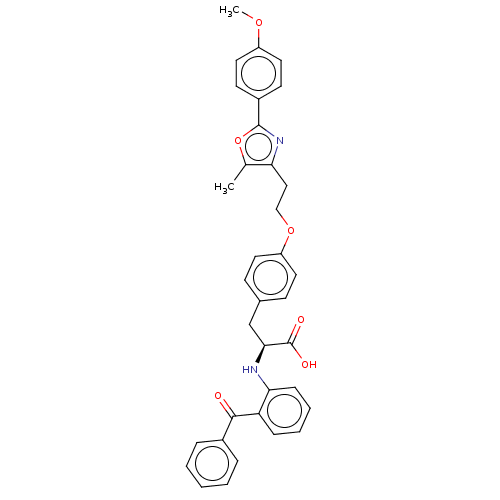
(CHEMBL356382)Show SMILES COc1ccc(cc1)-c1nc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)c(C)o1 Show InChI InChI=1S/C35H32N2O6/c1-23-30(37-34(43-23)26-14-18-27(41-2)19-15-26)20-21-42-28-16-12-24(13-17-28)22-32(35(39)40)36-31-11-7-6-10-29(31)33(38)25-8-4-3-5-9-25/h3-19,32,36H,20-22H2,1-2H3,(H,39,40)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 356: 312-20 (1997)
Article DOI: 10.1007/pl00005056
BindingDB Entry DOI: 10.7270/Q2KW5DKB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50098551
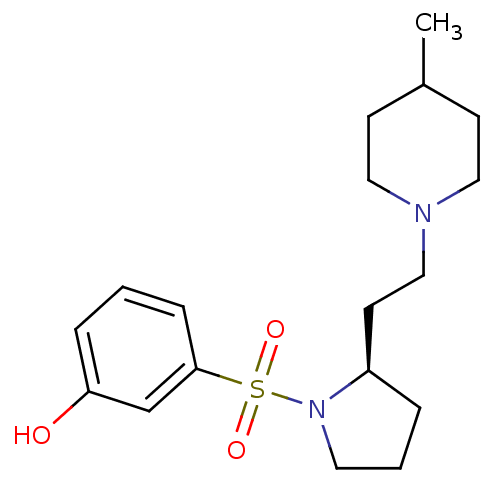
((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...)Show SMILES CC1CCN(CC[C@H]2CCCN2S(=O)(=O)c2cccc(O)c2)CC1 |r| Show InChI InChI=1S/C18H28N2O3S/c1-15-7-11-19(12-8-15)13-9-16-4-3-10-20(16)24(22,23)18-6-2-5-17(21)14-18/h2,5-6,14-16,21H,3-4,7-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Med Chem 43: 342-5 (2000)
Article DOI: 10.1021/jm991151j
BindingDB Entry DOI: 10.7270/Q28S4NGP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471966

(CHEMBL147935)Show SMILES Cc1cc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)nn1-c1ccccc1 Show InChI InChI=1S/C34H31N3O4/c1-24-22-27(36-37(24)28-12-6-3-7-13-28)20-21-41-29-18-16-25(17-19-29)23-32(34(39)40)35-31-15-9-8-14-30(31)33(38)26-10-4-2-5-11-26/h2-19,22,32,35H,20-21,23H2,1H3,(H,39,40)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471974

(CHEMBL148950)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccncc1 Show InChI InChI=1S/C33H29N3O5/c1-22-28(36-32(41-22)25-15-18-34-19-16-25)17-20-40-26-13-11-23(12-14-26)21-30(33(38)39)35-29-10-6-5-9-27(29)31(37)24-7-3-2-4-8-24/h2-16,18-19,30,35H,17,20-21H2,1H3,(H,38,39)/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472043
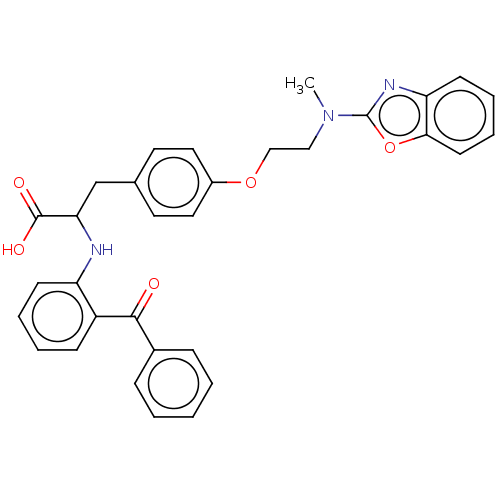
(CHEMBL148596)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C32H29N3O5/c1-35(32-34-27-13-7-8-14-29(27)40-32)19-20-39-24-17-15-22(16-18-24)21-28(31(37)38)33-26-12-6-5-11-25(26)30(36)23-9-3-2-4-10-23/h2-18,28,33H,19-21H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418564
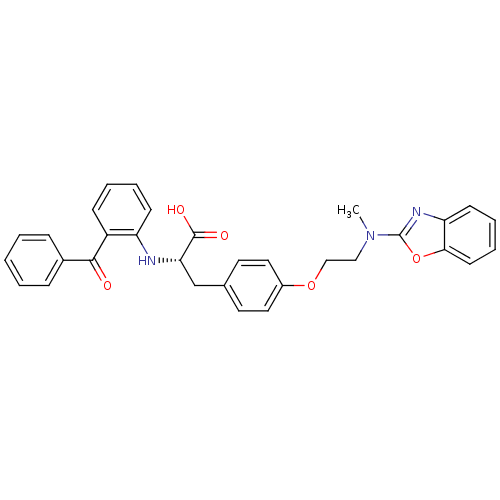
(CHEMBL423026)Show SMILES CN(CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C32H29N3O5/c1-35(32-34-27-13-7-8-14-29(27)40-32)19-20-39-24-17-15-22(16-18-24)21-28(31(37)38)33-26-12-6-5-11-25(26)30(36)23-9-3-2-4-10-23/h2-18,28,33H,19-21H2,1H3,(H,37,38)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50069035
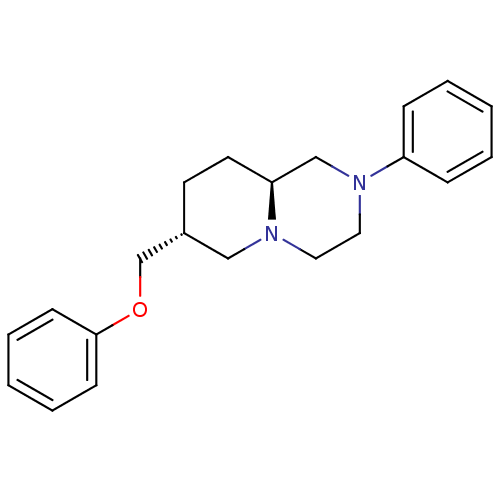
((7R,9aS)-7-Phenoxymethyl-2-phenyl-octahydro-pyrido...)Show SMILES C(Oc1ccccc1)[C@@H]1CC[C@H]2CN(CCN2C1)c1ccccc1 Show InChI InChI=1S/C21H26N2O/c1-3-7-19(8-4-1)23-14-13-22-15-18(11-12-20(22)16-23)17-24-21-9-5-2-6-10-21/h1-10,18,20H,11-17H2/t18-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human D4 dopamine receptor in CHO cells by [3H]-spiperone displacment. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471982
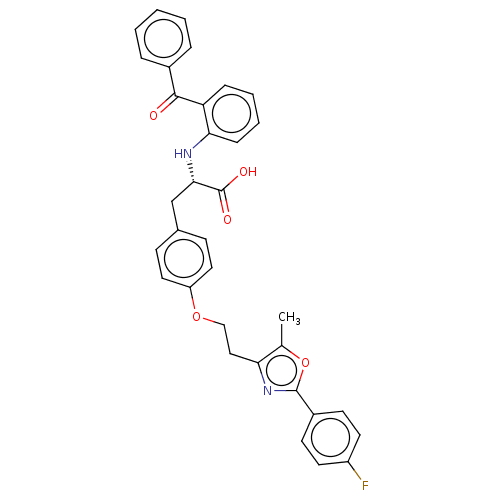
(CHEMBL147384)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C34H29FN2O5/c1-22-29(37-33(42-22)25-13-15-26(35)16-14-25)19-20-41-27-17-11-23(12-18-27)21-31(34(39)40)36-30-10-6-5-9-28(30)32(38)24-7-3-2-4-8-24/h2-18,31,36H,19-21H2,1H3,(H,39,40)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472035
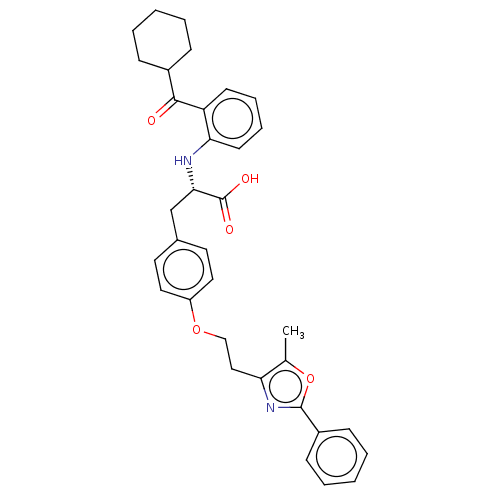
(CHEMBL2112870)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)C2CCCCC2)C(O)=O)cc1)-c1ccccc1 |r| Show InChI InChI=1S/C34H36N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h3,6-9,12-19,25,31,35H,2,4-5,10-11,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472034
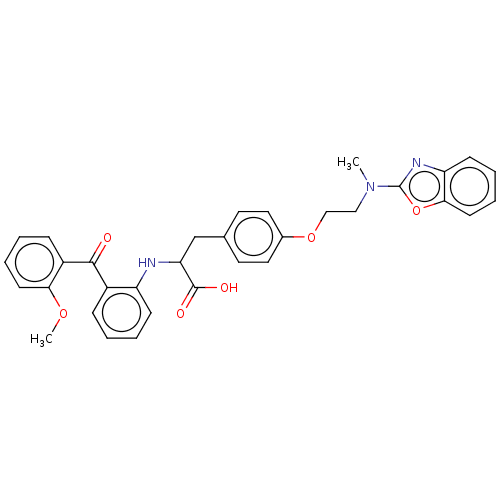
(CHEMBL147021)Show SMILES COc1ccccc1C(=O)c1ccccc1NC(Cc1ccc(OCCN(C)c2nc3ccccc3o2)cc1)C(O)=O Show InChI InChI=1S/C33H31N3O6/c1-36(33-35-27-12-6-8-14-30(27)42-33)19-20-41-23-17-15-22(16-18-23)21-28(32(38)39)34-26-11-5-3-9-24(26)31(37)25-10-4-7-13-29(25)40-2/h3-18,28,34H,19-21H2,1-2H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50069036
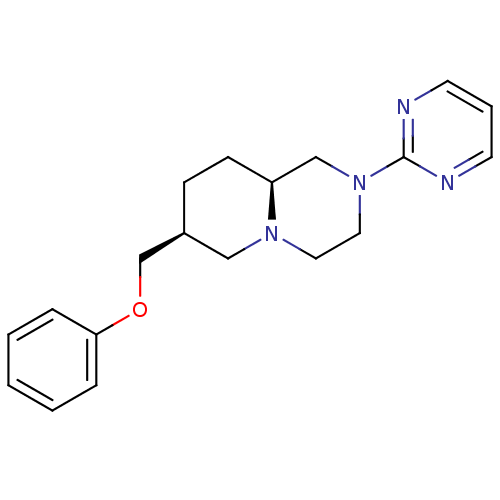
((7S,9aS)-7-Phenoxymethyl-2-pyrimidin-2-yl-octahydr...)Show SMILES C(Oc1ccccc1)[C@H]1CC[C@H]2CN(CCN2C1)c1ncccn1 Show InChI InChI=1S/C19H24N4O/c1-2-5-18(6-3-1)24-15-16-7-8-17-14-23(12-11-22(17)13-16)19-20-9-4-10-21-19/h1-6,9-10,16-17H,7-8,11-15H2/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human D4 dopamine receptor in CHO cells by [3H]-spiperone displacment. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50069037
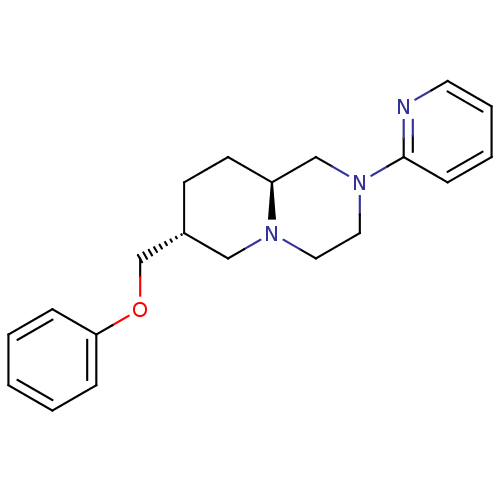
((7R,9aS)-7-Phenoxymethyl-2-pyridin-2-yl-octahydro-...)Show SMILES C(Oc1ccccc1)[C@@H]1CC[C@H]2CN(CCN2C1)c1ccccn1 Show InChI InChI=1S/C20H25N3O/c1-2-6-19(7-3-1)24-16-17-9-10-18-15-23(13-12-22(18)14-17)20-8-4-5-11-21-20/h1-8,11,17-18H,9-10,12-16H2/t17-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human D4 dopamine receptor in CHO cells by [3H]-spiperone displacment. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50069036

((7S,9aS)-7-Phenoxymethyl-2-pyrimidin-2-yl-octahydr...)Show SMILES C(Oc1ccccc1)[C@H]1CC[C@H]2CN(CCN2C1)c1ncccn1 Show InChI InChI=1S/C19H24N4O/c1-2-5-18(6-3-1)24-15-16-7-8-17-14-23(12-11-22(17)13-16)19-20-9-4-10-21-19/h1-6,9-10,16-17H,7-8,11-15H2/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human D4 dopamine receptor in CHO cells by [3H]-spiperone displacment. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471955
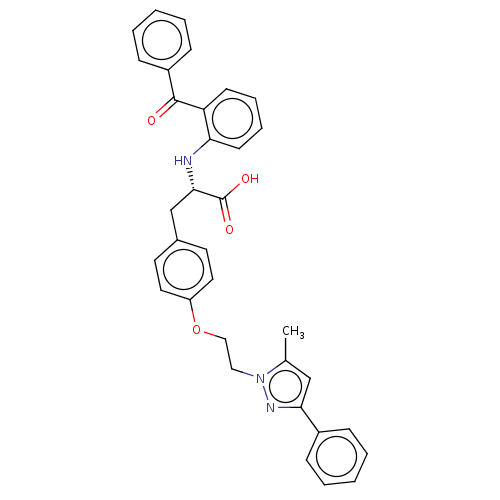
(CHEMBL148797)Show SMILES Cc1cc(nn1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H31N3O4/c1-24-22-31(26-10-4-2-5-11-26)36-37(24)20-21-41-28-18-16-25(17-19-28)23-32(34(39)40)35-30-15-9-8-14-29(30)33(38)27-12-6-3-7-13-27/h2-19,22,32,35H,20-21,23H2,1H3,(H,39,40)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472037

(CHEMBL414442)Show SMILES Cc1oc(nc1CCOc1ccc(CC(Nc2ccccc2C(=O)c2ccncc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C33H29N3O5/c1-22-28(36-32(41-22)25-7-3-2-4-8-25)17-20-40-26-13-11-23(12-14-26)21-30(33(38)39)35-29-10-6-5-9-27(29)31(37)24-15-18-34-19-16-24/h2-16,18-19,30,35H,17,20-21H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471970

(CHEMBL148459)Show SMILES Cc1ccsc1-c1nc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)c(C)o1 Show InChI InChI=1S/C33H30N2O5S/c1-21-17-19-41-31(21)32-35-27(22(2)40-32)16-18-39-25-14-12-23(13-15-25)20-29(33(37)38)34-28-11-7-6-10-26(28)30(36)24-8-4-3-5-9-24/h3-15,17,19,29,34H,16,18,20H2,1-2H3,(H,37,38)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50069039
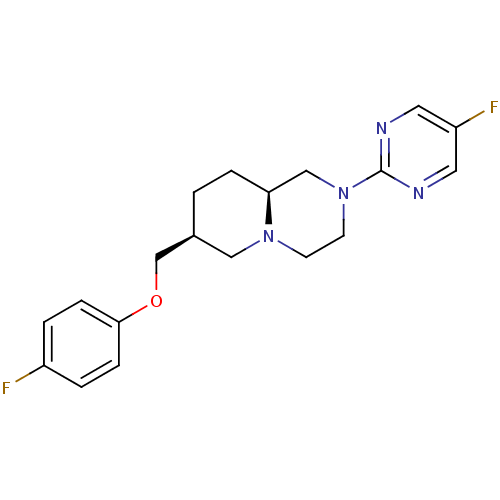
((7S,9aS)-7-(4-Fluoro-phenoxymethyl)-2-(5-fluoro-py...)Show SMILES Fc1ccc(OC[C@H]2CC[C@H]3CN(CCN3C2)c2ncc(F)cn2)cc1 Show InChI InChI=1S/C19H22F2N4O/c20-15-2-5-18(6-3-15)26-13-14-1-4-17-12-25(8-7-24(17)11-14)19-22-9-16(21)10-23-19/h2-3,5-6,9-10,14,17H,1,4,7-8,11-13H2/t14-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human D4 dopamine receptor in CHO cells by [3H]-spiperone displacment. |
Bioorg Med Chem Lett 8: 725-30 (1999)
BindingDB Entry DOI: 10.7270/Q29Z941K |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471948
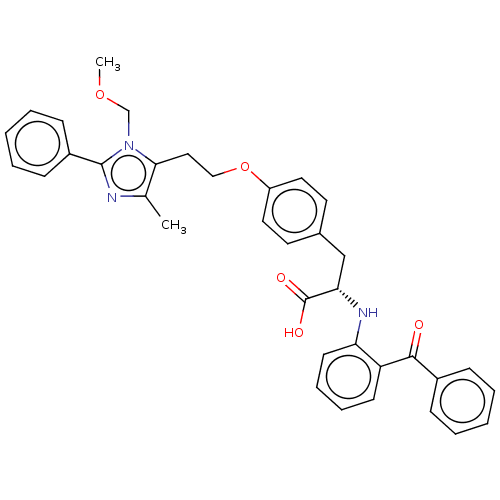
(CHEMBL149647)Show SMILES COCn1c(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)c(C)nc1-c1ccccc1 Show InChI InChI=1S/C36H35N3O5/c1-25-33(39(24-43-2)35(37-25)28-13-7-4-8-14-28)21-22-44-29-19-17-26(18-20-29)23-32(36(41)42)38-31-16-10-9-15-30(31)34(40)27-11-5-3-6-12-27/h3-20,32,38H,21-24H2,1-2H3,(H,41,42)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM85610
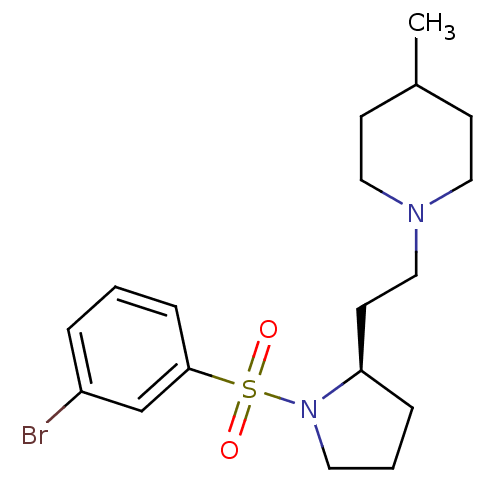
((R)-1-(2-(1-(3-bromo-benzenesulfonyl)-pyrrolidin-2...)Show SMILES [#6]-[#6]-1-[#6]-[#6]-[#7](-[#6]-[#6]-[#6@H]-2-[#6]-[#6]-[#6]-[#7]-2[S;v6](=O)(=O)c2cccc(Br)c2)-[#6]-[#6]-1 Show InChI InChI=1S/C18H27BrN2O2S/c1-15-7-11-20(12-8-15)13-9-17-5-3-10-21(17)24(22,23)18-6-2-4-16(19)14-18/h2,4,6,14-15,17H,3,5,7-13H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Med Chem 43: 342-5 (2000)
Article DOI: 10.1021/jm991151j
BindingDB Entry DOI: 10.7270/Q28S4NGP |
More data for this
Ligand-Target Pair | |
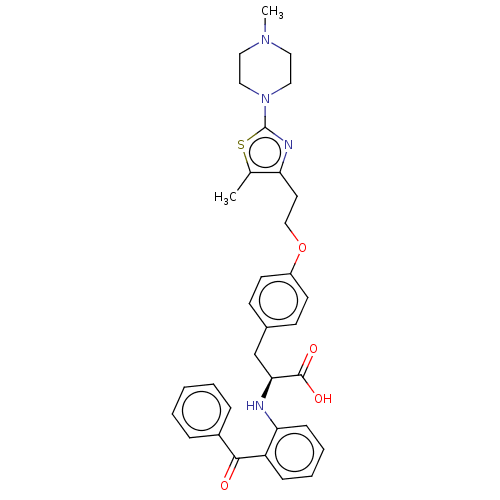
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471954
(CHEMBL358325)Show SMILES CN1CCN(CC1)c1nc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)c(C)s1 Show InChI InChI=1S/C33H36N4O4S/c1-23-28(35-33(42-23)37-19-17-36(2)18-20-37)16-21-41-26-14-12-24(13-15-26)22-30(32(39)40)34-29-11-7-6-10-27(29)31(38)25-8-4-3-5-9-25/h3-15,30,34H,16-22H2,1-2H3,(H,39,40)/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472047

(CHEMBL146355)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2C(=O)c2ccccc2C)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C33H31N3O5/c1-22-9-3-4-10-25(22)31(37)26-11-5-6-12-27(26)34-29(32(38)39)21-23-15-17-24(18-16-23)40-20-19-36(2)33-35-28-13-7-8-14-30(28)41-33/h3-18,29,34H,19-21H2,1-2H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472024

(CHEMBL145886)Show SMILES COc1cccc(c1)C(=O)c1ccccc1NC(Cc1ccc(OCCN(C)c2nc3ccccc3o2)cc1)C(O)=O Show InChI InChI=1S/C33H31N3O6/c1-36(33-35-28-12-5-6-13-30(28)42-33)18-19-41-24-16-14-22(15-17-24)20-29(32(38)39)34-27-11-4-3-10-26(27)31(37)23-8-7-9-25(21-23)40-2/h3-17,21,29,34H,18-20H2,1-2H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472048

(CHEMBL146562)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2C(=O)c2ccsc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C30H27N3O5S/c1-33(30-32-25-8-4-5-9-27(25)38-30)15-16-37-22-12-10-20(11-13-22)18-26(29(35)36)31-24-7-3-2-6-23(24)28(34)21-14-17-39-19-21/h2-14,17,19,26,31H,15-16,18H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472041

(CHEMBL147770)Show SMILES CCCOC(=O)c1ccccc1NC(Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C31H32N2O6/c1-3-18-38-31(36)25-11-7-8-12-27(25)32-28(30(34)35)20-22-13-15-24(16-14-22)37-19-17-26-21(2)39-29(33-26)23-9-5-4-6-10-23/h4-16,28,32H,3,17-20H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 125: 202-8 (1998)
Article DOI: 10.1038/sj.bjp.0702059
BindingDB Entry DOI: 10.7270/Q2PC30XV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 356: 312-20 (1997)
Article DOI: 10.1007/pl00005056
BindingDB Entry DOI: 10.7270/Q2KW5DKB |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471991
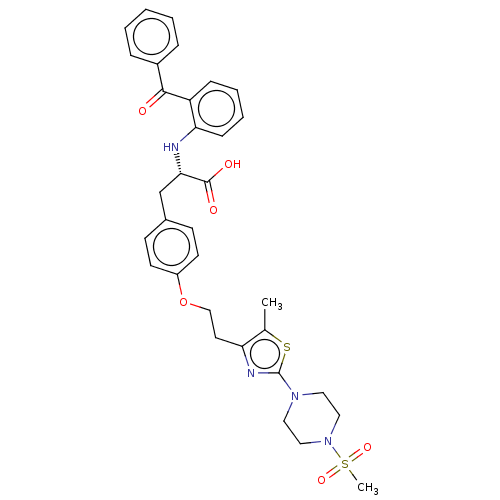
(CHEMBL146650)Show SMILES Cc1sc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C33H36N4O6S2/c1-23-28(35-33(44-23)36-17-19-37(20-18-36)45(2,41)42)16-21-43-26-14-12-24(13-15-26)22-30(32(39)40)34-29-11-7-6-10-27(29)31(38)25-8-4-3-5-9-25/h3-15,30,34H,16-22H2,1-2H3,(H,39,40)/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471989

(CHEMBL149835)Show SMILES Cc1[nH]c(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H31N3O4/c1-23-29(37-33(35-23)26-12-6-3-7-13-26)20-21-41-27-18-16-24(17-19-27)22-31(34(39)40)36-30-15-9-8-14-28(30)32(38)25-10-4-2-5-11-25/h2-19,31,36H,20-22H2,1H3,(H,35,37)(H,39,40)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472021

(CHEMBL146637)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2C(=O)c2cccc(c2)C(F)(F)F)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C33H28F3N3O5/c1-39(32-38-27-11-4-5-12-29(27)44-32)17-18-43-24-15-13-21(14-16-24)19-28(31(41)42)37-26-10-3-2-9-25(26)30(40)22-7-6-8-23(20-22)33(34,35)36/h2-16,20,28,37H,17-19H2,1H3,(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472029

(CHEMBL149780)Show SMILES CN(CCOc1ccc(CC(Nc2cscc2C(=O)c2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C30H27N3O5S/c1-33(30-32-24-9-5-6-10-27(24)38-30)15-16-37-22-13-11-20(12-14-22)17-25(29(35)36)31-26-19-39-18-23(26)28(34)21-7-3-2-4-8-21/h2-14,18-19,25,31H,15-17H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471963

(CHEMBL359285)Show SMILES COCCNc1nc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)c(C)s1 Show InChI InChI=1S/C31H33N3O5S/c1-21-26(34-31(40-21)32-17-19-38-2)16-18-39-24-14-12-22(13-15-24)20-28(30(36)37)33-27-11-7-6-10-25(27)29(35)23-8-4-3-5-9-23/h3-15,28,33H,16-20H2,1-2H3,(H,32,34)(H,36,37)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data