 Found 1106 hits with Last Name = 'cox' and Initial = 'p'
Found 1106 hits with Last Name = 'cox' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM356652
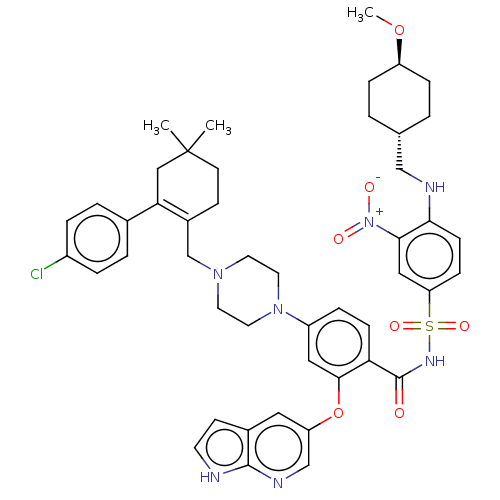
(US10213433, Compound 34 | US11369599, Compound 34 ...)Show SMILES CO[C@H]1CC[C@H](CNc2ccc(cc2[N+]([O-])=O)S(=O)(=O)NC(=O)c2ccc(cc2Oc2cnc3[nH]ccc3c2)N2CCN(CC3=C(CC(C)(C)CC3)c3ccc(Cl)cc3)CC2)CC1 |wU:5.5,wD:2.1,t:48,(-3.85,-16.17,;-2.52,-15.4,;-2.52,-13.86,;-1.18,-13.09,;-1.18,-11.55,;-2.52,-10.78,;-2.52,-9.24,;-1.18,-8.47,;-1.18,-6.93,;-2.52,-6.16,;-2.52,-4.62,;-1.18,-3.85,;.15,-4.62,;.15,-6.16,;1.48,-6.93,;2.82,-6.16,;1.48,-8.47,;-1.18,-2.31,;-2.72,-2.31,;.36,-2.31,;-1.18,-.77,;-2.52,,;-3.85,-.77,;-2.52,1.54,;-3.85,2.31,;-3.85,3.85,;-2.52,4.62,;-1.18,3.85,;-1.18,2.31,;.15,1.54,;1.48,2.31,;1.48,3.85,;2.82,4.62,;4.15,3.85,;5.61,4.33,;6.52,3.08,;5.61,1.83,;4.15,2.31,;2.82,1.54,;-2.52,6.16,;-3.85,6.93,;-3.85,8.47,;-2.52,9.24,;-2.52,10.78,;-1.18,11.55,;-1.18,13.09,;.15,13.86,;1.48,13.09,;2.25,14.42,;3.02,13.09,;1.48,11.55,;.15,10.78,;-2.52,13.86,;-3.85,13.09,;-5.19,13.86,;-5.19,15.4,;-6.52,16.17,;-3.85,16.17,;-2.52,15.4,;-1.18,8.47,;-1.18,6.93,;-3.85,-11.55,;-3.85,-13.09,)| Show InChI InChI=1S/C47H54ClN7O7S/c1-47(2)18-16-34(41(27-47)32-6-8-35(48)9-7-32)30-53-20-22-54(23-21-53)36-10-14-40(44(25-36)62-38-24-33-17-19-49-45(33)51-29-38)46(56)52-63(59,60)39-13-15-42(43(26-39)55(57)58)50-28-31-4-11-37(61-3)12-5-31/h6-10,13-15,17,19,24-26,29,31,37,50H,4-5,11-12,16,18,20-23,27-28,30H2,1-3H3,(H,49,51)(H,52,56)/t31-,37- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl2 (unknown origin) |
J Med Chem 61: 2636-2651 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00717
BindingDB Entry DOI: 10.7270/Q2ZK5K94 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl2 (unknown origin) |
J Med Chem 61: 2636-2651 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00717
BindingDB Entry DOI: 10.7270/Q2ZK5K94 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50270877
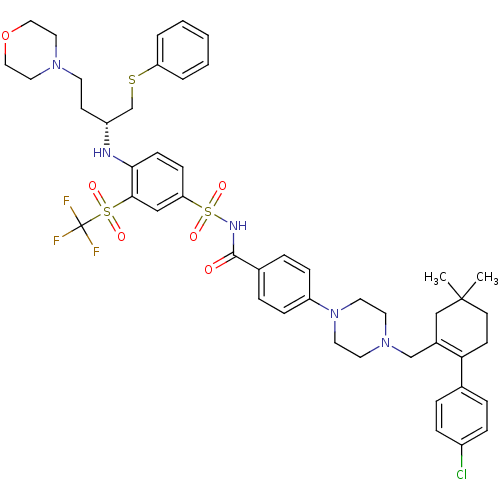
((R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex...)Show SMILES CC1(C)CCC(=C(CN2CCN(CC2)c2ccc(cc2)C(=O)NS(=O)(=O)c2ccc(N[C@H](CCN3CCOCC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)C1)c1ccc(Cl)cc1 |r,t:5| Show InChI InChI=1S/C47H55ClF3N5O6S3/c1-46(2)20-18-42(34-8-12-37(48)13-9-34)36(31-46)32-55-22-24-56(25-23-55)39-14-10-35(11-15-39)45(57)53-65(60,61)41-16-17-43(44(30-41)64(58,59)47(49,50)51)52-38(19-21-54-26-28-62-29-27-54)33-63-40-6-4-3-5-7-40/h3-17,30,38,52H,18-29,31-33H2,1-2H3,(H,53,57)/t38-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl2 (unknown origin) |
J Med Chem 61: 2636-2651 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00717
BindingDB Entry DOI: 10.7270/Q2ZK5K94 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50066789
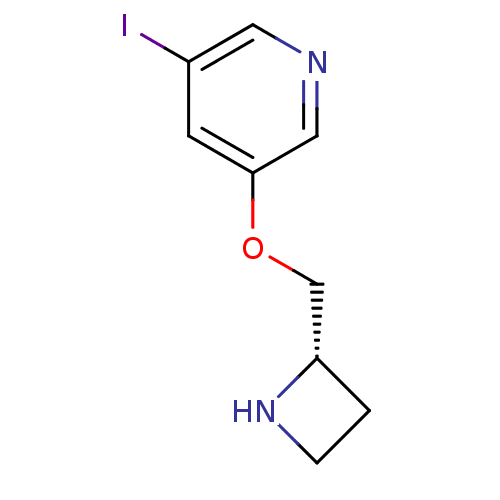
(3-((S)-1-Azetidin-2-ylmethoxy)-5-iodo-pyridine | A...)Show InChI InChI=1S/C9H11IN2O/c10-7-3-9(5-11-4-7)13-6-8-1-2-12-8/h3-5,8,12H,1-2,6H2/t8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat brain membrane |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50270877

((R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex...)Show SMILES CC1(C)CCC(=C(CN2CCN(CC2)c2ccc(cc2)C(=O)NS(=O)(=O)c2ccc(N[C@H](CCN3CCOCC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)C1)c1ccc(Cl)cc1 |r,t:5| Show InChI InChI=1S/C47H55ClF3N5O6S3/c1-46(2)20-18-42(34-8-12-37(48)13-9-34)36(31-46)32-55-22-24-56(25-23-55)39-14-10-35(11-15-39)45(57)53-65(60,61)41-16-17-43(44(30-41)64(58,59)47(49,50)51)52-38(19-21-54-26-28-62-29-27-54)33-63-40-6-4-3-5-7-40/h3-17,30,38,52H,18-29,31-33H2,1-2H3,(H,53,57)/t38-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-xL (unknown origin) |
J Med Chem 61: 2636-2651 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00717
BindingDB Entry DOI: 10.7270/Q2ZK5K94 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50166908

(5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...)Show InChI InChI=1S/C13H13N3/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1/h1-2,4-5,8-9,14H,3,6-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to alpha4beta2 nAChR in rat cortex |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against PDE4 was determined using [3H]- rolipram in guinea pig brain membrane binding assay |
Bioorg Med Chem Lett 8: 1867-72 (1998)
BindingDB Entry DOI: 10.7270/Q2CJ8GPB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Affinity for rolipram binding site of phosphodiesterase type IV (PDE4) |
Bioorg Med Chem Lett 8: 3053-8 (1998)
BindingDB Entry DOI: 10.7270/Q2PV6NK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM50067424
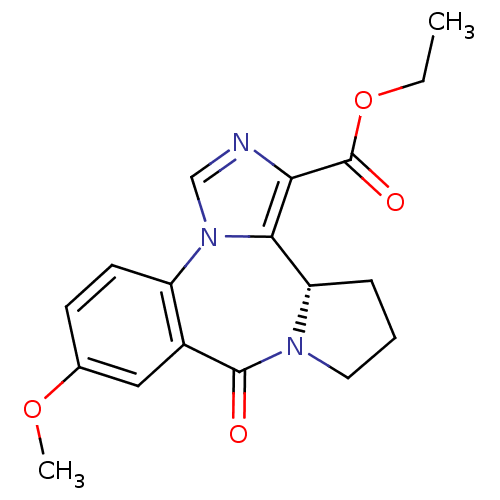
((S)-9-Methoxy-7-oxo-3b,4,5,6-tetrahydro-7H-2,6a,11...)Show SMILES CCOC(=O)c1ncn-2c1[C@@H]1CCCN1C(=O)c1cc(OC)ccc-21 Show InChI InChI=1S/C18H19N3O4/c1-3-25-18(23)15-16-14-5-4-8-20(14)17(22)12-9-11(24-2)6-7-13(12)21(16)10-19-15/h6-7,9-10,14H,3-5,8H2,1-2H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha5 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50142570
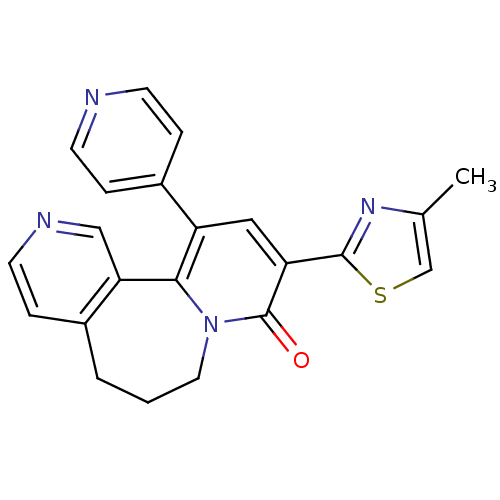
(9-(4-Methyl-thiazol-2-yl)-11-pyridin-4-yl-6,7-dihy...)Show SMILES Cc1csc(n1)-c1cc(-c2ccncc2)c2-c3cnccc3CCCn2c1=O Show InChI InChI=1S/C22H18N4OS/c1-14-13-28-21(25-14)18-11-17(16-4-7-23-8-5-16)20-19-12-24-9-6-15(19)3-2-10-26(20)22(18)27/h4-9,11-13H,2-3,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50142570

(9-(4-Methyl-thiazol-2-yl)-11-pyridin-4-yl-6,7-dihy...)Show SMILES Cc1csc(n1)-c1cc(-c2ccncc2)c2-c3cnccc3CCCn2c1=O Show InChI InChI=1S/C22H18N4OS/c1-14-13-28-21(25-14)18-11-17(16-4-7-23-8-5-16)20-19-12-24-9-6-15(19)3-2-10-26(20)22(18)27/h4-9,11-13H,2-3,10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50179998
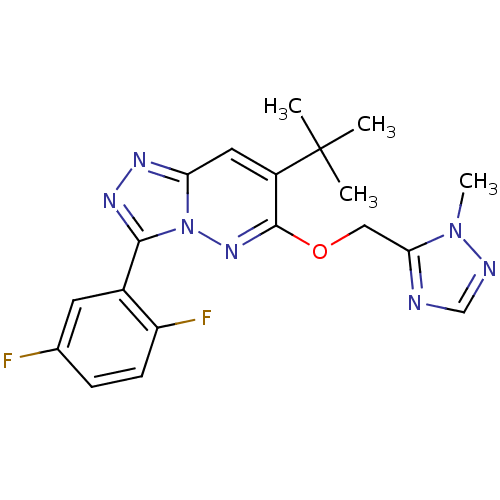
(7-tert-butyl-3-(2,5-difluorophenyl)-6-((2-methyl-2...)Show SMILES Cn1ncnc1COc1nn2c(nnc2cc1C(C)(C)C)-c1cc(F)ccc1F Show InChI InChI=1S/C19H19F2N7O/c1-19(2,3)13-8-15-24-25-17(12-7-11(20)5-6-14(12)21)28(15)26-18(13)29-9-16-22-10-23-27(16)4/h5-8,10H,9H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50179998

(7-tert-butyl-3-(2,5-difluorophenyl)-6-((2-methyl-2...)Show SMILES Cn1ncnc1COc1nn2c(nnc2cc1C(C)(C)C)-c1cc(F)ccc1F Show InChI InChI=1S/C19H19F2N7O/c1-19(2,3)13-8-15-24-25-17(12-7-11(20)5-6-14(12)21)28(15)26-18(13)29-9-16-22-10-23-27(16)4/h5-8,10H,9H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM21447

(4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C42H45ClN6O5S2/c1-46(2)23-22-35(30-55-37-9-4-3-5-10-37)44-40-21-20-38(28-41(40)49(51)52)56(53,54)45-42(50)32-14-18-36(19-15-32)48-26-24-47(25-27-48)29-33-8-6-7-11-39(33)31-12-16-34(43)17-13-31/h3-21,28,35,44H,22-27,29-30H2,1-2H3,(H,45,50)/t35-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-xL (unknown origin) |
J Med Chem 61: 2636-2651 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00717
BindingDB Entry DOI: 10.7270/Q2ZK5K94 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM21447

(4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C42H45ClN6O5S2/c1-46(2)23-22-35(30-55-37-9-4-3-5-10-37)44-40-21-20-38(28-41(40)49(51)52)56(53,54)45-42(50)32-14-18-36(19-15-32)48-26-24-47(25-27-48)29-33-8-6-7-11-39(33)31-12-16-34(43)17-13-31/h3-21,28,35,44H,22-27,29-30H2,1-2H3,(H,45,50)/t35-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl2 (unknown origin) |
J Med Chem 61: 2636-2651 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00717
BindingDB Entry DOI: 10.7270/Q2ZK5K94 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50142570

(9-(4-Methyl-thiazol-2-yl)-11-pyridin-4-yl-6,7-dihy...)Show SMILES Cc1csc(n1)-c1cc(-c2ccncc2)c2-c3cnccc3CCCn2c1=O Show InChI InChI=1S/C22H18N4OS/c1-14-13-28-21(25-14)18-11-17(16-4-7-23-8-5-16)20-19-12-24-9-6-15(19)3-2-10-26(20)22(18)27/h4-9,11-13H,2-3,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha5 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM50179998

(7-tert-butyl-3-(2,5-difluorophenyl)-6-((2-methyl-2...)Show SMILES Cn1ncnc1COc1nn2c(nnc2cc1C(C)(C)C)-c1cc(F)ccc1F Show InChI InChI=1S/C19H19F2N7O/c1-19(2,3)13-8-15-24-25-17(12-7-11(20)5-6-14(12)21)28(15)26-18(13)29-9-16-22-10-23-27(16)4/h5-8,10H,9H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha5 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Affinity for rolipram binding site of phosphodiesterase type IV (PDE4) |
Bioorg Med Chem Lett 8: 3053-8 (1998)
BindingDB Entry DOI: 10.7270/Q2PV6NK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against PDE4 was determined using [3H]- rolipram in guinea pig brain membrane binding assay |
Bioorg Med Chem Lett 8: 1867-72 (1998)
BindingDB Entry DOI: 10.7270/Q2CJ8GPB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50215950
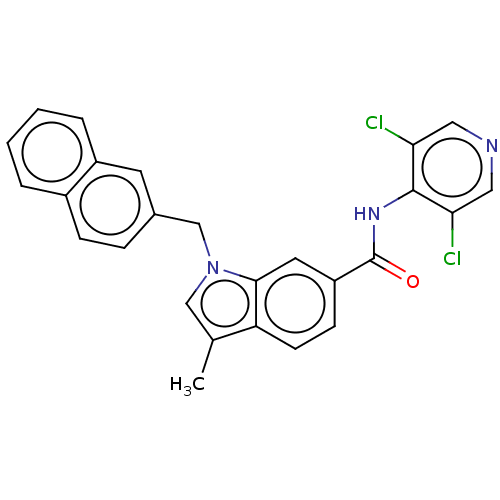
(CHEMBL55046)Show SMILES Cc1cn(Cc2ccc3ccccc3c2)c2cc(ccc12)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C26H19Cl2N3O/c1-16-14-31(15-17-6-7-18-4-2-3-5-19(18)10-17)24-11-20(8-9-21(16)24)26(32)30-25-22(27)12-29-13-23(25)28/h2-14H,15H2,1H3,(H,29,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against PDE4 was determined using [3H]- rolipram in guinea pig brain membrane binding assay |
Bioorg Med Chem Lett 8: 1867-72 (1998)
BindingDB Entry DOI: 10.7270/Q2CJ8GPB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50214362
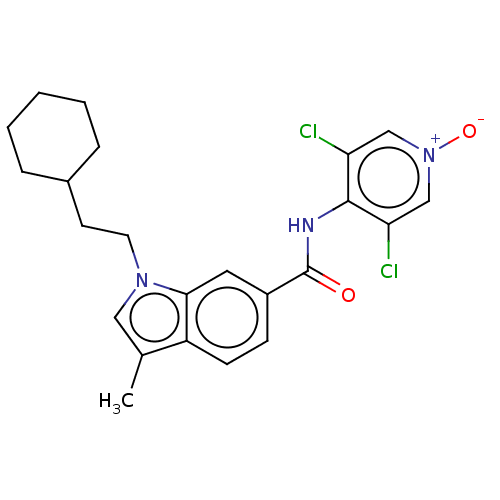
(CHEMBL102635)Show SMILES Cc1cn(CCC2CCCCC2)c2cc(ccc12)C(=O)Nc1c(Cl)c[n+]([O-])cc1Cl Show InChI InChI=1S/C23H25Cl2N3O2/c1-15-12-27(10-9-16-5-3-2-4-6-16)21-11-17(7-8-18(15)21)23(29)26-22-19(24)13-28(30)14-20(22)25/h7-8,11-14,16H,2-6,9-10H2,1H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Affinity for rolipram binding site of phosphodiesterase type IV (PDE4) |
Bioorg Med Chem Lett 8: 3053-8 (1998)
BindingDB Entry DOI: 10.7270/Q2PV6NK6 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50215947
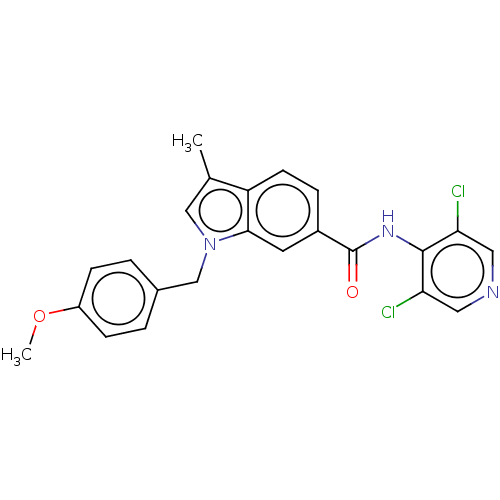
(CHEMBL53710)Show SMILES COc1ccc(Cn2cc(C)c3ccc(cc23)C(=O)Nc2c(Cl)cncc2Cl)cc1 Show InChI InChI=1S/C23H19Cl2N3O2/c1-14-12-28(13-15-3-6-17(30-2)7-4-15)21-9-16(5-8-18(14)21)23(29)27-22-19(24)10-26-11-20(22)25/h3-12H,13H2,1-2H3,(H,26,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against PDE4 was determined using [3H]- rolipram in guinea pig brain membrane binding assay |
Bioorg Med Chem Lett 8: 1867-72 (1998)
BindingDB Entry DOI: 10.7270/Q2CJ8GPB |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM356652

(US10213433, Compound 34 | US11369599, Compound 34 ...)Show SMILES CO[C@H]1CC[C@H](CNc2ccc(cc2[N+]([O-])=O)S(=O)(=O)NC(=O)c2ccc(cc2Oc2cnc3[nH]ccc3c2)N2CCN(CC3=C(CC(C)(C)CC3)c3ccc(Cl)cc3)CC2)CC1 |wU:5.5,wD:2.1,t:48,(-3.85,-16.17,;-2.52,-15.4,;-2.52,-13.86,;-1.18,-13.09,;-1.18,-11.55,;-2.52,-10.78,;-2.52,-9.24,;-1.18,-8.47,;-1.18,-6.93,;-2.52,-6.16,;-2.52,-4.62,;-1.18,-3.85,;.15,-4.62,;.15,-6.16,;1.48,-6.93,;2.82,-6.16,;1.48,-8.47,;-1.18,-2.31,;-2.72,-2.31,;.36,-2.31,;-1.18,-.77,;-2.52,,;-3.85,-.77,;-2.52,1.54,;-3.85,2.31,;-3.85,3.85,;-2.52,4.62,;-1.18,3.85,;-1.18,2.31,;.15,1.54,;1.48,2.31,;1.48,3.85,;2.82,4.62,;4.15,3.85,;5.61,4.33,;6.52,3.08,;5.61,1.83,;4.15,2.31,;2.82,1.54,;-2.52,6.16,;-3.85,6.93,;-3.85,8.47,;-2.52,9.24,;-2.52,10.78,;-1.18,11.55,;-1.18,13.09,;.15,13.86,;1.48,13.09,;2.25,14.42,;3.02,13.09,;1.48,11.55,;.15,10.78,;-2.52,13.86,;-3.85,13.09,;-5.19,13.86,;-5.19,15.4,;-6.52,16.17,;-3.85,16.17,;-2.52,15.4,;-1.18,8.47,;-1.18,6.93,;-3.85,-11.55,;-3.85,-13.09,)| Show InChI InChI=1S/C47H54ClN7O7S/c1-47(2)18-16-34(41(27-47)32-6-8-35(48)9-7-32)30-53-20-22-54(23-21-53)36-10-14-40(44(25-36)62-38-24-33-17-19-49-45(33)51-29-38)46(56)52-63(59,60)39-13-15-42(43(26-39)55(57)58)50-28-31-4-11-37(61-3)12-5-31/h6-10,13-15,17,19,24-26,29,31,37,50H,4-5,11-12,16,18,20-23,27-28,30H2,1-3H3,(H,49,51)(H,52,56)/t31-,37- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-xL (unknown origin) |
J Med Chem 61: 2636-2651 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00717
BindingDB Entry DOI: 10.7270/Q2ZK5K94 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50215945
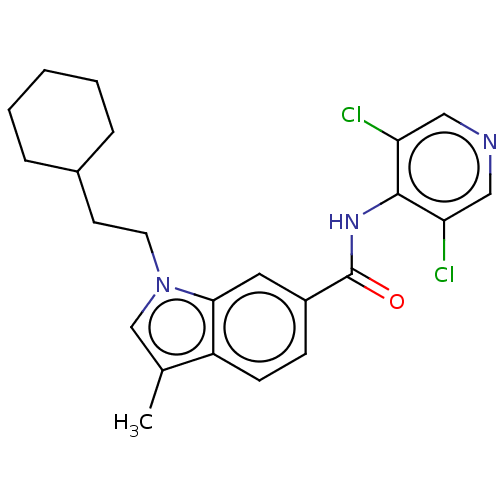
(CHEMBL54988)Show SMILES Cc1cn(CCC2CCCCC2)c2cc(ccc12)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C23H25Cl2N3O/c1-15-14-28(10-9-16-5-3-2-4-6-16)21-11-17(7-8-18(15)21)23(29)27-22-19(24)12-26-13-20(22)25/h7-8,11-14,16H,2-6,9-10H2,1H3,(H,26,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against PDE4 was determined using [3H]- rolipram in guinea pig brain membrane binding assay |
Bioorg Med Chem Lett 8: 1867-72 (1998)
BindingDB Entry DOI: 10.7270/Q2CJ8GPB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50215955
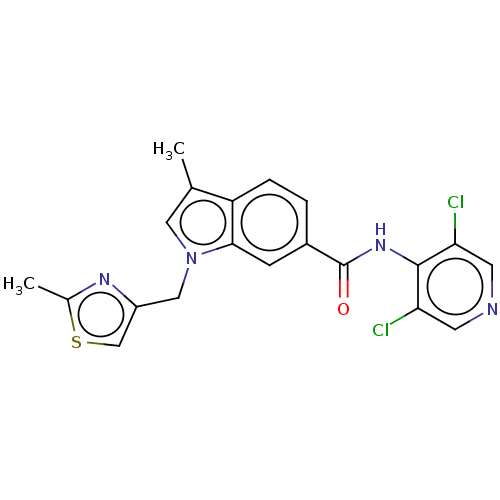
(CHEMBL301448)Show SMILES Cc1nc(Cn2cc(C)c3ccc(cc23)C(=O)Nc2c(Cl)cncc2Cl)cs1 Show InChI InChI=1S/C20H16Cl2N4OS/c1-11-8-26(9-14-10-28-12(2)24-14)18-5-13(3-4-15(11)18)20(27)25-19-16(21)6-23-7-17(19)22/h3-8,10H,9H2,1-2H3,(H,23,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against PDE4 was determined using [3H]- rolipram in guinea pig brain membrane binding assay |
Bioorg Med Chem Lett 8: 1867-72 (1998)
BindingDB Entry DOI: 10.7270/Q2CJ8GPB |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50067424

((S)-9-Methoxy-7-oxo-3b,4,5,6-tetrahydro-7H-2,6a,11...)Show SMILES CCOC(=O)c1ncn-2c1[C@@H]1CCCN1C(=O)c1cc(OC)ccc-21 Show InChI InChI=1S/C18H19N3O4/c1-3-25-18(23)15-16-14-5-4-8-20(14)17(22)12-9-11(24-2)6-7-13(12)21(16)10-19-15/h6-7,9-10,14H,3-5,8H2,1-2H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50067424

((S)-9-Methoxy-7-oxo-3b,4,5,6-tetrahydro-7H-2,6a,11...)Show SMILES CCOC(=O)c1ncn-2c1[C@@H]1CCCN1C(=O)c1cc(OC)ccc-21 Show InChI InChI=1S/C18H19N3O4/c1-3-25-18(23)15-16-14-5-4-8-20(14)17(22)12-9-11(24-2)6-7-13(12)21(16)10-19-15/h6-7,9-10,14H,3-5,8H2,1-2H3/t14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50215125
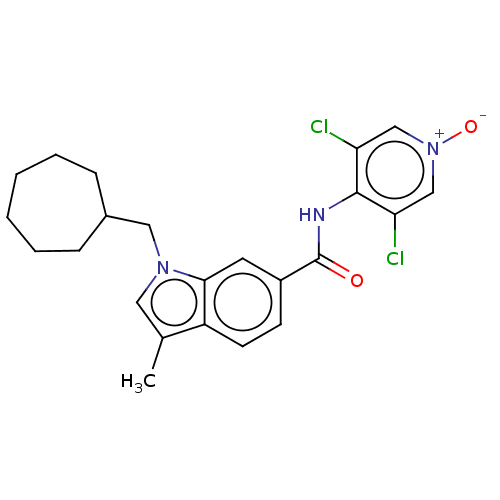
(CHEMBL102503)Show SMILES Cc1cn(CC2CCCCCC2)c2cc(ccc12)C(=O)Nc1c(Cl)c[n+]([O-])cc1Cl Show InChI InChI=1S/C23H25Cl2N3O2/c1-15-11-27(12-16-6-4-2-3-5-7-16)21-10-17(8-9-18(15)21)23(29)26-22-19(24)13-28(30)14-20(22)25/h8-11,13-14,16H,2-7,12H2,1H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Affinity for rolipram binding site of phosphodiesterase type IV (PDE4) |
Bioorg Med Chem Lett 8: 3053-8 (1998)
BindingDB Entry DOI: 10.7270/Q2PV6NK6 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50214422
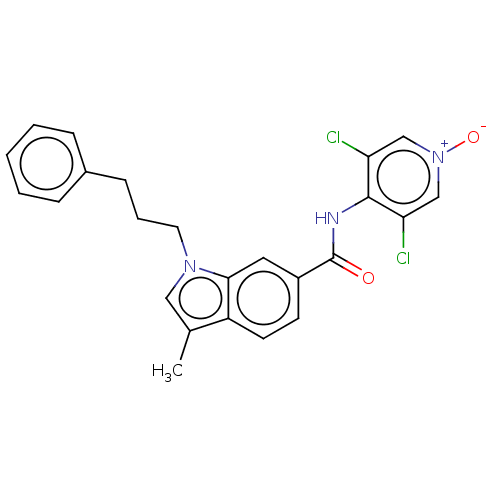
(CHEMBL101479)Show SMILES Cc1cn(CCCc2ccccc2)c2cc(ccc12)C(=O)Nc1c(Cl)c[n+]([O-])cc1Cl Show InChI InChI=1S/C24H21Cl2N3O2/c1-16-13-28(11-5-8-17-6-3-2-4-7-17)22-12-18(9-10-19(16)22)24(30)27-23-20(25)14-29(31)15-21(23)26/h2-4,6-7,9-10,12-15H,5,8,11H2,1H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Affinity for rolipram binding site of phosphodiesterase type IV (PDE4) |
Bioorg Med Chem Lett 8: 3053-8 (1998)
BindingDB Entry DOI: 10.7270/Q2PV6NK6 |
More data for this
Ligand-Target Pair | |
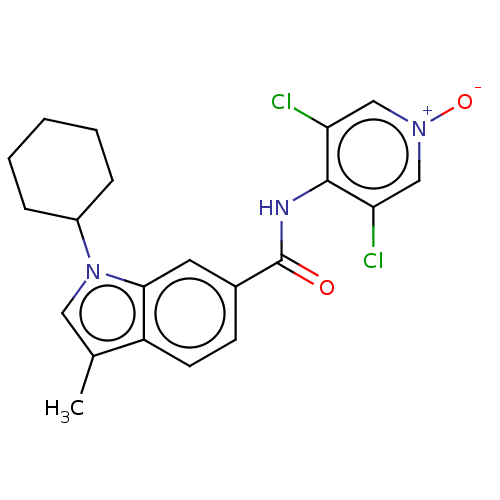
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50215127
(CHEMBL102310)Show SMILES Cc1cn(C2CCCCC2)c2cc(ccc12)C(=O)Nc1c(Cl)c[n+]([O-])cc1Cl Show InChI InChI=1S/C21H21Cl2N3O2/c1-13-10-26(15-5-3-2-4-6-15)19-9-14(7-8-16(13)19)21(27)24-20-17(22)11-25(28)12-18(20)23/h7-12,15H,2-6H2,1H3,(H,24,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Affinity for rolipram binding site of phosphodiesterase type IV (PDE4) |
Bioorg Med Chem Lett 8: 3053-8 (1998)
BindingDB Entry DOI: 10.7270/Q2PV6NK6 |
More data for this
Ligand-Target Pair | |
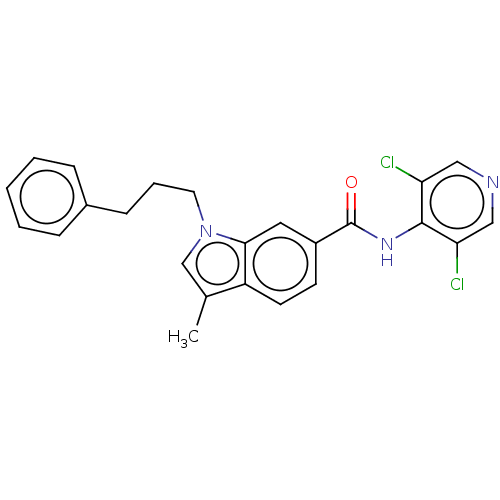
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50215948
(CHEMBL54361)Show SMILES Cc1cn(CCCc2ccccc2)c2cc(ccc12)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C24H21Cl2N3O/c1-16-15-29(11-5-8-17-6-3-2-4-7-17)22-12-18(9-10-19(16)22)24(30)28-23-20(25)13-27-14-21(23)26/h2-4,6-7,9-10,12-15H,5,8,11H2,1H3,(H,27,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against PDE4 was determined using [3H]- rolipram in guinea pig brain membrane binding assay |
Bioorg Med Chem Lett 8: 1867-72 (1998)
BindingDB Entry DOI: 10.7270/Q2CJ8GPB |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-xL (unknown origin) |
J Med Chem 61: 2636-2651 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00717
BindingDB Entry DOI: 10.7270/Q2ZK5K94 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50067424

((S)-9-Methoxy-7-oxo-3b,4,5,6-tetrahydro-7H-2,6a,11...)Show SMILES CCOC(=O)c1ncn-2c1[C@@H]1CCCN1C(=O)c1cc(OC)ccc-21 Show InChI InChI=1S/C18H19N3O4/c1-3-25-18(23)15-16-14-5-4-8-20(14)17(22)12-9-11(24-2)6-7-13(12)21(16)10-19-15/h6-7,9-10,14H,3-5,8H2,1-2H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50214506
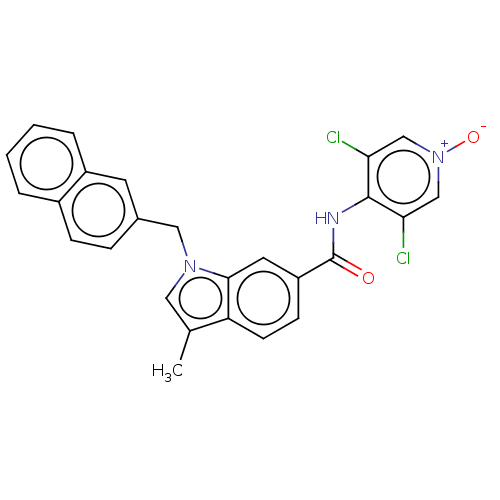
(CHEMBL323069)Show SMILES Cc1cn(Cc2ccc3ccccc3c2)c2cc(ccc12)C(=O)Nc1c(Cl)c[n+]([O-])cc1Cl Show InChI InChI=1S/C26H19Cl2N3O2/c1-16-12-30(13-17-6-7-18-4-2-3-5-19(18)10-17)24-11-20(8-9-21(16)24)26(32)29-25-22(27)14-31(33)15-23(25)28/h2-12,14-15H,13H2,1H3,(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Affinity for rolipram binding site of phosphodiesterase type IV (PDE4) |
Bioorg Med Chem Lett 8: 3053-8 (1998)
BindingDB Entry DOI: 10.7270/Q2PV6NK6 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50215953
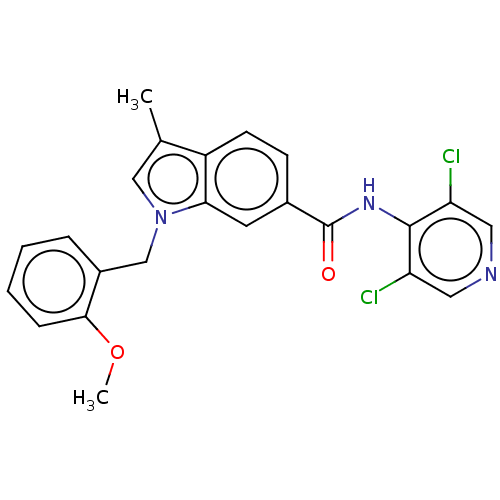
(CHEMBL292925)Show SMILES COc1ccccc1Cn1cc(C)c2ccc(cc12)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C23H19Cl2N3O2/c1-14-12-28(13-16-5-3-4-6-21(16)30-2)20-9-15(7-8-17(14)20)23(29)27-22-18(24)10-26-11-19(22)25/h3-12H,13H2,1-2H3,(H,26,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against PDE4 was determined using [3H]- rolipram in guinea pig brain membrane binding assay |
Bioorg Med Chem Lett 8: 1867-72 (1998)
BindingDB Entry DOI: 10.7270/Q2CJ8GPB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50214749
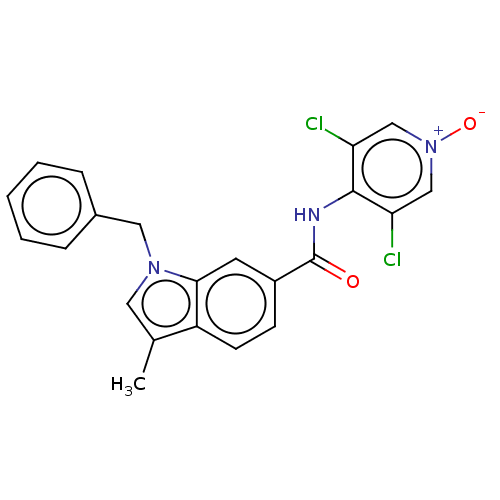
(CHEMBL102741)Show SMILES Cc1cn(Cc2ccccc2)c2cc(ccc12)C(=O)Nc1c(Cl)c[n+]([O-])cc1Cl Show InChI InChI=1S/C22H17Cl2N3O2/c1-14-10-26(11-15-5-3-2-4-6-15)20-9-16(7-8-17(14)20)22(28)25-21-18(23)12-27(29)13-19(21)24/h2-10,12-13H,11H2,1H3,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Affinity for rolipram binding site of phosphodiesterase type IV (PDE4) |
Bioorg Med Chem Lett 8: 3053-8 (1998)
BindingDB Entry DOI: 10.7270/Q2PV6NK6 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
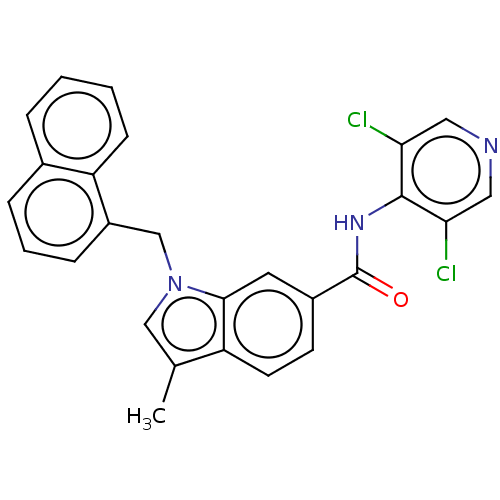
(Homo sapiens (Human)) | BDBM50215944
(CHEMBL54590)Show SMILES Cc1cn(Cc2cccc3ccccc23)c2cc(ccc12)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C26H19Cl2N3O/c1-16-14-31(15-19-7-4-6-17-5-2-3-8-21(17)19)24-11-18(9-10-20(16)24)26(32)30-25-22(27)12-29-13-23(25)28/h2-14H,15H2,1H3,(H,29,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against PDE4 was determined using [3H]- rolipram in guinea pig brain membrane binding assay |
Bioorg Med Chem Lett 8: 1867-72 (1998)
BindingDB Entry DOI: 10.7270/Q2CJ8GPB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50215057
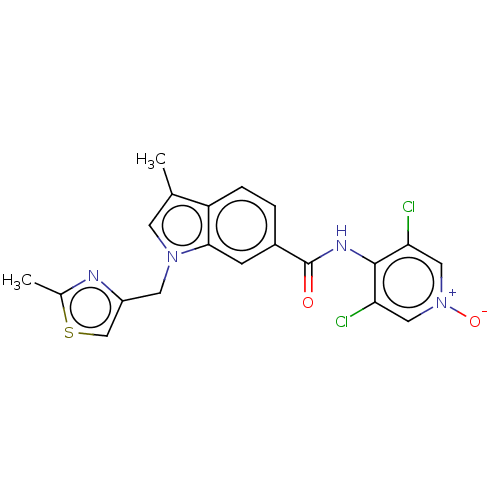
(CHEMBL322837)Show SMILES Cc1nc(Cn2cc(C)c3ccc(cc23)C(=O)Nc2c(Cl)c[n+]([O-])cc2Cl)cs1 Show InChI InChI=1S/C20H16Cl2N4O2S/c1-11-6-25(7-14-10-29-12(2)23-14)18-5-13(3-4-15(11)18)20(27)24-19-16(21)8-26(28)9-17(19)22/h3-6,8-10H,7H2,1-2H3,(H,24,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Affinity for rolipram binding site of phosphodiesterase type IV (PDE4) |
Bioorg Med Chem Lett 8: 3053-8 (1998)
BindingDB Entry DOI: 10.7270/Q2PV6NK6 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50215952
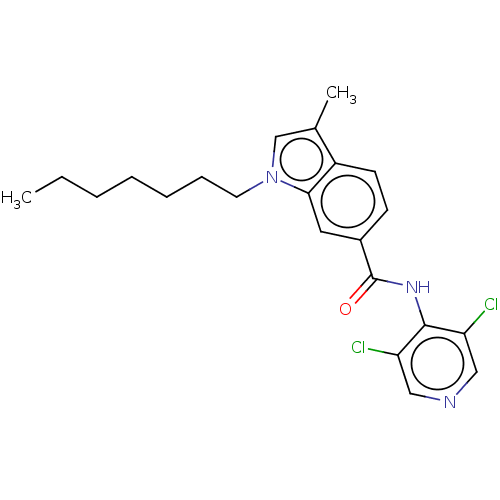
(CHEMBL55203)Show SMILES CCCCCCCn1cc(C)c2ccc(cc12)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C22H25Cl2N3O/c1-3-4-5-6-7-10-27-14-15(2)17-9-8-16(11-20(17)27)22(28)26-21-18(23)12-25-13-19(21)24/h8-9,11-14H,3-7,10H2,1-2H3,(H,25,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against PDE4 was determined using [3H]- rolipram in guinea pig brain membrane binding assay |
Bioorg Med Chem Lett 8: 1867-72 (1998)
BindingDB Entry DOI: 10.7270/Q2CJ8GPB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50215126
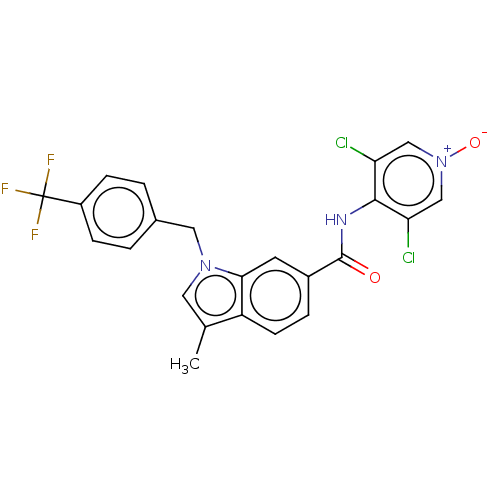
(CHEMBL414417)Show SMILES Cc1cn(Cc2ccc(cc2)C(F)(F)F)c2cc(ccc12)C(=O)Nc1c(Cl)c[n+]([O-])cc1Cl Show InChI InChI=1S/C23H16Cl2F3N3O2/c1-13-9-30(10-14-2-5-16(6-3-14)23(26,27)28)20-8-15(4-7-17(13)20)22(32)29-21-18(24)11-31(33)12-19(21)25/h2-9,11-12H,10H2,1H3,(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Affinity for rolipram binding site of phosphodiesterase type IV (PDE4) |
Bioorg Med Chem Lett 8: 3053-8 (1998)
BindingDB Entry DOI: 10.7270/Q2PV6NK6 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
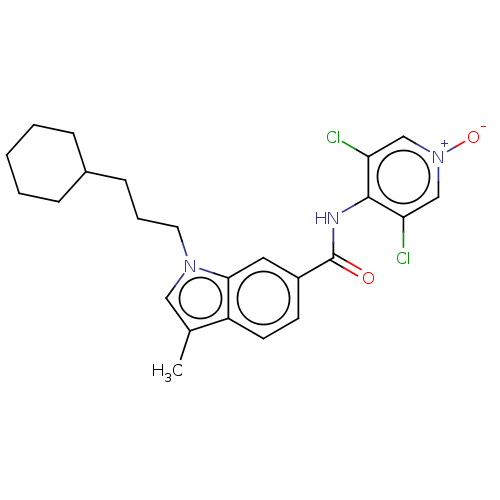
(Homo sapiens (Human)) | BDBM50214380
(CHEMBL323132)Show SMILES Cc1cn(CCCC2CCCCC2)c2cc(ccc12)C(=O)Nc1c(Cl)c[n+]([O-])cc1Cl Show InChI InChI=1S/C24H27Cl2N3O2/c1-16-13-28(11-5-8-17-6-3-2-4-7-17)22-12-18(9-10-19(16)22)24(30)27-23-20(25)14-29(31)15-21(23)26/h9-10,12-15,17H,2-8,11H2,1H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Affinity for rolipram binding site of phosphodiesterase type IV (PDE4) |
Bioorg Med Chem Lett 8: 3053-8 (1998)
BindingDB Entry DOI: 10.7270/Q2PV6NK6 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50214748
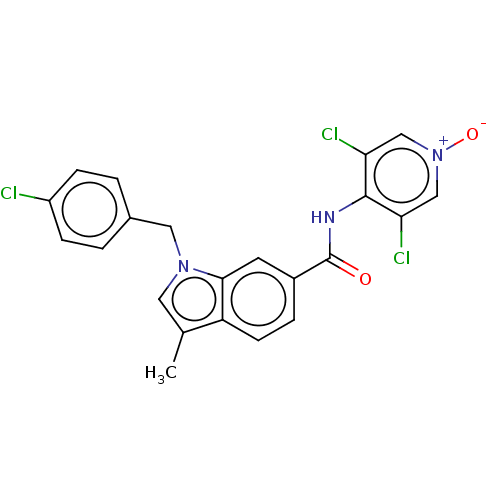
(CHEMBL103007)Show SMILES Cc1cn(Cc2ccc(Cl)cc2)c2cc(ccc12)C(=O)Nc1c(Cl)c[n+]([O-])cc1Cl Show InChI InChI=1S/C22H16Cl3N3O2/c1-13-9-27(10-14-2-5-16(23)6-3-14)20-8-15(4-7-17(13)20)22(29)26-21-18(24)11-28(30)12-19(21)25/h2-9,11-12H,10H2,1H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Affinity for rolipram binding site of phosphodiesterase type IV (PDE4) |
Bioorg Med Chem Lett 8: 3053-8 (1998)
BindingDB Entry DOI: 10.7270/Q2PV6NK6 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50215940
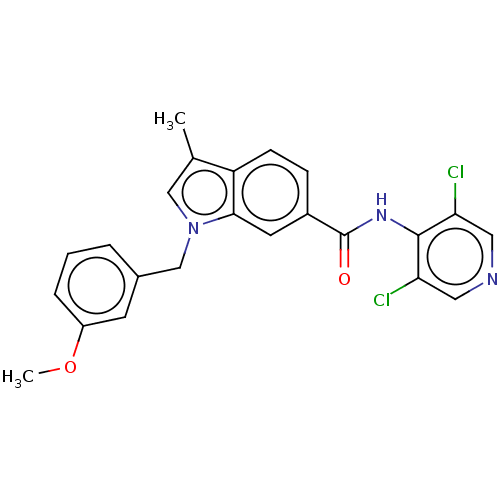
(CHEMBL53559)Show SMILES COc1cccc(Cn2cc(C)c3ccc(cc23)C(=O)Nc2c(Cl)cncc2Cl)c1 Show InChI InChI=1S/C23H19Cl2N3O2/c1-14-12-28(13-15-4-3-5-17(8-15)30-2)21-9-16(6-7-18(14)21)23(29)27-22-19(24)10-26-11-20(22)25/h3-12H,13H2,1-2H3,(H,26,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against PDE4 was determined using [3H]- rolipram in guinea pig brain membrane binding assay |
Bioorg Med Chem Lett 8: 1867-72 (1998)
BindingDB Entry DOI: 10.7270/Q2CJ8GPB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50215941
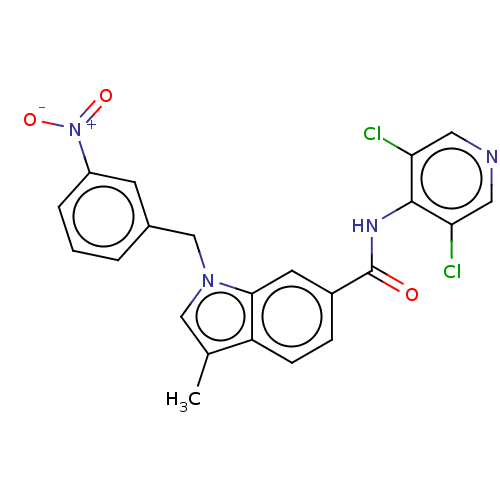
(CHEMBL300395)Show SMILES Cc1cn(Cc2cccc(c2)[N+]([O-])=O)c2cc(ccc12)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C22H16Cl2N4O3/c1-13-11-27(12-14-3-2-4-16(7-14)28(30)31)20-8-15(5-6-17(13)20)22(29)26-21-18(23)9-25-10-19(21)24/h2-11H,12H2,1H3,(H,25,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against PDE4 was determined using [3H]- rolipram in guinea pig brain membrane binding assay |
Bioorg Med Chem Lett 8: 1867-72 (1998)
BindingDB Entry DOI: 10.7270/Q2CJ8GPB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50215943
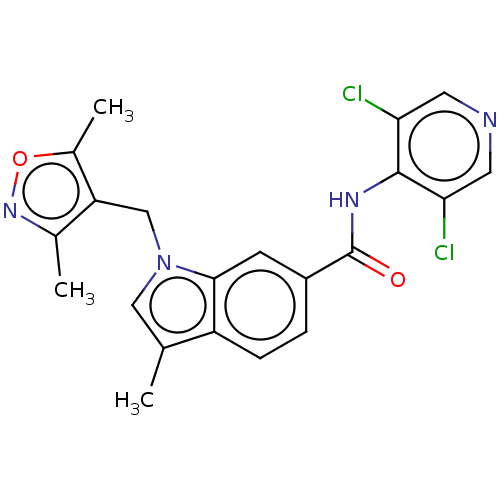
(CHEMBL417930)Show SMILES Cc1cn(Cc2c(C)noc2C)c2cc(ccc12)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C21H18Cl2N4O2/c1-11-9-27(10-16-12(2)26-29-13(16)3)19-6-14(4-5-15(11)19)21(28)25-20-17(22)7-24-8-18(20)23/h4-9H,10H2,1-3H3,(H,24,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against PDE4 was determined using [3H]- rolipram in guinea pig brain membrane binding assay |
Bioorg Med Chem Lett 8: 1867-72 (1998)
BindingDB Entry DOI: 10.7270/Q2CJ8GPB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50215128
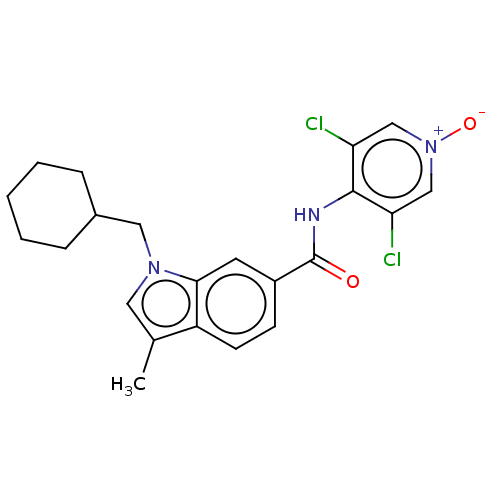
(CHEMBL318586)Show SMILES Cc1cn(CC2CCCCC2)c2cc(ccc12)C(=O)Nc1c(Cl)c[n+]([O-])cc1Cl Show InChI InChI=1S/C22H23Cl2N3O2/c1-14-10-26(11-15-5-3-2-4-6-15)20-9-16(7-8-17(14)20)22(28)25-21-18(23)12-27(29)13-19(21)24/h7-10,12-13,15H,2-6,11H2,1H3,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Affinity for rolipram binding site of phosphodiesterase type IV (PDE4) |
Bioorg Med Chem Lett 8: 3053-8 (1998)
BindingDB Entry DOI: 10.7270/Q2PV6NK6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data