

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipopolysaccharide heptosyltransferase 1 (Escherichia coli (strain K12)) | BDBM34103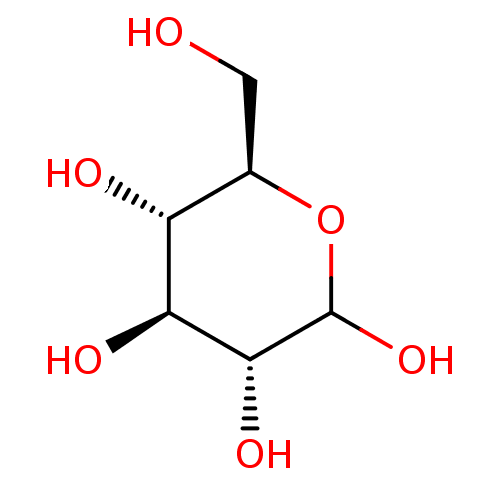 (D-glucose | dextrose | glucose) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Heptosyltransferase I assessed as reduction in ADP release using ODLA and ADP-heptose substrates in presence of phosph... | Bioorg Med Chem Lett 28: 594-600 (2018) Article DOI: 10.1016/j.bmcl.2018.01.040 BindingDB Entry DOI: 10.7270/Q2J38W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipopolysaccharide heptosyltransferase 1 (Escherichia coli (strain K12)) | BDBM50454776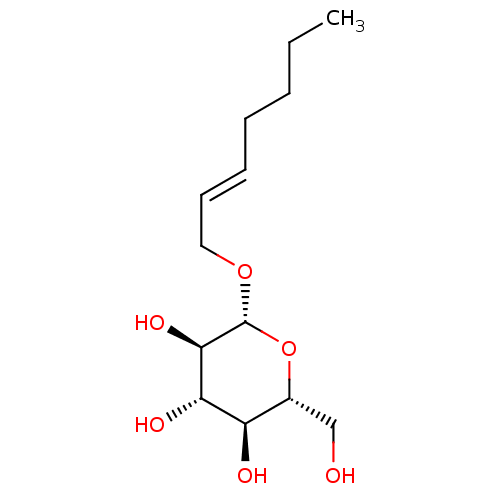 (CHEMBL4209246) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 3.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Heptosyltransferase I assessed as reduction in ADP release using ODLA and ADP-heptose substrates in presence of phosph... | Bioorg Med Chem Lett 28: 594-600 (2018) Article DOI: 10.1016/j.bmcl.2018.01.040 BindingDB Entry DOI: 10.7270/Q2J38W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipopolysaccharide heptosyltransferase 1 (Escherichia coli (strain K12)) | BDBM20876 ((2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-methoxyoxane-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Heptosyltransferase I assessed as reduction in ADP release using ODLA and ADP-heptose substrates in presence of phosph... | Bioorg Med Chem Lett 28: 594-600 (2018) Article DOI: 10.1016/j.bmcl.2018.01.040 BindingDB Entry DOI: 10.7270/Q2J38W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipopolysaccharide heptosyltransferase 1 (Escherichia coli (strain K12)) | BDBM228805 (Galactose) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Heptosyltransferase I assessed as reduction in ADP release using ODLA and ADP-heptose substrates in presence of phosph... | Bioorg Med Chem Lett 28: 594-600 (2018) Article DOI: 10.1016/j.bmcl.2018.01.040 BindingDB Entry DOI: 10.7270/Q2J38W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipopolysaccharide heptosyltransferase 1 (Escherichia coli (strain K12)) | BDBM50454780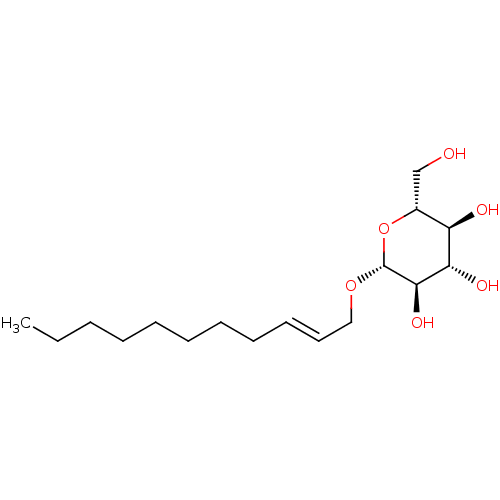 (CHEMBL4212177) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 5.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Heptosyltransferase I assessed as reduction in ADP release using ODLA and ADP-heptose substrates in presence of phosph... | Bioorg Med Chem Lett 28: 594-600 (2018) Article DOI: 10.1016/j.bmcl.2018.01.040 BindingDB Entry DOI: 10.7270/Q2J38W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipopolysaccharide heptosyltransferase 1 (Escherichia coli (strain K12)) | BDBM50454777 (CHEMBL4205643) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 5.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Heptosyltransferase I assessed as reduction in ADP release using ODLA and ADP-heptose substrates in presence of phosph... | Bioorg Med Chem Lett 28: 594-600 (2018) Article DOI: 10.1016/j.bmcl.2018.01.040 BindingDB Entry DOI: 10.7270/Q2J38W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipopolysaccharide heptosyltransferase 1 (Escherichia coli (strain K12)) | BDBM50454779 (CHEMBL4213641) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 6.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Heptosyltransferase I assessed as reduction in ADP release using ODLA and ADP-heptose substrates in presence of phosph... | Bioorg Med Chem Lett 28: 594-600 (2018) Article DOI: 10.1016/j.bmcl.2018.01.040 BindingDB Entry DOI: 10.7270/Q2J38W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipopolysaccharide heptosyltransferase 1 (Escherichia coli (strain K12)) | BDBM50454778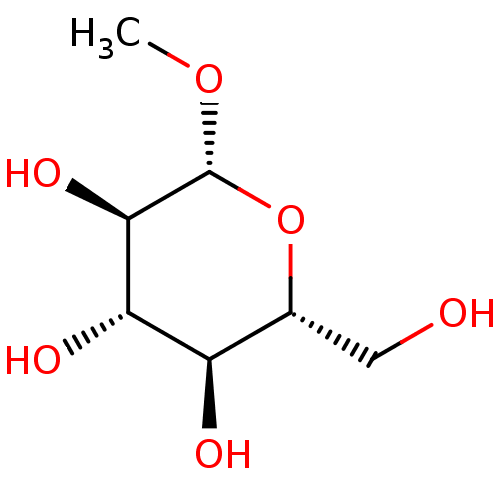 (CHEBI:320055 | CHEMBL132186) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | 6.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Heptosyltransferase I assessed as reduction in ADP release using ODLA and ADP-heptose substrates in presence of phosph... | Bioorg Med Chem Lett 28: 594-600 (2018) Article DOI: 10.1016/j.bmcl.2018.01.040 BindingDB Entry DOI: 10.7270/Q2J38W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50348226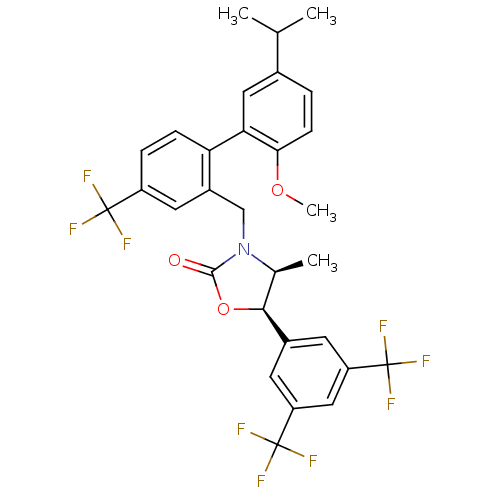 (CHEMBL1800622) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein using exogenous LDL and ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578772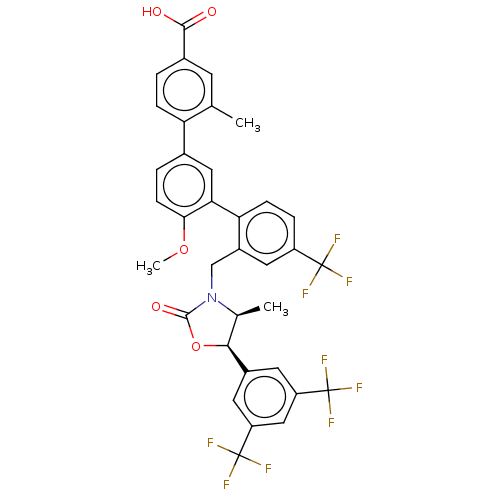 (CHEMBL4873416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein using exogenous LDL and ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578733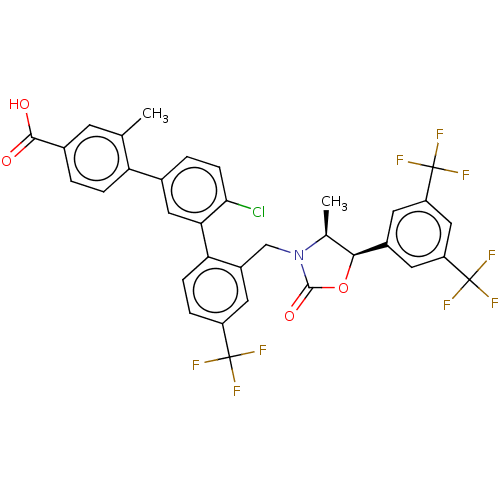 (CHEMBL4875851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein using exogenous LDL and ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578733 (CHEMBL4875851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50348228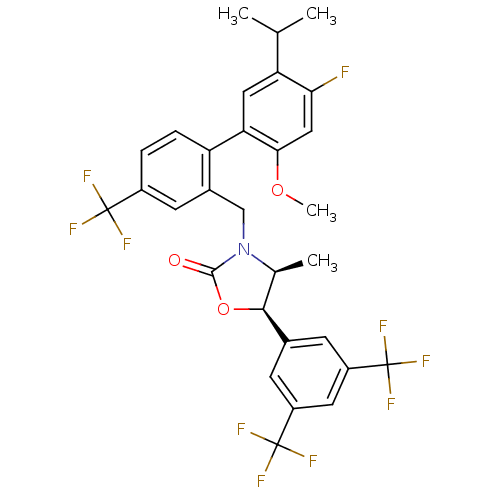 (CHEMBL1800807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein using exogenous LDL and ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM264531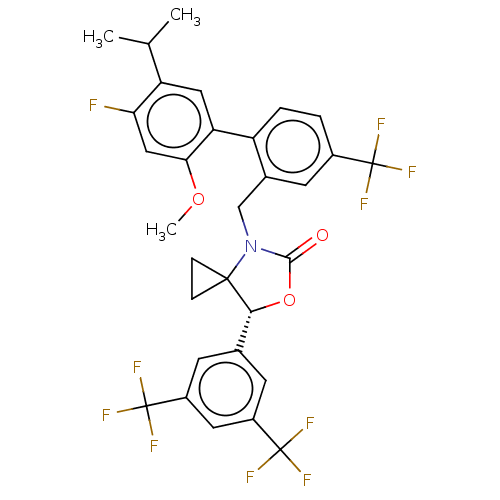 ((7R)-7-[3,5- bis(trifluoromethyl)phenyl]- 4-{[4'-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578756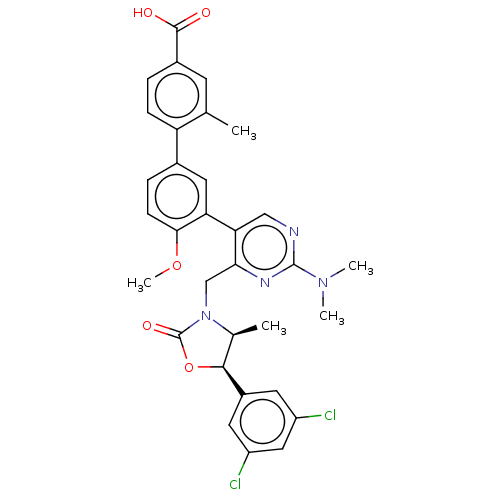 (CHEMBL4876430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum (isolate 3D7)) | BDBM50018008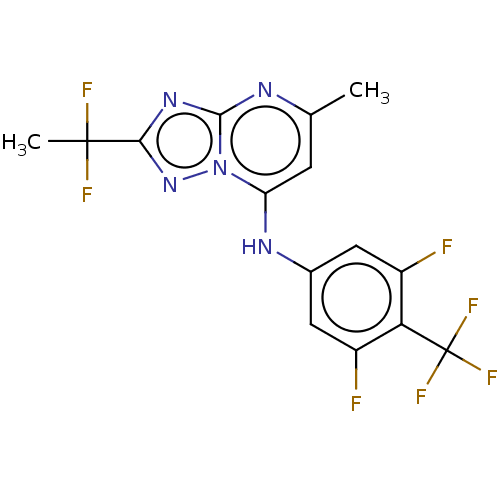 (CHEMBL3289671 | US9238653, Table 5, Compound 43) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.0 | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM; MMV MEDICINES FOR MALARIA VENTURE; UNIVERSITY OF WASHINGTON US Patent | Assay Description For studying inhibition of Plasmodium or human DHODH enzyme, two assays that are in routine use are described, for example, in Baldwin, et al. (2002)... | US Patent US9238653 (2016) BindingDB Entry DOI: 10.7270/Q2D79954 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578737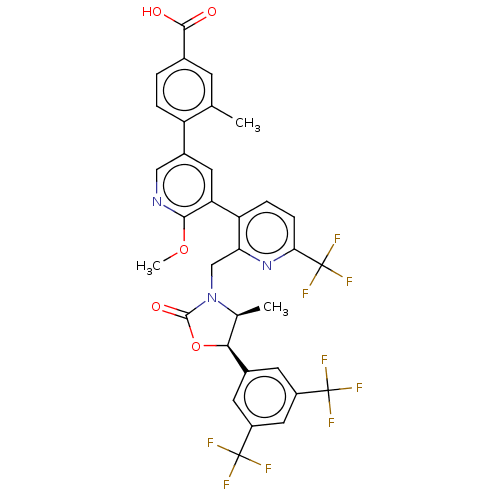 (CHEMBL4852134) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM138247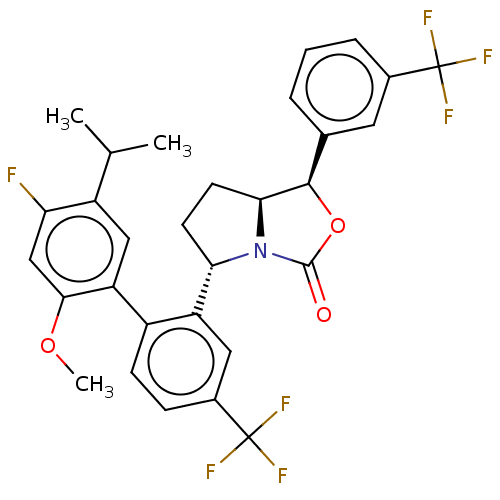 (US8871738, 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum (isolate 3D7)) | BDBM50018006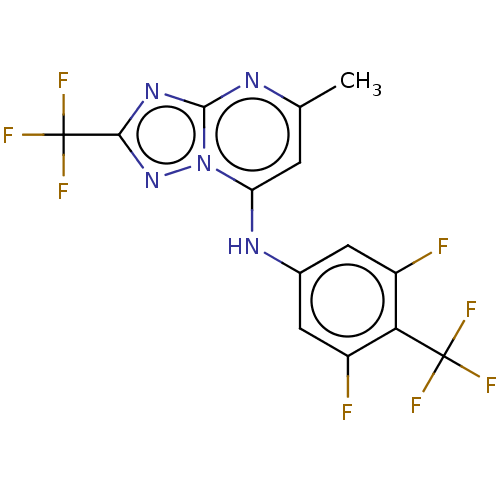 (CHEMBL3289672 | US9238653, Table 5, Compound 49) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 8.0 | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM; MMV MEDICINES FOR MALARIA VENTURE; UNIVERSITY OF WASHINGTON US Patent | Assay Description For studying inhibition of Plasmodium or human DHODH enzyme, two assays that are in routine use are described, for example, in Baldwin, et al. (2002)... | US Patent US9238653 (2016) BindingDB Entry DOI: 10.7270/Q2D79954 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578735 (CHEMBL4853626) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50348226 (CHEMBL1800622) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578754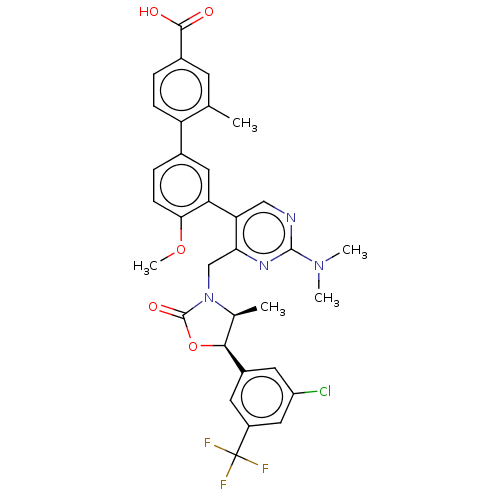 (CHEMBL4856008) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum (isolate 3D7)) | BDBM50365232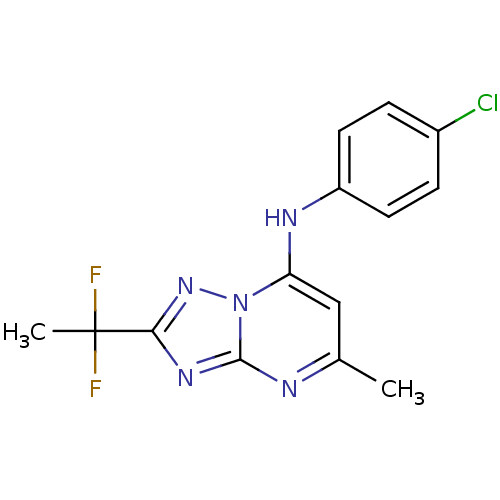 (CHEMBL1956283 | US9238653, Table 5, Compound 54) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 8.0 | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM; MMV MEDICINES FOR MALARIA VENTURE; UNIVERSITY OF WASHINGTON US Patent | Assay Description For studying inhibition of Plasmodium or human DHODH enzyme, two assays that are in routine use are described, for example, in Baldwin, et al. (2002)... | US Patent US9238653 (2016) BindingDB Entry DOI: 10.7270/Q2D79954 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578770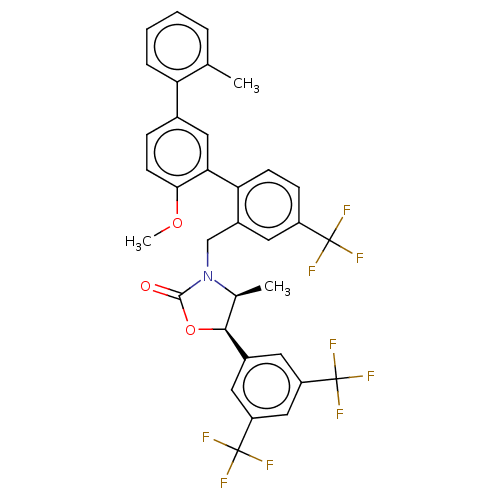 (CHEMBL4859118) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein using exogenous LDL and ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578767 (CHEMBL4876714) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein using exogenous LDL and ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum (isolate 3D7)) | BDBM50365230 (CHEMBL1956285 | US11903936, Compound DSM265 | US92...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 8.0 | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM; MMV MEDICINES FOR MALARIA VENTURE; UNIVERSITY OF WASHINGTON US Patent | Assay Description For studying inhibition of Plasmodium or human DHODH enzyme, two assays that are in routine use are described, for example, in Baldwin, et al. (2002)... | US Patent US9238653 (2016) BindingDB Entry DOI: 10.7270/Q2D79954 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578742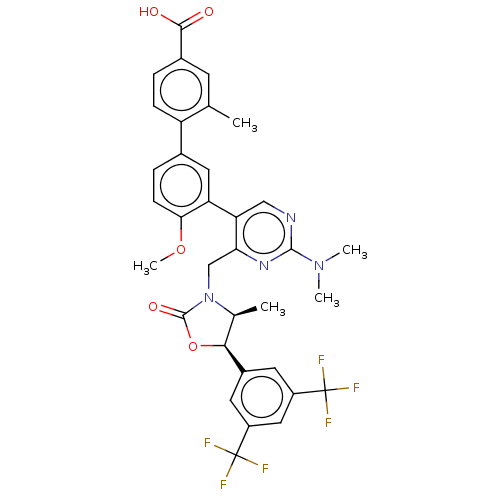 (CHEMBL4854376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum (isolate 3D7)) | BDBM50345441 (5-Methyl-N-(5,6,7,8-tetrahydronaphthalen-2yl)-[1,2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 8.0 | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM; MMV MEDICINES FOR MALARIA VENTURE; UNIVERSITY OF WASHINGTON US Patent | Assay Description For studying inhibition of Plasmodium or human DHODH enzyme, two assays that are in routine use are described, for example, in Baldwin, et al. (2002)... | US Patent US9238653 (2016) BindingDB Entry DOI: 10.7270/Q2D79954 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578736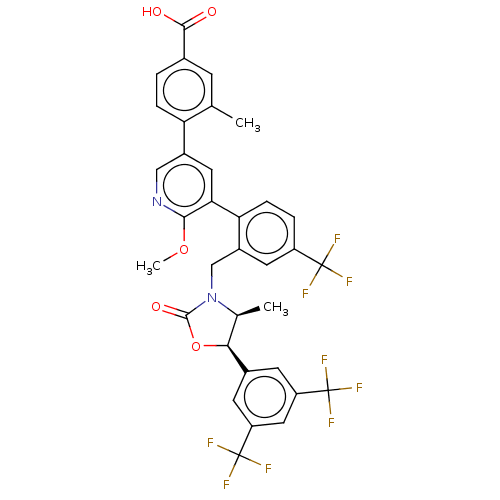 (CHEMBL4852538) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum (isolate 3D7)) | BDBM50365225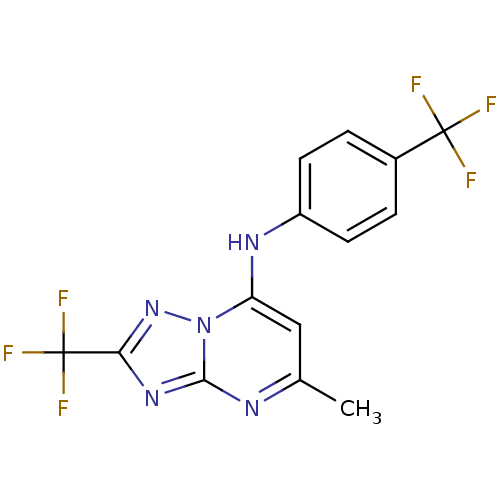 (CHEMBL1956290 | US9238653, Table 5, Compound 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 8.0 | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM; MMV MEDICINES FOR MALARIA VENTURE; UNIVERSITY OF WASHINGTON US Patent | Assay Description For studying inhibition of Plasmodium or human DHODH enzyme, two assays that are in routine use are described, for example, in Baldwin, et al. (2002)... | US Patent US9238653 (2016) BindingDB Entry DOI: 10.7270/Q2D79954 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum (isolate 3D7)) | BDBM50018007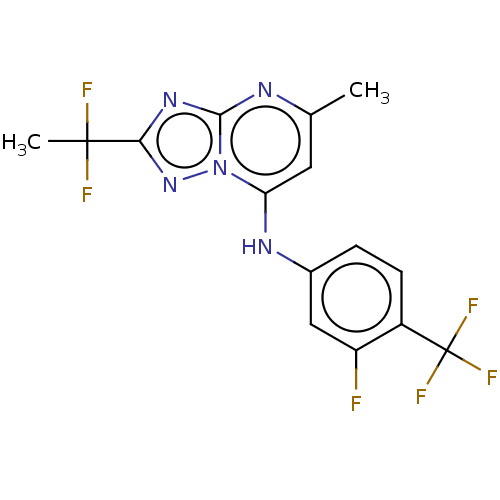 (CHEMBL3289670 | US9238653, Table 5, Compound 42) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 8.0 | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM; MMV MEDICINES FOR MALARIA VENTURE; UNIVERSITY OF WASHINGTON US Patent | Assay Description For studying inhibition of Plasmodium or human DHODH enzyme, two assays that are in routine use are described, for example, in Baldwin, et al. (2002)... | US Patent US9238653 (2016) BindingDB Entry DOI: 10.7270/Q2D79954 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum (isolate 3D7)) | BDBM50365231 (CHEMBL1738786 | US9238653, Table 5, Compound 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 8.0 | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM; MMV MEDICINES FOR MALARIA VENTURE; UNIVERSITY OF WASHINGTON US Patent | Assay Description For studying inhibition of Plasmodium or human DHODH enzyme, two assays that are in routine use are described, for example, in Baldwin, et al. (2002)... | US Patent US9238653 (2016) BindingDB Entry DOI: 10.7270/Q2D79954 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum (isolate 3D7)) | BDBM173505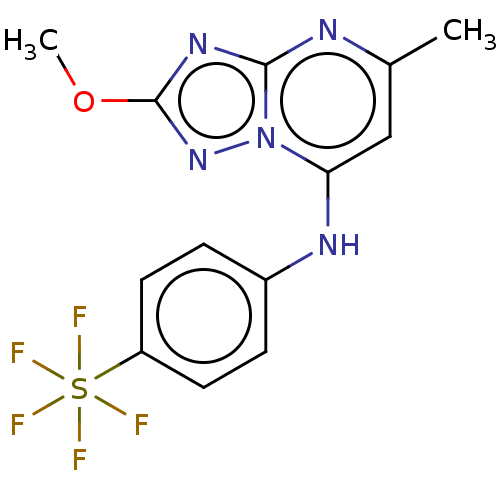 (US9238653, Table 5, Compound 44) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 8.0 | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM; MMV MEDICINES FOR MALARIA VENTURE; UNIVERSITY OF WASHINGTON US Patent | Assay Description For studying inhibition of Plasmodium or human DHODH enzyme, two assays that are in routine use are described, for example, in Baldwin, et al. (2002)... | US Patent US9238653 (2016) BindingDB Entry DOI: 10.7270/Q2D79954 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578748 (CHEMBL4848688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578769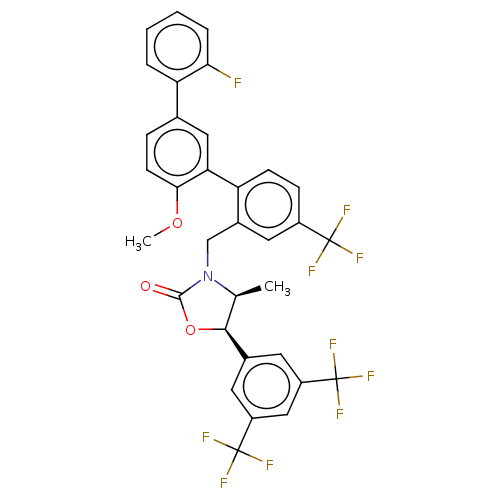 (CHEMBL4875002) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein using exogenous LDL and ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum (isolate 3D7)) | BDBM173492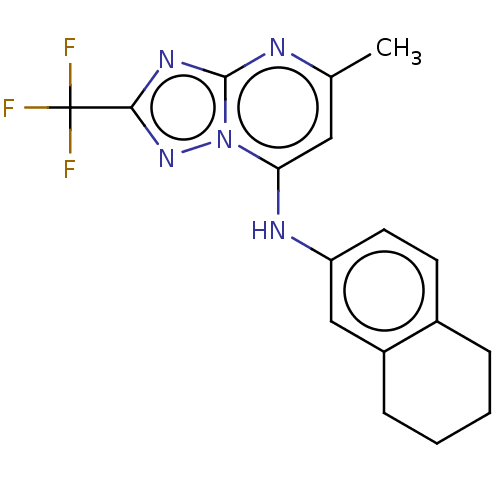 (US9238653, Table 5, Compound 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 43 | n/a | n/a | n/a | n/a | 8.0 | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM; MMV MEDICINES FOR MALARIA VENTURE; UNIVERSITY OF WASHINGTON US Patent | Assay Description For studying inhibition of Plasmodium or human DHODH enzyme, two assays that are in routine use are described, for example, in Baldwin, et al. (2002)... | US Patent US9238653 (2016) BindingDB Entry DOI: 10.7270/Q2D79954 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50348228 (CHEMBL1800807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578743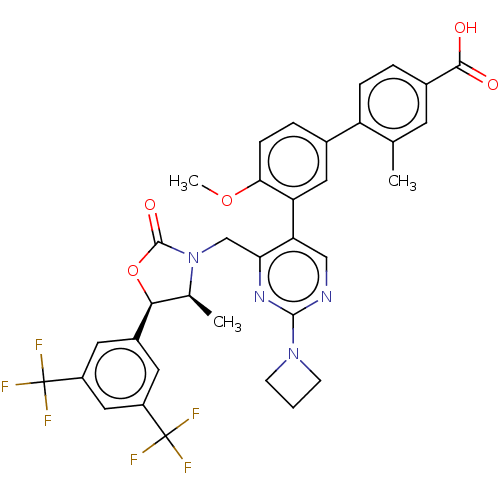 (CHEMBL4846106) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578749 (CHEMBL4851648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578755 (CHEMBL4855182) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578734 (CHEMBL4867412) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578750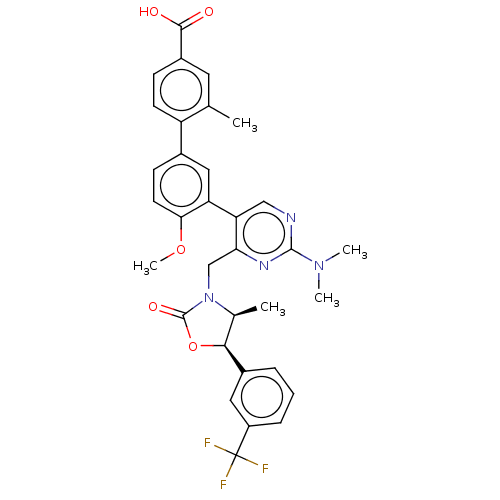 (CHEMBL4870761) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM138244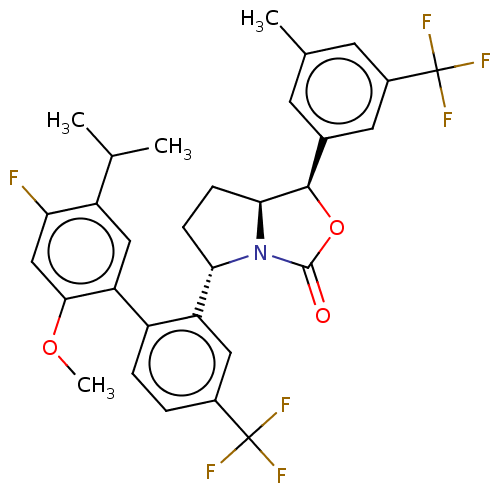 (US8871738, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum (isolate 3D7)) | BDBM50345406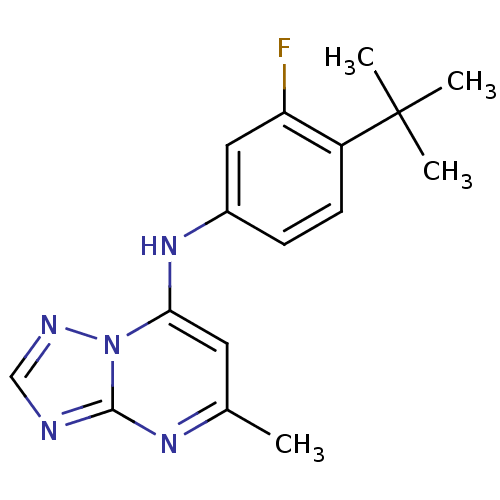 (CHEMBL1784574 | N-(4-tert-Butyl-3-fluorophenyl)-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 8.0 | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM; MMV MEDICINES FOR MALARIA VENTURE; UNIVERSITY OF WASHINGTON US Patent | Assay Description For studying inhibition of Plasmodium or human DHODH enzyme, two assays that are in routine use are described, for example, in Baldwin, et al. (2002)... | US Patent US9238653 (2016) BindingDB Entry DOI: 10.7270/Q2D79954 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578740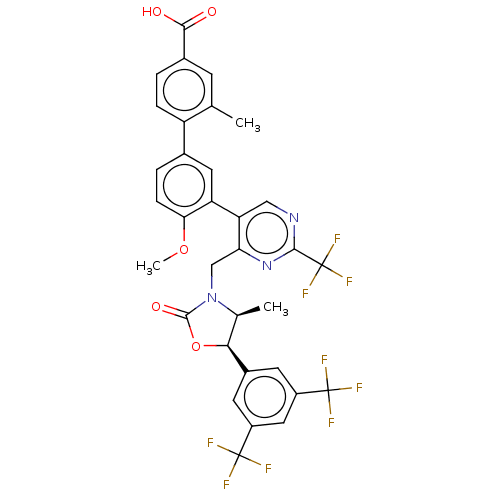 (CHEMBL4864055) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM138245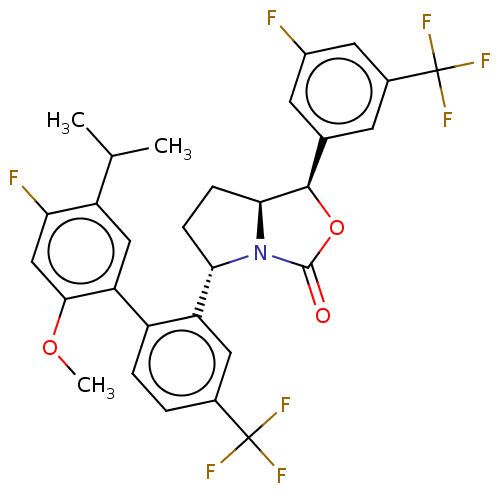 (US8871738, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum (isolate 3D7)) | BDBM50365226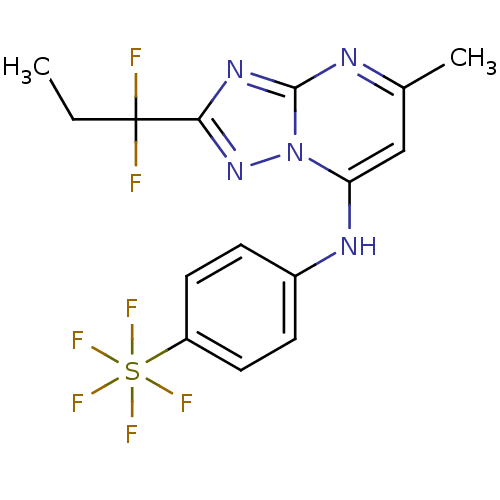 (CHEMBL1956289 | US9238653, Table 5, Compound 52) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 75 | n/a | n/a | n/a | n/a | 8.0 | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM; MMV MEDICINES FOR MALARIA VENTURE; UNIVERSITY OF WASHINGTON US Patent | Assay Description For studying inhibition of Plasmodium or human DHODH enzyme, two assays that are in routine use are described, for example, in Baldwin, et al. (2002)... | US Patent US9238653 (2016) BindingDB Entry DOI: 10.7270/Q2D79954 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum (isolate 3D7)) | BDBM50396723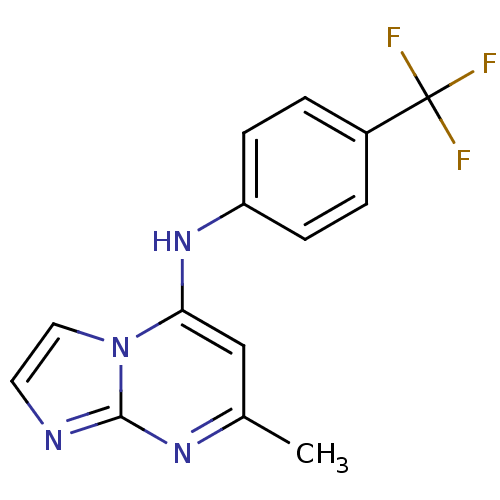 (CHEMBL2172226 | US9238653, Table 5, Compound 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 77 | n/a | n/a | n/a | n/a | 8.0 | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM; MMV MEDICINES FOR MALARIA VENTURE; UNIVERSITY OF WASHINGTON US Patent | Assay Description For studying inhibition of Plasmodium or human DHODH enzyme, two assays that are in routine use are described, for example, in Baldwin, et al. (2002)... | US Patent US9238653 (2016) BindingDB Entry DOI: 10.7270/Q2D79954 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578776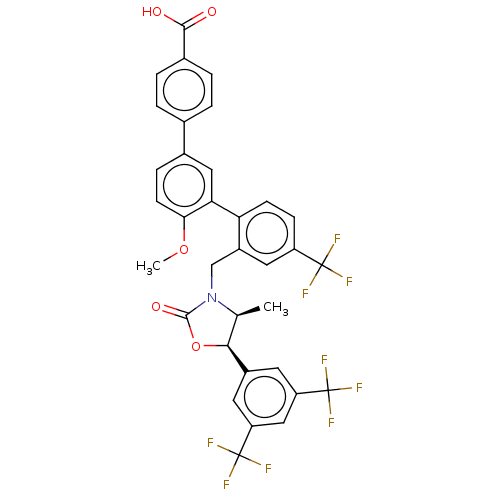 (CHEMBL4858285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein using exogenous LDL and ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578747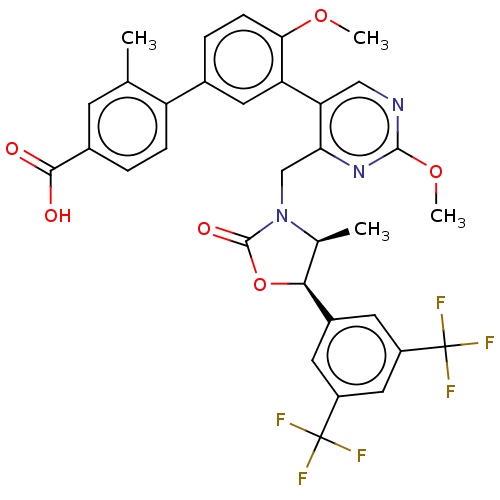 (CHEMBL4878757) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 347 total ) | Next | Last >> |