 Found 919 hits with Last Name = 'cornelius' and Initial = 'g'
Found 919 hits with Last Name = 'cornelius' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Androgen receptor
(Homo sapiens (Human)) | BDBM50122646

(CHEMBL3623125)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(=O)(=O)c1ccccc1F)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C24H19F4N3O5S/c1-22-10-17(30-37(34,35)16-6-4-3-5-15(16)25)23(2,36-22)19-18(22)20(32)31(21(19)33)13-8-7-12(11-29)14(9-13)24(26,27)28/h3-9,17-19,30H,10H2,1-2H3/t17-,18-,19+,22+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122650
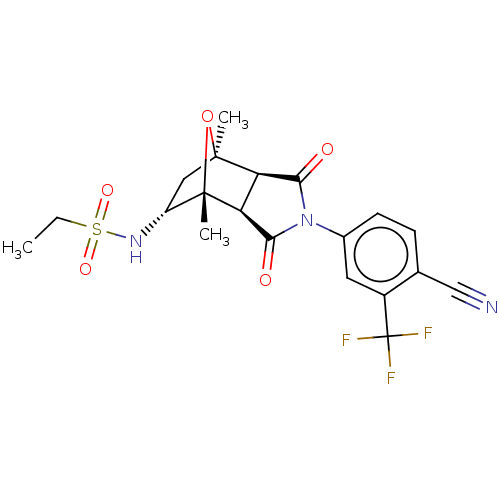
(CHEMBL3623127)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(=O)(=O)CC)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C20H20F3N3O5S/c1-4-32(29,30)25-13-8-18(2)14-15(19(13,3)31-18)17(28)26(16(14)27)11-6-5-10(9-24)12(7-11)20(21,22)23/h5-7,13-15,25H,4,8H2,1-3H3/t13-,14-,15+,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122647
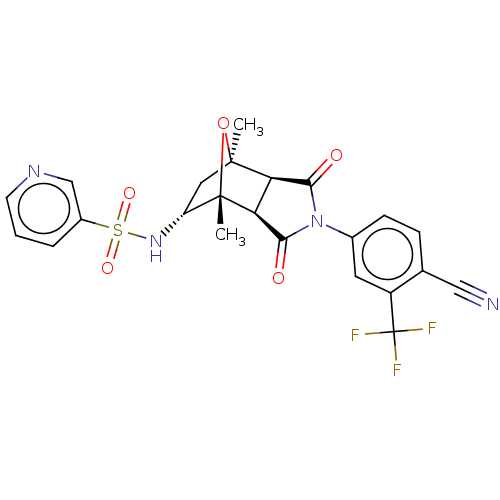
(CHEMBL3623126)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(=O)(=O)c1cccnc1)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C23H19F3N4O5S/c1-21-9-16(29-36(33,34)14-4-3-7-28-11-14)22(2,35-21)18-17(21)19(31)30(20(18)32)13-6-5-12(10-27)15(8-13)23(24,25)26/h3-8,11,16-18,29H,9H2,1-2H3/t16-,17-,18+,21+,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122640

(CHEMBL3623119)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NC(=O)CC)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C21H20F3N3O4/c1-4-14(28)26-13-8-19(2)15-16(20(13,3)31-19)18(30)27(17(15)29)11-6-5-10(9-25)12(7-11)21(22,23)24/h5-7,13,15-16H,4,8H2,1-3H3,(H,26,28)/t13-,15-,16+,19+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122643

(CHEMBL3623122)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NC(=O)ON1CCN(C)CC1)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C24H26F3N5O5/c1-22-11-16(29-21(35)36-31-8-6-30(3)7-9-31)23(2,37-22)18-17(22)19(33)32(20(18)34)14-5-4-13(12-28)15(10-14)24(25,26)27/h4-5,10,16-18H,6-9,11H2,1-3H3,(H,29,35)/t16-,17-,18+,22+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122635
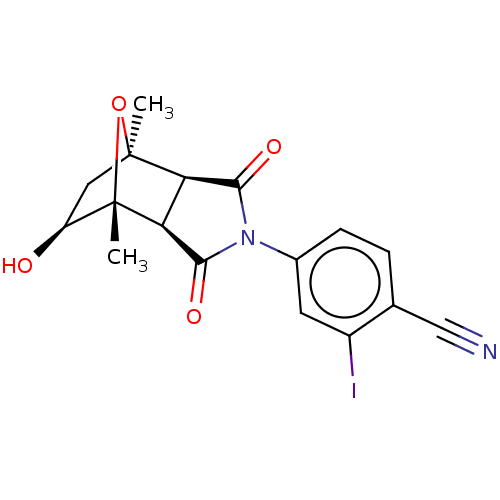
(CHEMBL3623114)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@@H]1O)c1ccc(C#N)c(I)c1 |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C17H15IN2O4/c1-16-6-11(21)17(2,24-16)13-12(16)14(22)20(15(13)23)9-4-3-8(7-19)10(18)5-9/h3-5,11-13,21H,6H2,1-2H3/t11-,12+,13-,16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122645
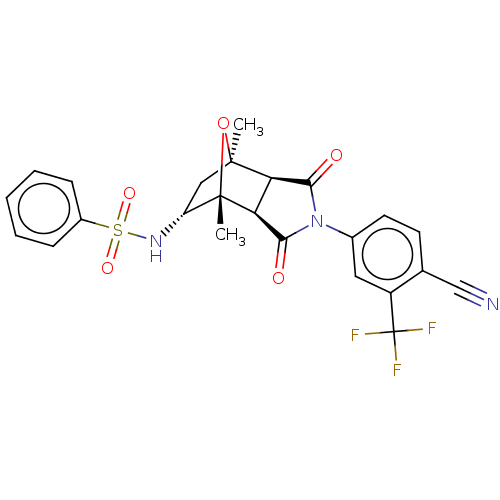
(CHEMBL3623124)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(=O)(=O)c1ccccc1)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C24H20F3N3O5S/c1-22-11-17(29-36(33,34)15-6-4-3-5-7-15)23(2,35-22)19-18(22)20(31)30(21(19)32)14-9-8-13(12-28)16(10-14)24(25,26)27/h3-10,17-19,29H,11H2,1-2H3/t17-,18-,19+,22+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122644
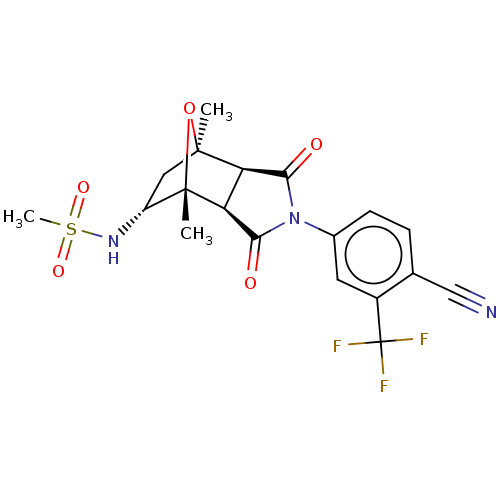
(CHEMBL3623123)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(C)(=O)=O)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C19H18F3N3O5S/c1-17-7-12(24-31(3,28)29)18(2,30-17)14-13(17)15(26)25(16(14)27)10-5-4-9(8-23)11(6-10)19(20,21)22/h4-6,12-14,24H,7H2,1-3H3/t12-,13-,14+,17+,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122637

(CHEMBL3623116)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1C(=O)OC(C)C)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C22H21F3N2O5/c1-10(2)31-19(30)14-8-20(3)15-16(21(14,4)32-20)18(29)27(17(15)28)12-6-5-11(9-26)13(7-12)22(23,24)25/h5-7,10,14-16H,8H2,1-4H3/t14-,15+,16-,20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122642

(CHEMBL3623121)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NC(=O)NC(C)C)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C22H23F3N4O4/c1-10(2)27-19(32)28-14-8-20(3)15-16(21(14,4)33-20)18(31)29(17(15)30)12-6-5-11(9-26)13(7-12)22(23,24)25/h5-7,10,14-16H,8H2,1-4H3,(H2,27,28,32)/t14-,15-,16+,20+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122639

(CHEMBL3623118)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1N)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C18H16F3N3O3/c1-16-6-11(23)17(2,27-16)13-12(16)14(25)24(15(13)26)9-4-3-8(7-22)10(5-9)18(19,20)21/h3-5,11-13H,6,23H2,1-2H3/t11-,12-,13+,16+,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122638
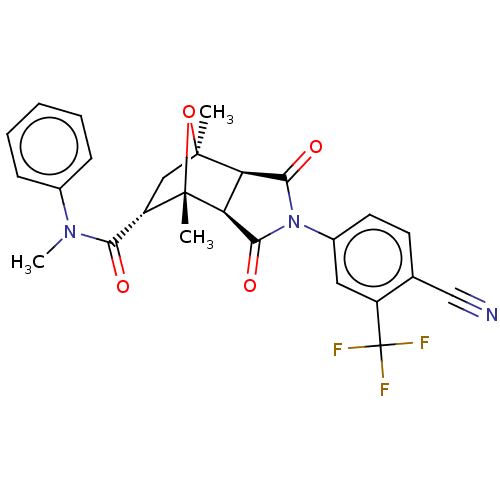
(CHEMBL3623117)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1C(=O)N(C)c1ccccc1)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C26H22F3N3O4/c1-24-12-18(21(33)31(3)15-7-5-4-6-8-15)25(2,36-24)20-19(24)22(34)32(23(20)35)16-10-9-14(13-30)17(11-16)26(27,28)29/h4-11,18-20H,12H2,1-3H3/t18-,19+,20-,24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122641

(CHEMBL3623120)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(=O)(=O)NC)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C19H19F3N4O5S/c1-17-7-12(25-32(29,30)24-3)18(2,31-17)14-13(17)15(27)26(16(14)28)10-5-4-9(8-23)11(6-10)19(20,21)22/h4-6,12-14,24-25H,7H2,1-3H3/t12-,13-,14+,17+,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18525
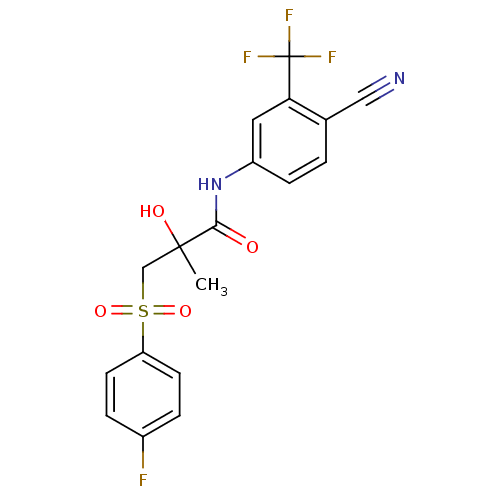
(Bicalutamide | CHEMBL409 | N-[4-cyano-3-(trifluoro...)Show SMILES CC(O)(CS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C18H14F4N2O4S/c1-17(26,10-29(27,28)14-6-3-12(19)4-7-14)16(25)24-13-5-2-11(9-23)15(8-13)18(20,21)22/h2-8,26H,10H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122636
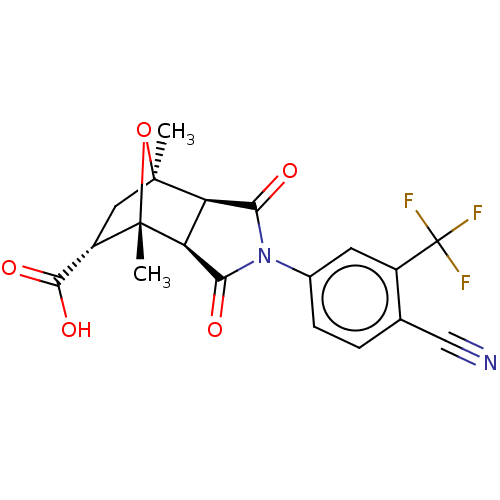
(CHEMBL3623115)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1C(O)=O)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C19H15F3N2O5/c1-17-6-11(16(27)28)18(2,29-17)13-12(17)14(25)24(15(13)26)9-4-3-8(7-23)10(5-9)19(20,21)22/h3-5,11-13H,6H2,1-2H3,(H,27,28)/t11-,12+,13-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DHT from androgen receptor in human MDA-MB-453 cells after 90 mins by TopCount analysis |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount scintillation counting metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01109
BindingDB Entry DOI: 10.7270/Q28919KX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of 33-P-S1P from from human S1P receptor expressed in CHO cell membranes after 50 mins by scintillation counting |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount scintillation counting metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01109
BindingDB Entry DOI: 10.7270/Q28919KX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of 33-P-S1P from from human S1P receptor expressed in CHO cell membranes after 50 mins by scintillation counting |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM258470
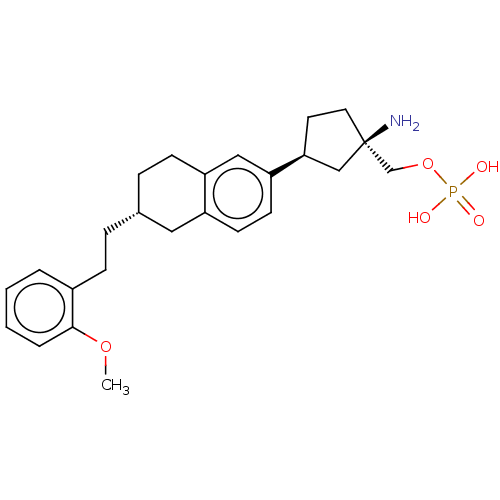
(US9522888, 697)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C25H34NO5P/c1-30-24-5-3-2-4-19(24)8-6-18-7-9-21-15-22(11-10-20(21)14-18)23-12-13-25(26,16-23)17-31-32(27,28)29/h2-5,10-11,15,18,23H,6-9,12-14,16-17,26H2,1H3,(H2,27,28,29)/t18-,23+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM258466
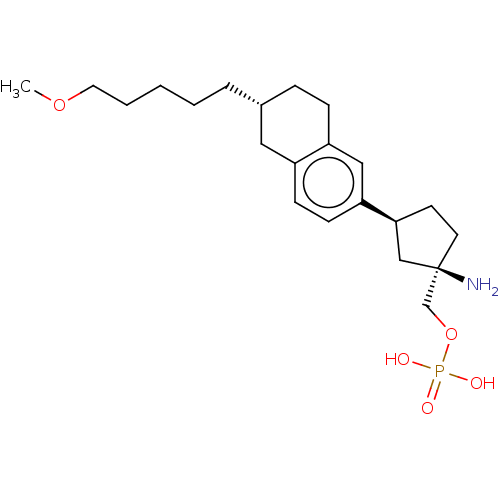
(US9522888, 689)Show SMILES COCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO5P/c1-27-12-4-2-3-5-17-6-7-19-14-20(9-8-18(19)13-17)21-10-11-22(23,15-21)16-28-29(24,25)26/h8-9,14,17,21H,2-7,10-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount scintillation counting metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01109
BindingDB Entry DOI: 10.7270/Q28919KX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50562873
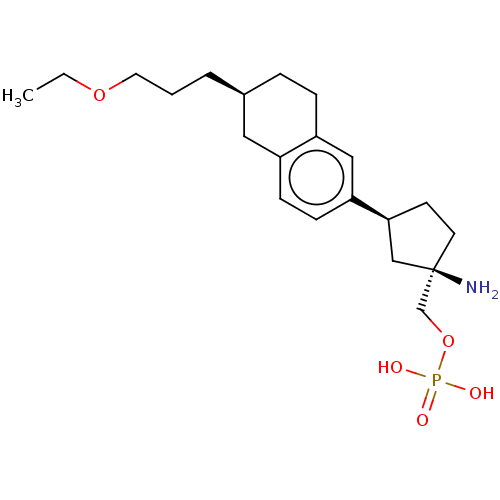
(CHEMBL4786296)Show SMILES CCOCCC[C@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount scintillation counting metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01109
BindingDB Entry DOI: 10.7270/Q28919KX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532543
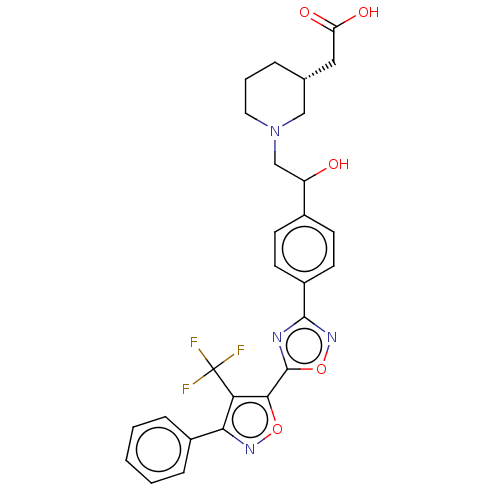
(CHEMBL4474984)Show SMILES OC(CN1CCC[C@H](CC(O)=O)C1)c1ccc(cc1)-c1noc(n1)-c1onc(c1C(F)(F)F)-c1ccccc1 |r| Show InChI InChI=1S/C27H25F3N4O5/c28-27(29,30)22-23(18-6-2-1-3-7-18)32-38-24(22)26-31-25(33-39-26)19-10-8-17(9-11-19)20(35)15-34-12-4-5-16(14-34)13-21(36)37/h1-3,6-11,16,20,35H,4-5,12-15H2,(H,36,37)/t16-,20?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532543

(CHEMBL4474984)Show SMILES OC(CN1CCC[C@H](CC(O)=O)C1)c1ccc(cc1)-c1noc(n1)-c1onc(c1C(F)(F)F)-c1ccccc1 |r| Show InChI InChI=1S/C27H25F3N4O5/c28-27(29,30)22-23(18-6-2-1-3-7-18)32-38-24(22)26-31-25(33-39-26)19-10-8-17(9-11-19)20(35)15-34-12-4-5-16(14-34)13-21(36)37/h1-3,6-11,16,20,35H,4-5,12-15H2,(H,36,37)/t16-,20?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532539
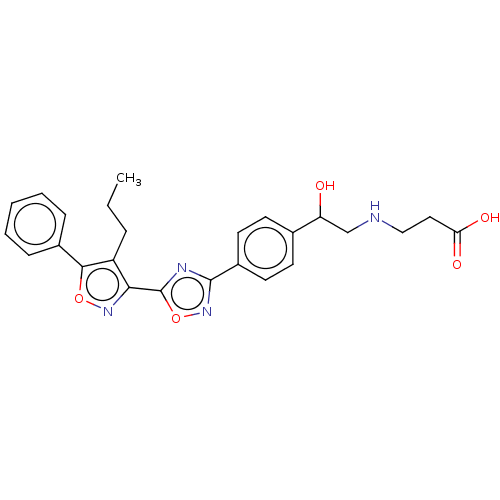
(CHEMBL4569675)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CNCCC(O)=O Show InChI InChI=1S/C25H26N4O5/c1-2-6-19-22(28-33-23(19)17-7-4-3-5-8-17)25-27-24(29-34-25)18-11-9-16(10-12-18)20(30)15-26-14-13-21(31)32/h3-5,7-12,20,26,30H,2,6,13-15H2,1H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532539

(CHEMBL4569675)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CNCCC(O)=O Show InChI InChI=1S/C25H26N4O5/c1-2-6-19-22(28-33-23(19)17-7-4-3-5-8-17)25-27-24(29-34-25)18-11-9-16(10-12-18)20(30)15-26-14-13-21(31)32/h3-5,7-12,20,26,30H,2,6,13-15H2,1H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532532

(CHEMBL4457691)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN1CCC[C@H](CC(O)=O)C1 |r| Show InChI InChI=1S/C29H32N4O5/c1-2-7-23-26(31-37-27(23)21-9-4-3-5-10-21)29-30-28(32-38-29)22-13-11-20(12-14-22)24(34)18-33-15-6-8-19(17-33)16-25(35)36/h3-5,9-14,19,24,34H,2,6-8,15-18H2,1H3,(H,35,36)/t19-,24?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532532

(CHEMBL4457691)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN1CCC[C@H](CC(O)=O)C1 |r| Show InChI InChI=1S/C29H32N4O5/c1-2-7-23-26(31-37-27(23)21-9-4-3-5-10-21)29-30-28(32-38-29)22-13-11-20(12-14-22)24(34)18-33-15-6-8-19(17-33)16-25(35)36/h3-5,9-14,19,24,34H,2,6-8,15-18H2,1H3,(H,35,36)/t19-,24?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM197654

(US9216972, 7)Show SMILES OC(=O)C1CN(Cc2ccc3-c4noc(c4CCc3c2)-c2onc(c2C(F)(F)F)-c2ccccc2)C1 Show InChI InChI=1S/C26H20F3N3O4/c27-26(28,29)20-21(15-4-2-1-3-5-15)30-36-24(20)23-19-9-7-16-10-14(6-8-18(16)22(19)31-35-23)11-32-12-17(13-32)25(33)34/h1-6,8,10,17H,7,9,11-13H2,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human S1P1 |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24X5CD0 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532542
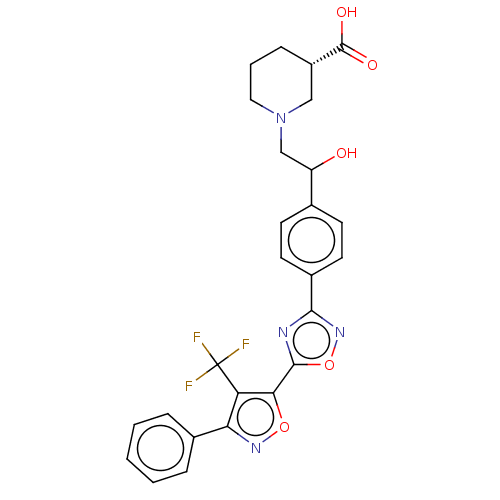
(CHEMBL4461520)Show SMILES OC(CN1CCC[C@@H](C1)C(O)=O)c1ccc(cc1)-c1noc(n1)-c1onc(c1C(F)(F)F)-c1ccccc1 |r| Show InChI InChI=1S/C26H23F3N4O5/c27-26(28,29)20-21(16-5-2-1-3-6-16)31-37-22(20)24-30-23(32-38-24)17-10-8-15(9-11-17)19(34)14-33-12-4-7-18(13-33)25(35)36/h1-3,5-6,8-11,18-19,34H,4,7,12-14H2,(H,35,36)/t18-,19?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532542

(CHEMBL4461520)Show SMILES OC(CN1CCC[C@@H](C1)C(O)=O)c1ccc(cc1)-c1noc(n1)-c1onc(c1C(F)(F)F)-c1ccccc1 |r| Show InChI InChI=1S/C26H23F3N4O5/c27-26(28,29)20-21(16-5-2-1-3-6-16)31-37-22(20)24-30-23(32-38-24)17-10-8-15(9-11-17)19(34)14-33-12-4-7-18(13-33)25(35)36/h1-3,5-6,8-11,18-19,34H,4,7,12-14H2,(H,35,36)/t18-,19?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532534

(CHEMBL4469843)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN[C@H]1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C28H30N4O5/c1-2-7-21-24(31-36-25(21)18-8-4-3-5-9-18)27-30-26(32-37-27)19-14-12-17(13-15-19)23(33)16-29-22-11-6-10-20(22)28(34)35/h3-5,8-9,12-15,20,22-23,29,33H,2,6-7,10-11,16H2,1H3,(H,34,35)/t20-,22+,23?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532534

(CHEMBL4469843)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN[C@H]1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C28H30N4O5/c1-2-7-21-24(31-36-25(21)18-8-4-3-5-9-18)27-30-26(32-37-27)19-14-12-17(13-15-19)23(33)16-29-22-11-6-10-20(22)28(34)35/h3-5,8-9,12-15,20,22-23,29,33H,2,6-7,10-11,16H2,1H3,(H,34,35)/t20-,22+,23?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532526
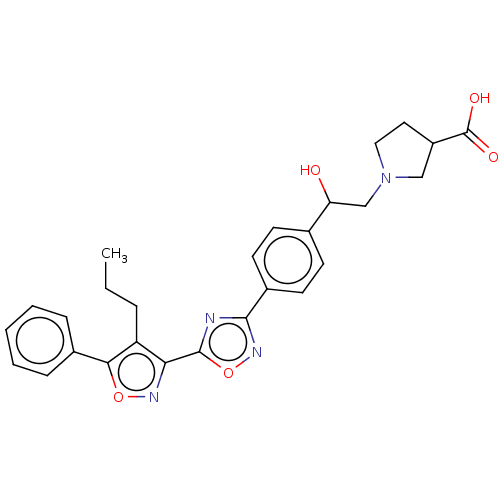
(CHEMBL4440968)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN1CCC(C1)C(O)=O Show InChI InChI=1S/C27H28N4O5/c1-2-6-21-23(29-35-24(21)18-7-4-3-5-8-18)26-28-25(30-36-26)19-11-9-17(10-12-19)22(32)16-31-14-13-20(15-31)27(33)34/h3-5,7-12,20,22,32H,2,6,13-16H2,1H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532526

(CHEMBL4440968)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN1CCC(C1)C(O)=O Show InChI InChI=1S/C27H28N4O5/c1-2-6-21-23(29-35-24(21)18-7-4-3-5-8-18)26-28-25(30-36-26)19-11-9-17(10-12-19)22(32)16-31-14-13-20(15-31)27(33)34/h3-5,7-12,20,22,32H,2,6,13-16H2,1H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50122645

(CHEMBL3623124)Show SMILES [H][C@]12C(=O)N(C(=O)[C@@]1([H])[C@@]1(C)O[C@]2(C)C[C@H]1NS(=O)(=O)c1ccccc1)c1ccc(C#N)c(c1)C(F)(F)F |r,TLB:16:15:1.7:11| Show InChI InChI=1S/C24H20F3N3O5S/c1-22-11-17(29-36(33,34)15-6-4-3-5-7-15)23(2,35-22)19-18(22)20(31)30(21(19)32)14-9-8-13(12-28)16(10-14)24(25,26)27/h3-10,17-19,29H,11H2,1-2H3/t17-,18-,19+,22+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor in human MDA-MB-453 cells assessed as inhibition of DHT-induced PSA expression by alkaline phosphatase repor... |
ACS Med Chem Lett 6: 908-12 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00173
BindingDB Entry DOI: 10.7270/Q2QV3P96 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532538
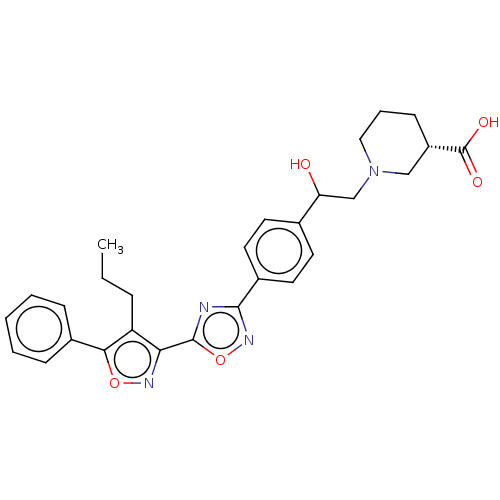
(CHEMBL4587424)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN1CCC[C@@H](C1)C(O)=O |r| Show InChI InChI=1S/C28H30N4O5/c1-2-7-22-24(30-36-25(22)19-8-4-3-5-9-19)27-29-26(31-37-27)20-13-11-18(12-14-20)23(33)17-32-15-6-10-21(16-32)28(34)35/h3-5,8-9,11-14,21,23,33H,2,6-7,10,15-17H2,1H3,(H,34,35)/t21-,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532538

(CHEMBL4587424)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN1CCC[C@@H](C1)C(O)=O |r| Show InChI InChI=1S/C28H30N4O5/c1-2-7-22-24(30-36-25(22)19-8-4-3-5-9-19)27-29-26(31-37-27)20-13-11-18(12-14-20)23(33)17-32-15-6-10-21(16-32)28(34)35/h3-5,8-9,11-14,21,23,33H,2,6-7,10,15-17H2,1H3,(H,34,35)/t21-,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532540

(CHEMBL4475594)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN Show InChI InChI=1S/C22H22N4O3/c1-2-6-17-19(25-28-20(17)15-7-4-3-5-8-15)22-24-21(26-29-22)16-11-9-14(10-12-16)18(27)13-23/h3-5,7-12,18,27H,2,6,13,23H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532540

(CHEMBL4475594)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN Show InChI InChI=1S/C22H22N4O3/c1-2-6-17-19(25-28-20(17)15-7-4-3-5-8-15)22-24-21(26-29-22)16-11-9-14(10-12-16)18(27)13-23/h3-5,7-12,18,27H,2,6,13,23H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532541
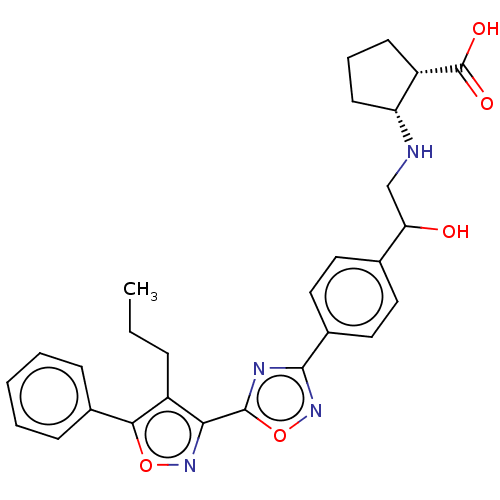
(CHEMBL4452807)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN[C@@H]1CCC[C@@H]1C(O)=O |r| Show InChI InChI=1S/C28H30N4O5/c1-2-7-21-24(31-36-25(21)18-8-4-3-5-9-18)27-30-26(32-37-27)19-14-12-17(13-15-19)23(33)16-29-22-11-6-10-20(22)28(34)35/h3-5,8-9,12-15,20,22-23,29,33H,2,6-7,10-11,16H2,1H3,(H,34,35)/t20-,22+,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532541

(CHEMBL4452807)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN[C@@H]1CCC[C@@H]1C(O)=O |r| Show InChI InChI=1S/C28H30N4O5/c1-2-7-21-24(31-36-25(21)18-8-4-3-5-9-18)27-30-26(32-37-27)19-14-12-17(13-15-19)23(33)16-29-22-11-6-10-20(22)28(34)35/h3-5,8-9,12-15,20,22-23,29,33H,2,6-7,10-11,16H2,1H3,(H,34,35)/t20-,22+,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50112346
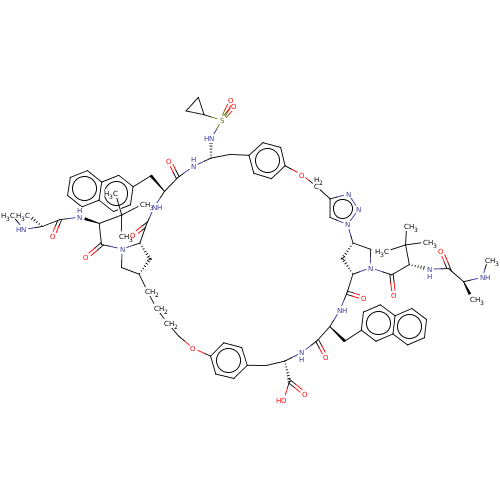
(CHEMBL3609325)Show SMILES [H][C@@]12CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)[C@@]([H])(C1)C(=O)N[C@@H](Cc1ccc3ccccc3c1)C(=O)N[C@@H](Cc1ccc(OCc3cn(nn3)[C@]3([H])CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)[C@@]([H])(C3)C(=O)N[C@@H](Cc3ccc4ccccc4c3)C(=O)N[C@@H](Cc3ccc(OCCCC2)cc3)C(O)=O)cc1)NS(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C83H106N14O14S/c1-49(84-9)73(98)90-71(82(3,4)5)79(104)95-45-55-17-15-16-36-110-62-30-24-51(25-31-62)39-67(81(106)107)88-75(100)65(40-53-22-28-56-18-11-13-20-58(56)37-53)86-78(103)69-44-61(47-96(69)80(105)72(83(6,7)8)91-74(99)50(2)85-10)97-46-60(92-94-97)48-111-63-32-26-52(27-33-63)43-70(93-112(108,109)64-34-35-64)89-76(101)66(87-77(102)68(95)42-55)41-54-23-29-57-19-12-14-21-59(57)38-54/h11-14,18-33,37-38,46,49-50,55,61,64-72,84-85,93H,15-17,34-36,39-45,47-48H2,1-10H3,(H,86,103)(H,87,102)(H,88,100)(H,89,101)(H,90,98)(H,91,99)(H,106,107)/t49-,50-,55-,61-,65-,66-,67-,68-,69-,70+,71+,72+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of cIAP BIR2-3 domain (unknown origin) |
ACS Med Chem Lett 6: 770-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00091
BindingDB Entry DOI: 10.7270/Q29P33D2 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50112346

(CHEMBL3609325)Show SMILES [H][C@@]12CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)[C@@]([H])(C1)C(=O)N[C@@H](Cc1ccc3ccccc3c1)C(=O)N[C@@H](Cc1ccc(OCc3cn(nn3)[C@]3([H])CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)[C@@]([H])(C3)C(=O)N[C@@H](Cc3ccc4ccccc4c3)C(=O)N[C@@H](Cc3ccc(OCCCC2)cc3)C(O)=O)cc1)NS(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C83H106N14O14S/c1-49(84-9)73(98)90-71(82(3,4)5)79(104)95-45-55-17-15-16-36-110-62-30-24-51(25-31-62)39-67(81(106)107)88-75(100)65(40-53-22-28-56-18-11-13-20-58(56)37-53)86-78(103)69-44-61(47-96(69)80(105)72(83(6,7)8)91-74(99)50(2)85-10)97-46-60(92-94-97)48-111-63-32-26-52(27-33-63)43-70(93-112(108,109)64-34-35-64)89-76(101)66(87-77(102)68(95)42-55)41-54-23-29-57-19-12-14-21-59(57)38-54/h11-14,18-33,37-38,46,49-50,55,61,64-72,84-85,93H,15-17,34-36,39-45,47-48H2,1-10H3,(H,86,103)(H,87,102)(H,88,100)(H,89,101)(H,90,98)(H,91,99)(H,106,107)/t49-,50-,55-,61-,65-,66-,67-,68-,69-,70+,71+,72+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of XIAP BIR3 domain (unknown origin) |
ACS Med Chem Lett 6: 770-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00091
BindingDB Entry DOI: 10.7270/Q29P33D2 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532527
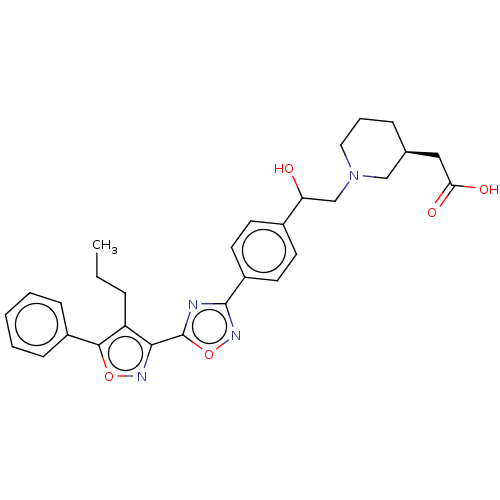
(CHEMBL4464631)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN1CCC[C@@H](CC(O)=O)C1 |r| Show InChI InChI=1S/C29H32N4O5/c1-2-7-23-26(31-37-27(23)21-9-4-3-5-10-21)29-30-28(32-38-29)22-13-11-20(12-14-22)24(34)18-33-15-6-8-19(17-33)16-25(35)36/h3-5,9-14,19,24,34H,2,6-8,15-18H2,1H3,(H,35,36)/t19-,24?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532527

(CHEMBL4464631)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN1CCC[C@@H](CC(O)=O)C1 |r| Show InChI InChI=1S/C29H32N4O5/c1-2-7-23-26(31-37-27(23)21-9-4-3-5-10-21)29-30-28(32-38-29)22-13-11-20(12-14-22)24(34)18-33-15-6-8-19(17-33)16-25(35)36/h3-5,9-14,19,24,34H,2,6-8,15-18H2,1H3,(H,35,36)/t19-,24?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532535

(CHEMBL4545842)Show SMILES OC(CN1CC(C1)C(O)=O)c1ccc(cc1)-c1noc(n1)-c1onc(c1C(F)(F)F)-c1ccccc1 Show InChI InChI=1S/C24H19F3N4O5/c25-24(26,27)18-19(14-4-2-1-3-5-14)29-35-20(18)22-28-21(30-36-22)15-8-6-13(7-9-15)17(32)12-31-10-16(11-31)23(33)34/h1-9,16-17,32H,10-12H2,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532535

(CHEMBL4545842)Show SMILES OC(CN1CC(C1)C(O)=O)c1ccc(cc1)-c1noc(n1)-c1onc(c1C(F)(F)F)-c1ccccc1 Show InChI InChI=1S/C24H19F3N4O5/c25-24(26,27)18-19(14-4-2-1-3-5-14)29-35-20(18)22-28-21(30-36-22)15-8-6-13(7-9-15)17(32)12-31-10-16(11-31)23(33)34/h1-9,16-17,32H,10-12H2,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data