 Found 205 hits with Last Name = 'condon' and Initial = 'sl'
Found 205 hits with Last Name = 'condon' and Initial = 'sl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Renin
(Homo sapiens (Human)) | BDBM50046798
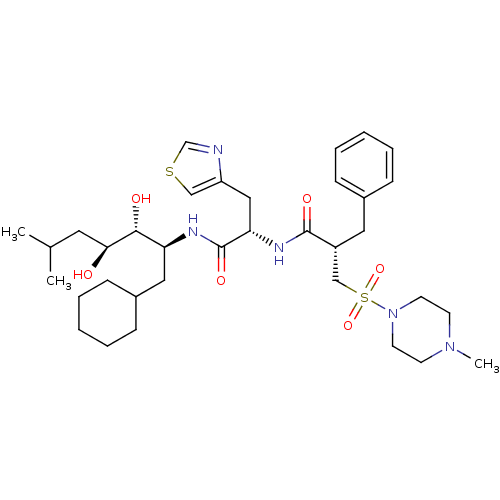
(2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@H](Cc1ccccc1)CS(=O)(=O)N1CCN(C)CC1 |r| Show InChI InChI=1S/C35H55N5O6S2/c1-25(2)18-32(41)33(42)30(20-27-12-8-5-9-13-27)37-35(44)31(21-29-22-47-24-36-29)38-34(43)28(19-26-10-6-4-7-11-26)23-48(45,46)40-16-14-39(3)15-17-40/h4,6-7,10-11,22,24-25,27-28,30-33,41-42H,5,8-9,12-21,23H2,1-3H3,(H,37,44)(H,38,43)/t28-,30+,31+,32+,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against monkey plasma renin at pH 7.4 |
J Med Chem 36: 460-7 (1993)
BindingDB Entry DOI: 10.7270/Q2VT1R5C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006122
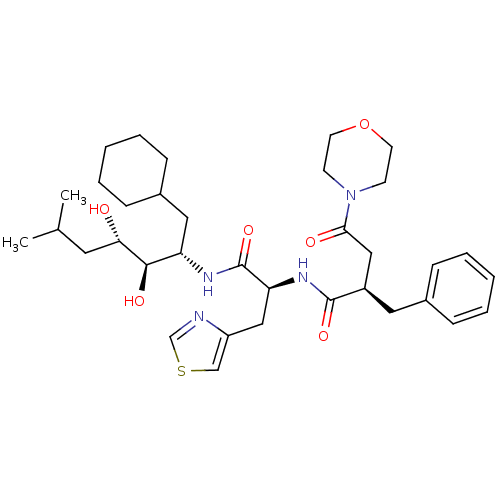
(2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@@H](CC(=O)N1CCOCC1)Cc1ccccc1 Show InChI InChI=1S/C35H52N4O6S/c1-24(2)17-31(40)33(42)29(19-26-11-7-4-8-12-26)37-35(44)30(21-28-22-46-23-36-28)38-34(43)27(18-25-9-5-3-6-10-25)20-32(41)39-13-15-45-16-14-39/h3,5-6,9-10,22-24,26-27,29-31,33,40,42H,4,7-8,11-21H2,1-2H3,(H,37,44)(H,38,43)/t27-,29+,30+,31+,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | 6.0 | n/a |
University of Texas
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human renin at a pH of 6.0. |
J Med Chem 35: 1710-21 (1992)
BindingDB Entry DOI: 10.7270/Q2GT5M4C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50280221

((1S,2S,3R)-2-Phenyl-3-(propane-2-sulfonyl)-cyclopr...)Show SMILES CCCC[C@H](NC(=O)[C@@H]1[C@H]([C@H]1S(=O)(=O)C(C)C)c1ccccc1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C33H54N2O6S/c1-6-7-18-25(32(38)35-26(20-23-14-10-8-11-15-23)30(37)27(36)19-21(2)3)34-33(39)29-28(24-16-12-9-13-17-24)31(29)42(40,41)22(4)5/h9,12-13,16-17,21-23,25-31,36-37H,6-8,10-11,14-15,18-20H2,1-5H3,(H,34,39)(H,35,38)/t25-,26-,27-,28+,29+,30+,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of human plasma renin at PH 7.4 |
Bioorg Med Chem Lett 2: 1405-1410 (1992)
Article DOI: 10.1016/S0960-894X(00)80522-3
BindingDB Entry DOI: 10.7270/Q2VD6ZBV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50280229

((1S,2S,3R)-2-Phenyl-3-(propane-2-sulfonyl)-cyclopr...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@@H]1[C@H]([C@H]1S(=O)(=O)C(C)C)c1ccccc1 Show InChI InChI=1S/C33H49N3O6S2/c1-20(2)15-27(37)30(38)25(16-22-11-7-5-8-12-22)35-32(39)26(17-24-18-43-19-34-24)36-33(40)29-28(23-13-9-6-10-14-23)31(29)44(41,42)21(3)4/h6,9-10,13-14,18-22,25-31,37-38H,5,7-8,11-12,15-17H2,1-4H3,(H,35,39)(H,36,40)/t25-,26-,27-,28+,29+,30+,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human renin at PH 6.0 |
Bioorg Med Chem Lett 2: 1405-1410 (1992)
Article DOI: 10.1016/S0960-894X(00)80522-3
BindingDB Entry DOI: 10.7270/Q2VD6ZBV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006125
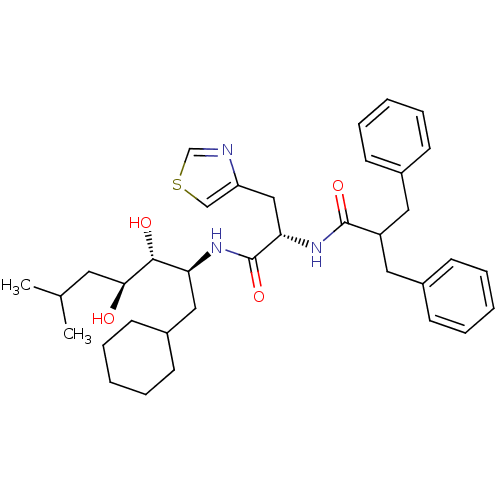
(2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)C(Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C36H49N3O4S/c1-25(2)18-33(40)34(41)31(21-28-16-10-5-11-17-28)38-36(43)32(22-30-23-44-24-37-30)39-35(42)29(19-26-12-6-3-7-13-26)20-27-14-8-4-9-15-27/h3-4,6-9,12-15,23-25,28-29,31-34,40-41H,5,10-11,16-22H2,1-2H3,(H,38,43)(H,39,42)/t31-,32-,33-,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | 6.0 | n/a |
University of Texas
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human renin at a pH of 6.0. |
J Med Chem 35: 1710-21 (1992)
BindingDB Entry DOI: 10.7270/Q2GT5M4C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50280237

((1S,2R,3S)-2-(2-Methoxymethoxy-ethanesulfonyl)-3-p...)Show SMILES COCOCCS(=O)(=O)[C@H]1[C@@H]([C@H]1c1ccccc1)C(=O)N[C@@H](Cc1cscn1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C34H51N3O8S2/c1-22(2)16-28(38)31(39)26(17-23-10-6-4-7-11-23)36-33(40)27(18-25-19-46-20-35-25)37-34(41)30-29(24-12-8-5-9-13-24)32(30)47(42,43)15-14-45-21-44-3/h5,8-9,12-13,19-20,22-23,26-32,38-39H,4,6-7,10-11,14-18,21H2,1-3H3,(H,36,40)(H,37,41)/t26-,27-,28-,29+,30+,31+,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human renin at PH 6.0 |
Bioorg Med Chem Lett 2: 1405-1410 (1992)
Article DOI: 10.1016/S0960-894X(00)80522-3
BindingDB Entry DOI: 10.7270/Q2VD6ZBV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50280235

((1S,2S,3R)-2-Phenyl-3-(propane-2-sulfonyl)-cyclopr...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1[C@H]([C@H]1S(=O)(=O)C(C)C)c1ccccc1 Show InChI InChI=1S/C33H54N2O6S/c1-20(2)17-26(32(38)34-25(19-23-13-9-7-10-14-23)30(37)27(36)18-21(3)4)35-33(39)29-28(24-15-11-8-12-16-24)31(29)42(40,41)22(5)6/h8,11-12,15-16,20-23,25-31,36-37H,7,9-10,13-14,17-19H2,1-6H3,(H,34,38)(H,35,39)/t25-,26-,27-,28+,29+,30+,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human renin at PH 6.0 |
Bioorg Med Chem Lett 2: 1405-1410 (1992)
Article DOI: 10.1016/S0960-894X(00)80522-3
BindingDB Entry DOI: 10.7270/Q2VD6ZBV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50280222
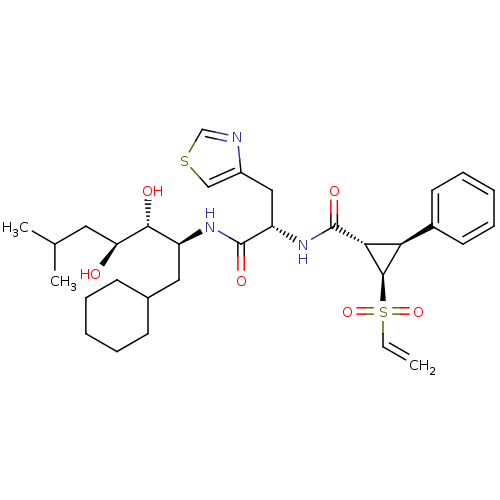
((1S,2R,3S)-2-Ethenesulfonyl-3-phenyl-cyclopropanec...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@@H]1[C@H]([C@H]1S(=O)(=O)C=C)c1ccccc1 Show InChI InChI=1S/C32H45N3O6S2/c1-4-43(40,41)30-27(22-13-9-6-10-14-22)28(30)32(39)35-25(17-23-18-42-19-33-23)31(38)34-24(16-21-11-7-5-8-12-21)29(37)26(36)15-20(2)3/h4,6,9-10,13-14,18-21,24-30,36-37H,1,5,7-8,11-12,15-17H2,2-3H3,(H,34,38)(H,35,39)/t24-,25-,26-,27+,28+,29+,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human renin at PH 6.0 |
Bioorg Med Chem Lett 2: 1405-1410 (1992)
Article DOI: 10.1016/S0960-894X(00)80522-3
BindingDB Entry DOI: 10.7270/Q2VD6ZBV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50280224

((1S,2R,3S)-2-Methanesulfonyl-3-phenyl-cyclopropane...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@@H]1[C@H]([C@H]1S(C)(=O)=O)c1ccccc1 Show InChI InChI=1S/C31H45N3O6S2/c1-19(2)14-25(35)28(36)23(15-20-10-6-4-7-11-20)33-30(37)24(16-22-17-41-18-32-22)34-31(38)27-26(29(27)42(3,39)40)21-12-8-5-9-13-21/h5,8-9,12-13,17-20,23-29,35-36H,4,6-7,10-11,14-16H2,1-3H3,(H,33,37)(H,34,38)/t23-,24-,25-,26+,27+,28+,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human renin at PH 6.0 |
Bioorg Med Chem Lett 2: 1405-1410 (1992)
Article DOI: 10.1016/S0960-894X(00)80522-3
BindingDB Entry DOI: 10.7270/Q2VD6ZBV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50280227
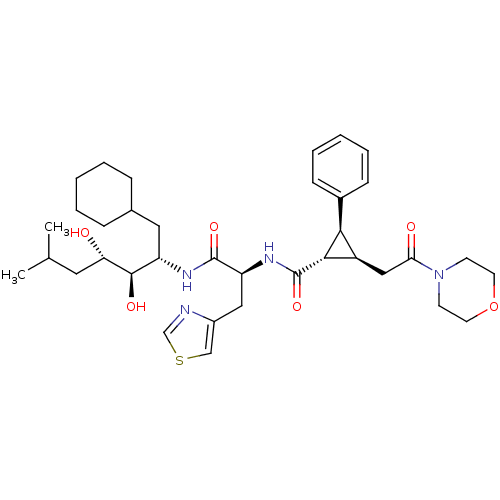
((1S,2R,3S)-2-(2-Morpholin-4-yl-2-oxo-ethyl)-3-phen...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@H]1[C@H](CC(=O)N2CCOCC2)[C@@H]1c1ccccc1 Show InChI InChI=1S/C36H52N4O6S/c1-23(2)17-30(41)34(43)28(18-24-9-5-3-6-10-24)38-35(44)29(19-26-21-47-22-37-26)39-36(45)33-27(32(33)25-11-7-4-8-12-25)20-31(42)40-13-15-46-16-14-40/h4,7-8,11-12,21-24,27-30,32-34,41,43H,3,5-6,9-10,13-20H2,1-2H3,(H,38,44)(H,39,45)/t27-,28+,29+,30+,32+,33+,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human renin at PH 6.0 |
Bioorg Med Chem Lett 2: 1405-1410 (1992)
Article DOI: 10.1016/S0960-894X(00)80522-3
BindingDB Entry DOI: 10.7270/Q2VD6ZBV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006138
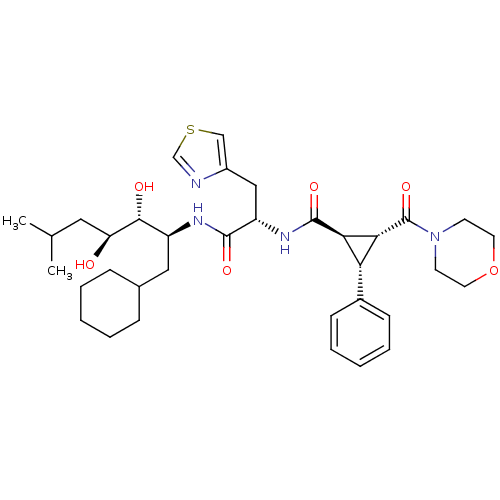
(2-(Morpholine-4-carbonyl)-3-phenyl-cyclopropanecar...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@H]1[C@@H]([C@@H]1c1ccccc1)C(=O)N1CCOCC1 Show InChI InChI=1S/C35H50N4O6S/c1-22(2)17-28(40)32(41)26(18-23-9-5-3-6-10-23)37-33(42)27(19-25-20-46-21-36-25)38-34(43)30-29(24-11-7-4-8-12-24)31(30)35(44)39-13-15-45-16-14-39/h4,7-8,11-12,20-23,26-32,40-41H,3,5-6,9-10,13-19H2,1-2H3,(H,37,42)(H,38,43)/t26-,27-,28-,29+,30+,31+,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 6.0 | n/a |
University of Texas
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at pH 7.4 |
J Med Chem 35: 1710-21 (1992)
BindingDB Entry DOI: 10.7270/Q2GT5M4C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50046799
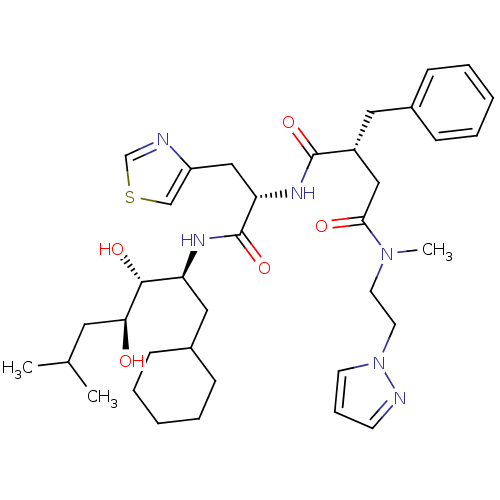
(2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@@H](CC(=O)N(C)CCn1cccn1)Cc1ccccc1 Show InChI InChI=1S/C37H54N6O5S/c1-26(2)19-33(44)35(46)31(21-28-13-8-5-9-14-28)40-37(48)32(23-30-24-49-25-38-30)41-36(47)29(20-27-11-6-4-7-12-27)22-34(45)42(3)17-18-43-16-10-15-39-43/h4,6-7,10-12,15-16,24-26,28-29,31-33,35,44,46H,5,8-9,13-14,17-23H2,1-3H3,(H,40,48)(H,41,47)/t29-,31+,32+,33+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma renin at pH 7.4 |
J Med Chem 36: 460-7 (1993)
BindingDB Entry DOI: 10.7270/Q2VT1R5C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50046794
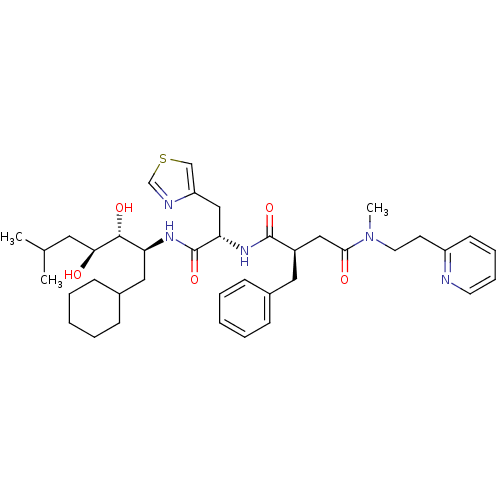
(2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@@H](CC(=O)N(C)CCc1ccccn1)Cc1ccccc1 Show InChI InChI=1S/C39H55N5O5S/c1-27(2)20-35(45)37(47)33(22-29-14-8-5-9-15-29)42-39(49)34(24-32-25-50-26-41-32)43-38(48)30(21-28-12-6-4-7-13-28)23-36(46)44(3)19-17-31-16-10-11-18-40-31/h4,6-7,10-13,16,18,25-27,29-30,33-35,37,45,47H,5,8-9,14-15,17,19-24H2,1-3H3,(H,42,49)(H,43,48)/t30-,33+,34+,35+,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against monkey plasma renin at pH 7.4 |
J Med Chem 36: 460-7 (1993)
BindingDB Entry DOI: 10.7270/Q2VT1R5C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50280219

(3-Benzenesulfonyl-2-benzyl-N-[(S)-1-((1S,2R,3S)-1-...)Show SMILES CO[C@@H](CC(C)C)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)C(Cc1ccccc1)CS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C37H51N3O6S2/c1-26(2)19-34(46-3)35(41)32(21-28-15-9-5-10-16-28)39-37(43)33(22-30-23-47-25-38-30)40-36(42)29(20-27-13-7-4-8-14-27)24-48(44,45)31-17-11-6-12-18-31/h4,6-8,11-14,17-18,23,25-26,28-29,32-35,41H,5,9-10,15-16,19-22,24H2,1-3H3,(H,39,43)(H,40,42)/t29?,32-,33-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human renin at PH 6.0 |
Bioorg Med Chem Lett 2: 1405-1410 (1992)
Article DOI: 10.1016/S0960-894X(00)80522-3
BindingDB Entry DOI: 10.7270/Q2VD6ZBV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50280231
((1S,2R,3R)-2-Cyclohexyl-3-(propane-2-sulfonyl)-cyc...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@@H]1[C@H]([C@H]1S(=O)(=O)C(C)C)C1CCCCC1 Show InChI InChI=1S/C33H55N3O6S2/c1-20(2)15-27(37)30(38)25(16-22-11-7-5-8-12-22)35-32(39)26(17-24-18-43-19-34-24)36-33(40)29-28(23-13-9-6-10-14-23)31(29)44(41,42)21(3)4/h18-23,25-31,37-38H,5-17H2,1-4H3,(H,35,39)(H,36,40)/t25-,26-,27-,28+,29+,30+,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human renin at PH 6.0 |
Bioorg Med Chem Lett 2: 1405-1410 (1992)
Article DOI: 10.1016/S0960-894X(00)80522-3
BindingDB Entry DOI: 10.7270/Q2VD6ZBV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50046802

(2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...)Show SMILES COCCOCOCCN(C)C(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C38H61N5O8/c1-27(2)19-34(44)36(46)32(21-29-13-9-6-10-14-29)41-38(48)33(23-31-24-39-25-40-31)42-37(47)30(20-28-11-7-5-8-12-28)22-35(45)43(3)15-16-50-26-51-18-17-49-4/h5,7-8,11-12,24-25,27,29-30,32-34,36,44,46H,6,9-10,13-23,26H2,1-4H3,(H,39,40)(H,41,48)(H,42,47)/t30-,32+,33+,34+,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma renin at pH 7.4 |
J Med Chem 36: 460-7 (1993)
BindingDB Entry DOI: 10.7270/Q2VT1R5C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50046796

((S)-N-[(S)-1-((1S,2R,3S)-1-Cyclohexylmethyl-2,3-di...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@H](Cc1ccccc1)CS(=O)(=O)C(C)(C)C Show InChI InChI=1S/C34H53N3O6S2/c1-23(2)16-30(38)31(39)28(18-25-14-10-7-11-15-25)36-33(41)29(19-27-20-44-22-35-27)37-32(40)26(17-24-12-8-6-9-13-24)21-45(42,43)34(3,4)5/h6,8-9,12-13,20,22-23,25-26,28-31,38-39H,7,10-11,14-19,21H2,1-5H3,(H,36,41)(H,37,40)/t26-,28+,29+,30+,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma renin at pH 7.4 |
J Med Chem 36: 460-7 (1993)
BindingDB Entry DOI: 10.7270/Q2VT1R5C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50046796

((S)-N-[(S)-1-((1S,2R,3S)-1-Cyclohexylmethyl-2,3-di...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@H](Cc1ccccc1)CS(=O)(=O)C(C)(C)C Show InChI InChI=1S/C34H53N3O6S2/c1-23(2)16-30(38)31(39)28(18-25-14-10-7-11-15-25)36-33(41)29(19-27-20-44-22-35-27)37-32(40)26(17-24-12-8-6-9-13-24)21-45(42,43)34(3,4)5/h6,8-9,12-13,20,22-23,25-26,28-31,38-39H,7,10-11,14-19,21H2,1-5H3,(H,36,41)(H,37,40)/t26-,28+,29+,30+,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | 7.4 | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of human plasma renin at PH 7.4 |
Bioorg Med Chem Lett 2: 1405-1410 (1992)
Article DOI: 10.1016/S0960-894X(00)80522-3
BindingDB Entry DOI: 10.7270/Q2VD6ZBV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006157
(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCCNC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCC)N[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(C)C Show InChI InChI=1S/C41H70N4O6/c1-6-8-20-35(43-37(27-32-18-14-11-15-19-32)41(49)45-24-21-33(22-25-45)51-29-50-5)40(48)44-36(26-31-16-12-10-13-17-31)38(46)28-34(30(3)4)39(47)42-23-9-7-2/h11,14-15,18-19,30-31,33-38,43,46H,6-10,12-13,16-17,20-29H2,1-5H3,(H,42,47)(H,44,48)/t34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against plasma renin at a pH of 7.4 |
J Med Chem 35: 1722-34 (1992)
BindingDB Entry DOI: 10.7270/Q2C24VCH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50006147
(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C34H56N2O7/c1-5-30(33(39)35-28(21-25-12-8-6-9-13-25)32(38)29(37)20-24(2)3)43-31(22-26-14-10-7-11-15-26)34(40)36-18-16-27(17-19-36)42-23-41-4/h7,10-11,14-15,24-25,27-32,37-38H,5-6,8-9,12-13,16-23H2,1-4H3,(H,35,39)/t28-,29-,30-,31-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against purified renin at a pH of 6.0. |
J Med Chem 35: 1722-34 (1992)
BindingDB Entry DOI: 10.7270/Q2C24VCH |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50280226

((1R,2S,3R)-2-(2-Methoxymethoxy-ethanesulfonyl)-3-p...)Show SMILES COCOCCS(=O)(=O)[C@@H]1[C@H]([C@@H]1c1ccccc1)C(=O)N[C@@H](Cc1cscn1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C34H51N3O8S2/c1-22(2)16-28(38)31(39)26(17-23-10-6-4-7-11-23)36-33(40)27(18-25-19-46-20-35-25)37-34(41)30-29(24-12-8-5-9-13-24)32(30)47(42,43)15-14-45-21-44-3/h5,8-9,12-13,19-20,22-23,26-32,38-39H,4,6-7,10-11,14-18,21H2,1-3H3,(H,36,40)(H,37,41)/t26-,27-,28-,29-,30-,31+,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human renin at PH 6.0 |
Bioorg Med Chem Lett 2: 1405-1410 (1992)
Article DOI: 10.1016/S0960-894X(00)80522-3
BindingDB Entry DOI: 10.7270/Q2VD6ZBV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006152
(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C36H60N2O7/c1-5-6-17-32(35(41)37-30(23-27-13-9-7-10-14-27)34(40)31(39)22-26(2)3)45-33(24-28-15-11-8-12-16-28)36(42)38-20-18-29(19-21-38)44-25-43-4/h8,11-12,15-16,26-27,29-34,39-40H,5-7,9-10,13-14,17-25H2,1-4H3,(H,37,41)/t30-,31-,32-,33-,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against plasma renin at a pH of 7.4 |
J Med Chem 35: 1722-34 (1992)
BindingDB Entry DOI: 10.7270/Q2C24VCH |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50046798

(2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@H](Cc1ccccc1)CS(=O)(=O)N1CCN(C)CC1 |r| Show InChI InChI=1S/C35H55N5O6S2/c1-25(2)18-32(41)33(42)30(20-27-12-8-5-9-13-27)37-35(44)31(21-29-22-47-24-36-29)38-34(43)28(19-26-10-6-4-7-11-26)23-48(45,46)40-16-14-39(3)15-17-40/h4,6-7,10-11,22,24-25,27-28,30-33,41-42H,5,8-9,12-21,23H2,1-3H3,(H,37,44)(H,38,43)/t28-,30+,31+,32+,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against human plasma renin at pH 7.4 |
J Med Chem 36: 460-7 (1993)
BindingDB Entry DOI: 10.7270/Q2VT1R5C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006123
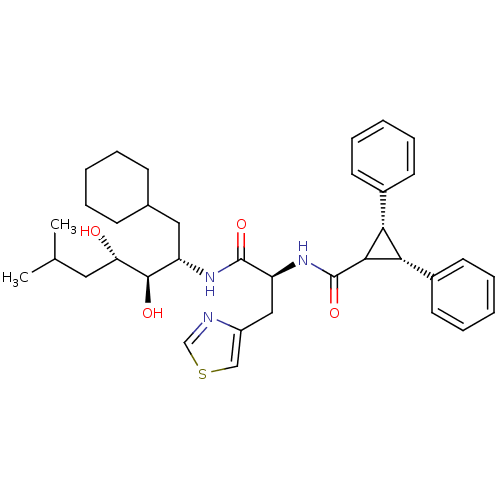
(2,3-Diphenyl-cyclopropanecarboxylic acid [1-(1-cyc...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)C1[C@@H]([C@@H]1c1ccccc1)c1ccccc1 Show InChI InChI=1S/C36H47N3O4S/c1-23(2)18-30(40)34(41)28(19-24-12-6-3-7-13-24)38-35(42)29(20-27-21-44-22-37-27)39-36(43)33-31(25-14-8-4-9-15-25)32(33)26-16-10-5-11-17-26/h4-5,8-11,14-17,21-24,28-34,40-41H,3,6-7,12-13,18-20H2,1-2H3,(H,38,42)(H,39,43)/t28-,29-,30-,31-,32+,33?,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 6.0 | n/a |
University of Texas
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human renin at a pH of 6.0. |
J Med Chem 35: 1710-21 (1992)
BindingDB Entry DOI: 10.7270/Q2GT5M4C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50046798

(2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@H](Cc1ccccc1)CS(=O)(=O)N1CCN(C)CC1 |r| Show InChI InChI=1S/C35H55N5O6S2/c1-25(2)18-32(41)33(42)30(20-27-12-8-5-9-13-27)37-35(44)31(21-29-22-47-24-36-29)38-34(43)28(19-26-10-6-4-7-11-26)23-48(45,46)40-16-14-39(3)15-17-40/h4,6-7,10-11,22,24-25,27-28,30-33,41-42H,5,8-9,12-21,23H2,1-3H3,(H,37,44)(H,38,43)/t28-,30+,31+,32+,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against human plasma renin at pH 7.4 |
J Med Chem 36: 460-7 (1993)
BindingDB Entry DOI: 10.7270/Q2VT1R5C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006161
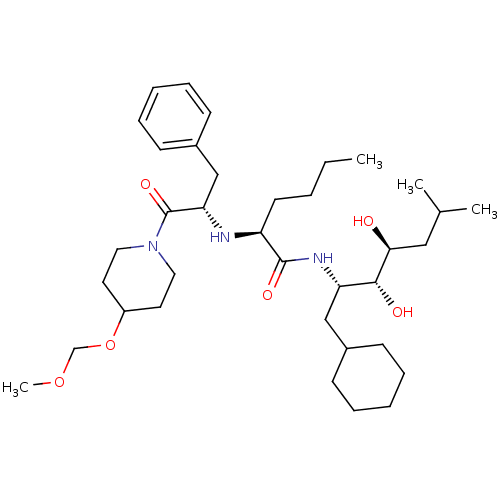
(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](N[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C36H61N3O6/c1-5-6-17-30(35(42)38-31(23-27-13-9-7-10-14-27)34(41)33(40)22-26(2)3)37-32(24-28-15-11-8-12-16-28)36(43)39-20-18-29(19-21-39)45-25-44-4/h8,11-12,15-16,26-27,29-34,37,40-41H,5-7,9-10,13-14,17-25H2,1-4H3,(H,38,42)/t30-,31-,32-,33-,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against purified renin at a pH of 7.4 |
J Med Chem 35: 1722-34 (1992)
BindingDB Entry DOI: 10.7270/Q2C24VCH |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50280236
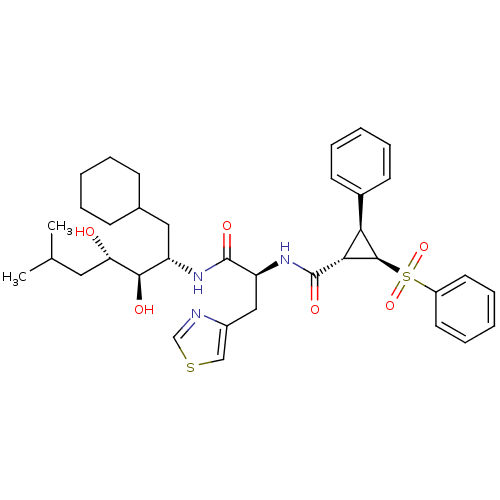
((1S,2R,3S)-2-Benzenesulfonyl-3-phenyl-cyclopropane...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@@H]1[C@H]([C@H]1S(=O)(=O)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C36H47N3O6S2/c1-23(2)18-30(40)33(41)28(19-24-12-6-3-7-13-24)38-35(42)29(20-26-21-46-22-37-26)39-36(43)32-31(25-14-8-4-9-15-25)34(32)47(44,45)27-16-10-5-11-17-27/h4-5,8-11,14-17,21-24,28-34,40-41H,3,6-7,12-13,18-20H2,1-2H3,(H,38,42)(H,39,43)/t28-,29-,30-,31+,32+,33+,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human renin at PH 6.0 |
Bioorg Med Chem Lett 2: 1405-1410 (1992)
Article DOI: 10.1016/S0960-894X(00)80522-3
BindingDB Entry DOI: 10.7270/Q2VD6ZBV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006148

(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCCNC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCC)O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(C)C Show InChI InChI=1S/C41H69N3O7/c1-6-8-20-37(51-38(27-32-18-14-11-15-19-32)41(48)44-24-21-33(22-25-44)50-29-49-5)40(47)43-35(26-31-16-12-10-13-17-31)36(45)28-34(30(3)4)39(46)42-23-9-7-2/h11,14-15,18-19,30-31,33-38,45H,6-10,12-13,16-17,20-29H2,1-5H3,(H,42,46)(H,43,47)/t34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against purified renin at a pH of 7.4 |
J Med Chem 35: 1722-34 (1992)
BindingDB Entry DOI: 10.7270/Q2C24VCH |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006150
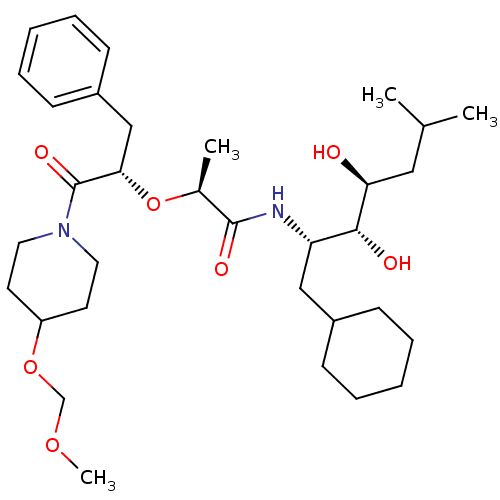
(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES COCOC1CCN(CC1)C(=O)[C@H](Cc1ccccc1)O[C@@H](C)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C33H54N2O7/c1-23(2)19-29(36)31(37)28(20-25-11-7-5-8-12-25)34-32(38)24(3)42-30(21-26-13-9-6-10-14-26)33(39)35-17-15-27(16-18-35)41-22-40-4/h6,9-10,13-14,23-25,27-31,36-37H,5,7-8,11-12,15-22H2,1-4H3,(H,34,38)/t24-,28-,29-,30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against plasma renin at a pH of 7.4 |
J Med Chem 35: 1722-34 (1992)
BindingDB Entry DOI: 10.7270/Q2C24VCH |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50046801
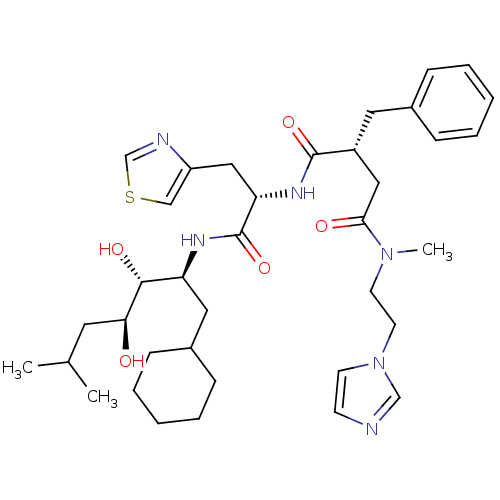
(2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@@H](CC(=O)N(C)CCn1ccnc1)Cc1ccccc1 Show InChI InChI=1S/C37H54N6O5S/c1-26(2)18-33(44)35(46)31(20-28-12-8-5-9-13-28)40-37(48)32(22-30-23-49-25-39-30)41-36(47)29(19-27-10-6-4-7-11-27)21-34(45)42(3)16-17-43-15-14-38-24-43/h4,6-7,10-11,14-15,23-26,28-29,31-33,35,44,46H,5,8-9,12-13,16-22H2,1-3H3,(H,40,48)(H,41,47)/t29-,31+,32+,33+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma renin at pH 7.4 |
J Med Chem 36: 460-7 (1993)
BindingDB Entry DOI: 10.7270/Q2VT1R5C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50280234
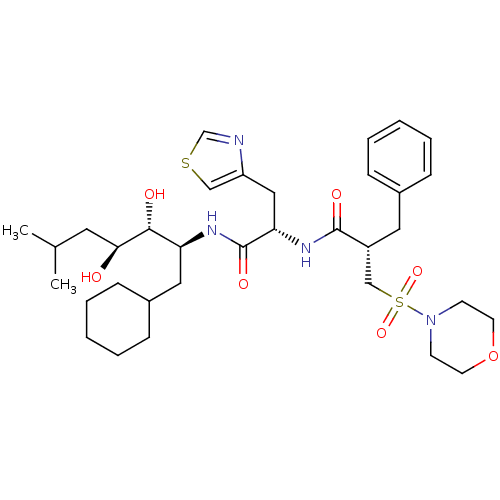
((S)-2-Benzyl-N-[(S)-1-((1S,2R,3S)-1-cyclohexylmeth...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@H](Cc1ccccc1)CS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C34H52N4O7S2/c1-24(2)17-31(39)32(40)29(19-26-11-7-4-8-12-26)36-34(42)30(20-28-21-46-23-35-28)37-33(41)27(18-25-9-5-3-6-10-25)22-47(43,44)38-13-15-45-16-14-38/h3,5-6,9-10,21,23-24,26-27,29-32,39-40H,4,7-8,11-20,22H2,1-2H3,(H,36,42)(H,37,41)/t27-,29+,30+,31+,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of human plasma renin at PH 7.4 |
Bioorg Med Chem Lett 2: 1405-1410 (1992)
Article DOI: 10.1016/S0960-894X(00)80522-3
BindingDB Entry DOI: 10.7270/Q2VD6ZBV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50280230

((1S,2S,3R)-2-Phenyl-3-(propane-2-sulfonyl)-cyclopr...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cn1ccnc1)NC(=O)[C@@H]1[C@H]([C@H]1S(=O)(=O)C(C)C)c1ccccc1 Show InChI InChI=1S/C33H50N4O6S/c1-21(2)17-27(38)30(39)25(18-23-11-7-5-8-12-23)35-32(40)26(19-37-16-15-34-20-37)36-33(41)29-28(24-13-9-6-10-14-24)31(29)44(42,43)22(3)4/h6,9-10,13-16,20-23,25-31,38-39H,5,7-8,11-12,17-19H2,1-4H3,(H,35,40)(H,36,41)/t25-,26-,27-,28+,29+,30+,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human renin at PH 6.0 |
Bioorg Med Chem Lett 2: 1405-1410 (1992)
Article DOI: 10.1016/S0960-894X(00)80522-3
BindingDB Entry DOI: 10.7270/Q2VD6ZBV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006141

(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C35H58N2O7/c1-5-12-31(34(40)36-29(22-26-13-8-6-9-14-26)33(39)30(38)21-25(2)3)44-32(23-27-15-10-7-11-16-27)35(41)37-19-17-28(18-20-37)43-24-42-4/h7,10-11,15-16,25-26,28-33,38-39H,5-6,8-9,12-14,17-24H2,1-4H3,(H,36,40)/t29-,30-,31-,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against plasma renin at a pH of 6.0 |
J Med Chem 35: 1722-34 (1992)
BindingDB Entry DOI: 10.7270/Q2C24VCH |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50046808
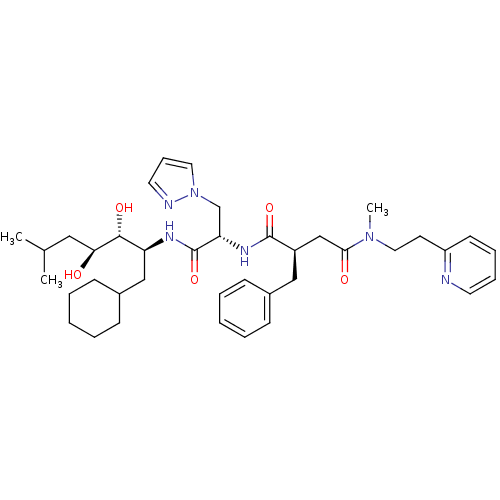
(2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cn1cccn1)NC(=O)[C@@H](CC(=O)N(C)CCc1ccccn1)Cc1ccccc1 Show InChI InChI=1S/C39H56N6O5/c1-28(2)23-35(46)37(48)33(25-30-15-8-5-9-16-30)42-39(50)34(27-45-21-12-20-41-45)43-38(49)31(24-29-13-6-4-7-14-29)26-36(47)44(3)22-18-32-17-10-11-19-40-32/h4,6-7,10-14,17,19-21,28,30-31,33-35,37,46,48H,5,8-9,15-16,18,22-27H2,1-3H3,(H,42,50)(H,43,49)/t31-,33+,34+,35+,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma renin at pH 7.4 |
J Med Chem 36: 460-7 (1993)
BindingDB Entry DOI: 10.7270/Q2VT1R5C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50046806
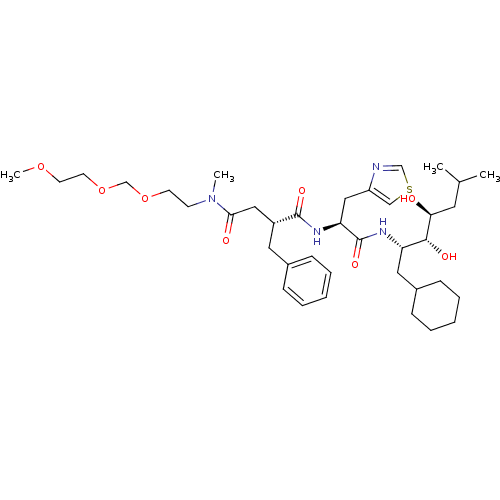
(2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...)Show SMILES COCCOCOCCN(C)C(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cscn1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C38H60N4O8S/c1-27(2)19-34(43)36(45)32(21-29-13-9-6-10-14-29)40-38(47)33(23-31-24-51-25-39-31)41-37(46)30(20-28-11-7-5-8-12-28)22-35(44)42(3)15-16-49-26-50-18-17-48-4/h5,7-8,11-12,24-25,27,29-30,32-34,36,43,45H,6,9-10,13-23,26H2,1-4H3,(H,40,47)(H,41,46)/t30-,32+,33+,34+,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma renin at pH 7.4 |
J Med Chem 36: 460-7 (1993)
BindingDB Entry DOI: 10.7270/Q2VT1R5C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50046805

(2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@H](Cc1ccccc1)CS(=O)(=O)N(C)CCc1ccccn1 Show InChI InChI=1S/C38H55N5O6S2/c1-27(2)20-35(44)36(45)33(22-29-14-8-5-9-15-29)41-38(47)34(23-32-24-50-26-40-32)42-37(46)30(21-28-12-6-4-7-13-28)25-51(48,49)43(3)19-17-31-16-10-11-18-39-31/h4,6-7,10-13,16,18,24,26-27,29-30,33-36,44-45H,5,8-9,14-15,17,19-23,25H2,1-3H3,(H,41,47)(H,42,46)/t30-,33+,34+,35+,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma renin at pH 7.4 |
J Med Chem 36: 460-7 (1993)
BindingDB Entry DOI: 10.7270/Q2VT1R5C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006174
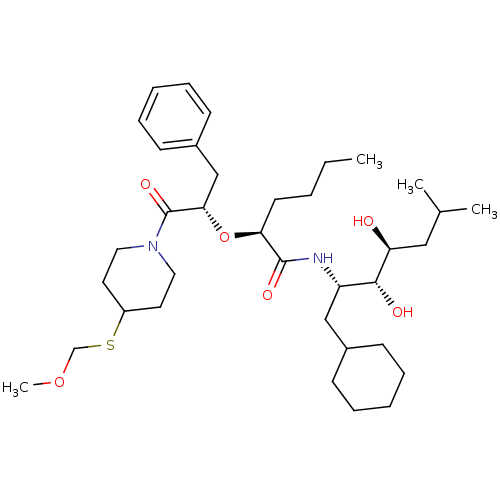
(2-[1-Benzyl-2-(4-methoxymethylsulfanyl-piperidin-1...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)SCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C36H60N2O6S/c1-5-6-17-32(35(41)37-30(23-27-13-9-7-10-14-27)34(40)31(39)22-26(2)3)44-33(24-28-15-11-8-12-16-28)36(42)38-20-18-29(19-21-38)45-25-43-4/h8,11-12,15-16,26-27,29-34,39-40H,5-7,9-10,13-14,17-25H2,1-4H3,(H,37,41)/t30-,31-,32-,33-,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against plasma renin at a pH of 7.4 |
J Med Chem 35: 1722-34 (1992)
BindingDB Entry DOI: 10.7270/Q2C24VCH |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50280232

((1R,2R,3S)-2-Phenyl-3-(propane-2-sulfonyl)-cyclopr...)Show SMILES CCCC[C@H](NC(=O)[C@H]1[C@@H]([C@@H]1S(=O)(=O)C(C)C)c1ccccc1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C33H54N2O6S/c1-6-7-18-25(32(38)35-26(20-23-14-10-8-11-15-23)30(37)27(36)19-21(2)3)34-33(39)29-28(24-16-12-9-13-17-24)31(29)42(40,41)22(4)5/h9,12-13,16-17,21-23,25-31,36-37H,6-8,10-11,14-15,18-20H2,1-5H3,(H,34,39)(H,35,38)/t25-,26-,27-,28-,29-,30+,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human renin at PH 6.0 |
Bioorg Med Chem Lett 2: 1405-1410 (1992)
Article DOI: 10.1016/S0960-894X(00)80522-3
BindingDB Entry DOI: 10.7270/Q2VD6ZBV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006128
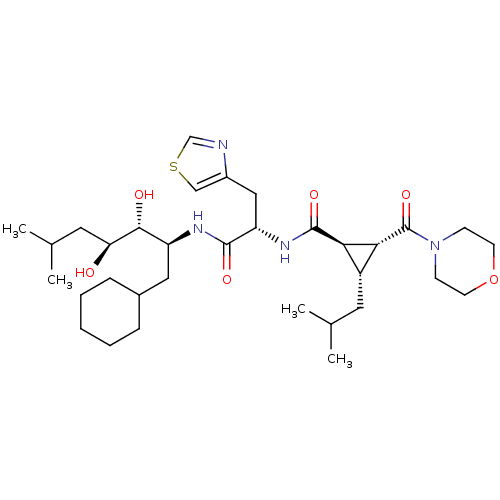
(2-Isobutyl-3-(morpholine-4-carbonyl)-cyclopropanec...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@@H]1[C@@H](CC(C)C)[C@H]1C(=O)N1CCOCC1 Show InChI InChI=1S/C33H54N4O6S/c1-20(2)14-24-28(29(24)33(42)37-10-12-43-13-11-37)32(41)36-26(17-23-18-44-19-34-23)31(40)35-25(16-22-8-6-5-7-9-22)30(39)27(38)15-21(3)4/h18-22,24-30,38-39H,5-17H2,1-4H3,(H,35,40)(H,36,41)/t24-,25+,26+,27+,28-,29-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 6.0 | n/a |
University of Texas
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human renin at a pH of 6.0. |
J Med Chem 35: 1710-21 (1992)
BindingDB Entry DOI: 10.7270/Q2GT5M4C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006132
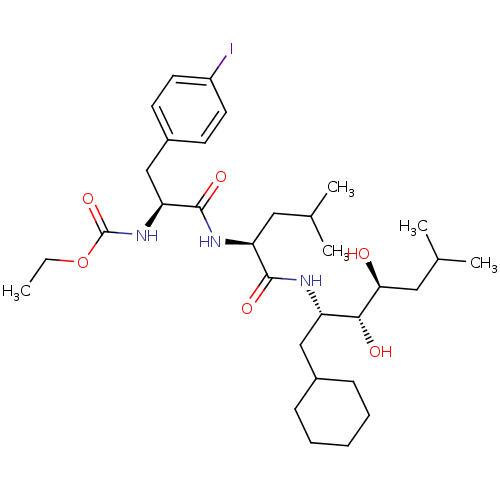
(CHEMBL297929 | [1-[1-(1-Cyclohexylmethyl-2,3-dihyd...)Show SMILES CCOC(=O)N[C@@H](Cc1ccc(I)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C32H52IN3O6/c1-6-42-32(41)36-27(19-23-12-14-24(33)15-13-23)31(40)35-26(16-20(2)3)30(39)34-25(18-22-10-8-7-9-11-22)29(38)28(37)17-21(4)5/h12-15,20-22,25-29,37-38H,6-11,16-19H2,1-5H3,(H,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 6.0 | n/a |
University of Texas
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human renin at a pH of 6.0. |
J Med Chem 35: 1710-21 (1992)
BindingDB Entry DOI: 10.7270/Q2GT5M4C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50280238
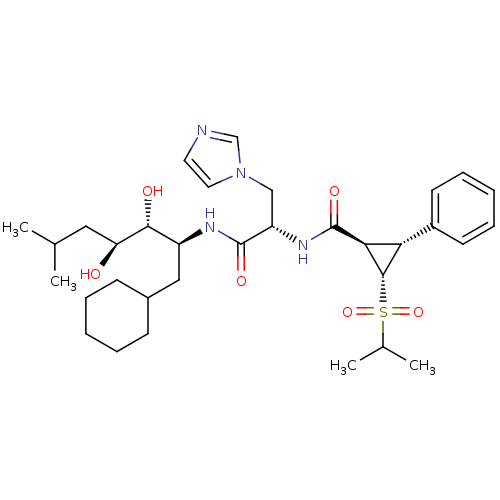
((1R,2R,3S)-2-Phenyl-3-(propane-2-sulfonyl)-cyclopr...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cn1ccnc1)NC(=O)[C@H]1[C@@H]([C@@H]1S(=O)(=O)C(C)C)c1ccccc1 Show InChI InChI=1S/C33H50N4O6S/c1-21(2)17-27(38)30(39)25(18-23-11-7-5-8-12-23)35-32(40)26(19-37-16-15-34-20-37)36-33(41)29-28(24-13-9-6-10-14-24)31(29)44(42,43)22(3)4/h6,9-10,13-16,20-23,25-31,38-39H,5,7-8,11-12,17-19H2,1-4H3,(H,35,40)(H,36,41)/t25-,26-,27-,28-,29-,30+,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of human plasma renin at PH 7.4 |
Bioorg Med Chem Lett 2: 1405-1410 (1992)
Article DOI: 10.1016/S0960-894X(00)80522-3
BindingDB Entry DOI: 10.7270/Q2VD6ZBV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50046794

(2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@@H](CC(=O)N(C)CCc1ccccn1)Cc1ccccc1 Show InChI InChI=1S/C39H55N5O5S/c1-27(2)20-35(45)37(47)33(22-29-14-8-5-9-15-29)42-39(49)34(24-32-25-50-26-41-32)43-38(48)30(21-28-12-6-4-7-13-28)23-36(46)44(3)19-17-31-16-10-11-18-40-31/h4,6-7,10-13,16,18,25-27,29-30,33-35,37,45,47H,5,8-9,14-15,17,19-24H2,1-3H3,(H,42,49)(H,43,48)/t30-,33+,34+,35+,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against human plasma renin at pH 7.4 |
J Med Chem 36: 460-7 (1993)
BindingDB Entry DOI: 10.7270/Q2VT1R5C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006161

(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](N[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C36H61N3O6/c1-5-6-17-30(35(42)38-31(23-27-13-9-7-10-14-27)34(41)33(40)22-26(2)3)37-32(24-28-15-11-8-12-16-28)36(43)39-20-18-29(19-21-39)45-25-44-4/h8,11-12,15-16,26-27,29-34,37,40-41H,5-7,9-10,13-14,17-25H2,1-4H3,(H,38,42)/t30-,31-,32-,33-,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against purified renin at a pH of 7.4 |
J Med Chem 35: 1722-34 (1992)
BindingDB Entry DOI: 10.7270/Q2C24VCH |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006152

(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C36H60N2O7/c1-5-6-17-32(35(41)37-30(23-27-13-9-7-10-14-27)34(40)31(39)22-26(2)3)45-33(24-28-15-11-8-12-16-28)36(42)38-20-18-29(19-21-38)44-25-43-4/h8,11-12,15-16,26-27,29-34,39-40H,5-7,9-10,13-14,17-25H2,1-4H3,(H,37,41)/t30-,31-,32-,33-,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against plasma renin at a pH of 6.0 |
J Med Chem 35: 1722-34 (1992)
BindingDB Entry DOI: 10.7270/Q2C24VCH |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50046800

(2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@@H](CC(=O)N(C)CCc1ccncc1)Cc1ccccc1 Show InChI InChI=1S/C39H55N5O5S/c1-27(2)20-35(45)37(47)33(22-30-12-8-5-9-13-30)42-39(49)34(24-32-25-50-26-41-32)43-38(48)31(21-29-10-6-4-7-11-29)23-36(46)44(3)19-16-28-14-17-40-18-15-28/h4,6-7,10-11,14-15,17-18,25-27,30-31,33-35,37,45,47H,5,8-9,12-13,16,19-24H2,1-3H3,(H,42,49)(H,43,48)/t31-,33+,34+,35+,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma renin at pH 7.4 |
J Med Chem 36: 460-7 (1993)
BindingDB Entry DOI: 10.7270/Q2VT1R5C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006152

(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C36H60N2O7/c1-5-6-17-32(35(41)37-30(23-27-13-9-7-10-14-27)34(40)31(39)22-26(2)3)45-33(24-28-15-11-8-12-16-28)36(42)38-20-18-29(19-21-38)44-25-43-4/h8,11-12,15-16,26-27,29-34,39-40H,5-7,9-10,13-14,17-25H2,1-4H3,(H,37,41)/t30-,31-,32-,33-,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against purified renin at a pH of 6.0. |
J Med Chem 35: 1722-34 (1992)
BindingDB Entry DOI: 10.7270/Q2C24VCH |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006141

(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C35H58N2O7/c1-5-12-31(34(40)36-29(22-26-13-8-6-9-14-26)33(39)30(38)21-25(2)3)44-32(23-27-15-10-7-11-16-27)35(41)37-19-17-28(18-20-37)43-24-42-4/h7,10-11,15-16,25-26,28-33,38-39H,5-6,8-9,12-14,17-24H2,1-4H3,(H,36,40)/t29-,30-,31-,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against plasma renin at a pH of 6.0 |
J Med Chem 35: 1722-34 (1992)
BindingDB Entry DOI: 10.7270/Q2C24VCH |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006161

(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](N[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C36H61N3O6/c1-5-6-17-30(35(42)38-31(23-27-13-9-7-10-14-27)34(41)33(40)22-26(2)3)37-32(24-28-15-11-8-12-16-28)36(43)39-20-18-29(19-21-39)45-25-44-4/h8,11-12,15-16,26-27,29-34,37,40-41H,5-7,9-10,13-14,17-25H2,1-4H3,(H,38,42)/t30-,31-,32-,33-,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against purified renin at a pH of 6.0. |
J Med Chem 35: 1722-34 (1992)
BindingDB Entry DOI: 10.7270/Q2C24VCH |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50046809
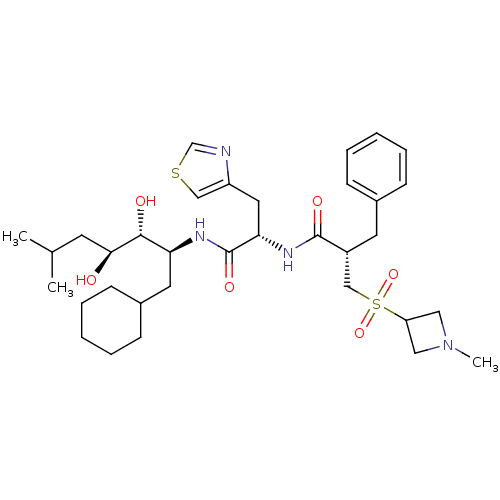
(2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@H](Cc1ccccc1)CS(=O)(=O)C1CN(C)C1 Show InChI InChI=1S/C34H52N4O6S2/c1-23(2)14-31(39)32(40)29(16-25-12-8-5-9-13-25)36-34(42)30(17-27-20-45-22-35-27)37-33(41)26(15-24-10-6-4-7-11-24)21-46(43,44)28-18-38(3)19-28/h4,6-7,10-11,20,22-23,25-26,28-32,39-40H,5,8-9,12-19,21H2,1-3H3,(H,36,42)(H,37,41)/t26-,29+,30+,31+,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma renin at pH 7.4 |
J Med Chem 36: 460-7 (1993)
BindingDB Entry DOI: 10.7270/Q2VT1R5C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50046811
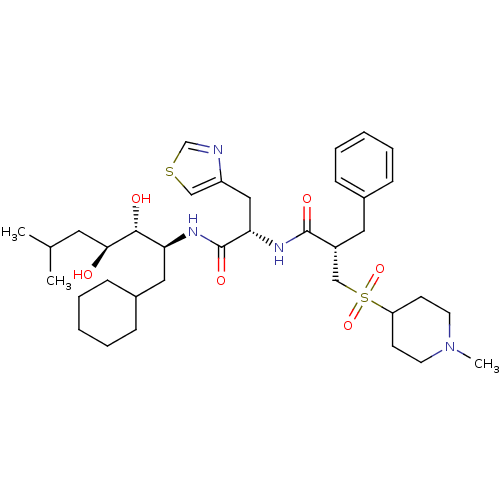
(2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@H](Cc1ccccc1)CS(=O)(=O)C1CCN(C)CC1 Show InChI InChI=1S/C36H56N4O6S2/c1-25(2)18-33(41)34(42)31(20-27-12-8-5-9-13-27)38-36(44)32(21-29-22-47-24-37-29)39-35(43)28(19-26-10-6-4-7-11-26)23-48(45,46)30-14-16-40(3)17-15-30/h4,6-7,10-11,22,24-25,27-28,30-34,41-42H,5,8-9,12-21,23H2,1-3H3,(H,38,44)(H,39,43)/t28-,31+,32+,33+,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma renin at pH 7.4 |
J Med Chem 36: 460-7 (1993)
BindingDB Entry DOI: 10.7270/Q2VT1R5C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data