 Found 657 hits with Last Name = 'cusack' and Initial = 'k'
Found 657 hits with Last Name = 'cusack' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM620450
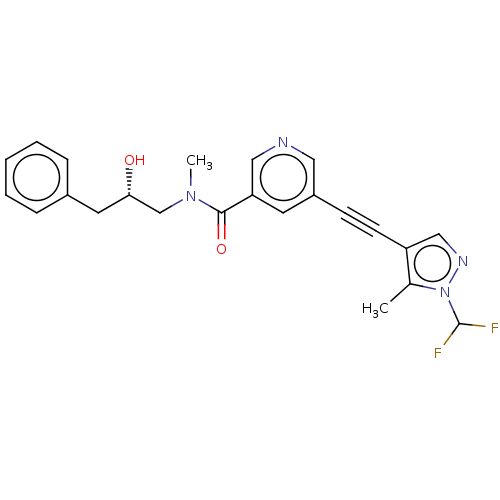
((S)-5-((1-(difluoromethyl)-5-methyl-1H-pyrazol-4-y...)Show SMILES CN(C[C@@H](O)Cc1ccccc1)C(=O)c1cncc(c1)C#Cc1cnn(C(F)F)c1C | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM620443

(US11767310, Example 11)Show SMILES CN(C[C@@H](O)Cc1ccccc1)C(=O)c1cncc(c1)C#Cc1cnn(C)c1C1CC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM620438

(US11767310, Example 6)Show SMILES CN(C[C@@H](O)Cc1ccccc1)C(=O)c1cncc(c1)C#Cc1cnn(c1)C1CCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM620434
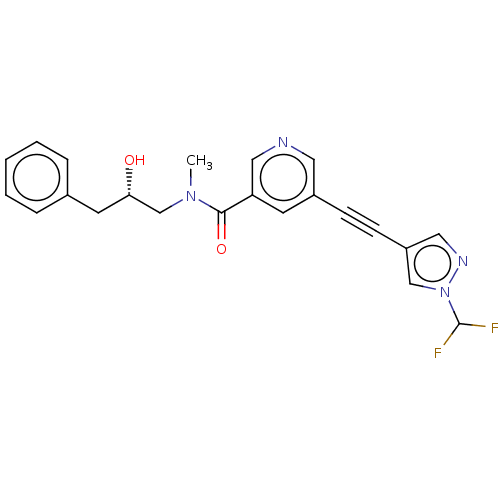
(US11767310, Example 2)Show SMILES CN(C[C@@H](O)Cc1ccccc1)C(=O)c1cncc(c1)C#Cc1cnn(c1)C(F)F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM620440

(US11767310, Example 8)Show SMILES CN(C[C@@H](O)Cc1ccccc1)C(=O)c1cncc(c1)C#Cc1cnn2CCOCc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM620439
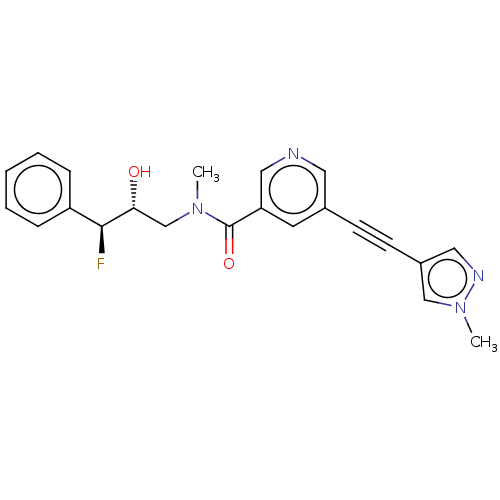
(US11767310, Example 7)Show SMILES CN(C[C@@H](O)[C@@H](F)c1ccccc1)C(=O)c1cncc(c1)C#Cc1cnn(C)c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM620446

(US11767310, Example 14)Show SMILES CN(C[C@@H](O)Cc1ccccc1)C(=O)c1cnc(C)c(c1)C#Cc1cnn(C)c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323351

(6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-isobutyl-6,7-d...)Show SMILES CC(C)CC1n2cncc2CN([C@@H](C)c2ccc(Cl)cc2)S1(=O)=O |r| Show InChI InChI=1S/C17H22ClN3O2S/c1-12(2)8-17-20-11-19-9-16(20)10-21(24(17,22)23)13(3)14-4-6-15(18)7-5-14/h4-7,9,11-13,17H,8,10H2,1-3H3/t13-,17?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 |
Bioorg Med Chem Lett 23: 5471-83 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.003
BindingDB Entry DOI: 10.7270/Q2R49S6F |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 8
(Homo sapiens (Human)) | BDBM50264866

(4'-(2-(2H-tetrazol-5-yl)thieno[2,3-c]pyridin-4-ylo...)Show SMILES NC(=O)c1ccc(cc1)-c1ccc(Oc2cncc3sc(cc23)-c2nnn[nH]2)cc1 Show InChI InChI=1S/C21H14N6O2S/c22-20(28)14-3-1-12(2-4-14)13-5-7-15(8-6-13)29-17-10-23-11-19-16(17)9-18(30-19)21-24-26-27-25-21/h1-11H,(H2,22,28)(H,24,25,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COT by HTRF-based assay |
Bioorg Med Chem Lett 18: 4952-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.037
BindingDB Entry DOI: 10.7270/Q23J3CTH |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM620433

(US11767310, Example 1)Show SMILES CN(C[C@@H](O)Cc1ccccc1)C(=O)c1cncc(c1)C#Cc1cnn(C)c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323353
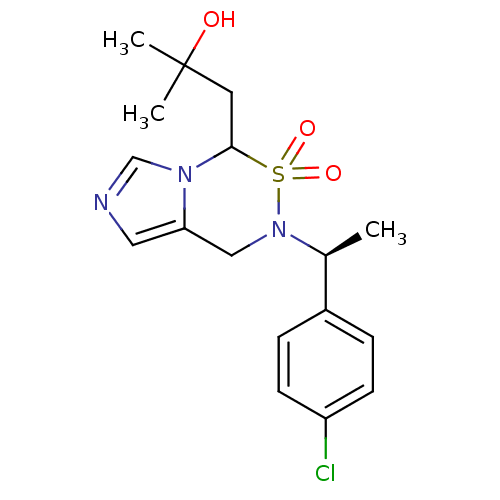
(1-{6-[(S)-1-(4-Chloro-phenyl)-ethyl]-5,5-dioxo-4,5...)Show SMILES C[C@H](N1Cc2cncn2C(CC(C)(C)O)S1(=O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C17H22ClN3O3S/c1-12(13-4-6-14(18)7-5-13)21-10-15-9-19-11-20(15)16(25(21,23)24)8-17(2,3)22/h4-7,9,11-12,16,22H,8,10H2,1-3H3/t12-,16?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 |
Bioorg Med Chem Lett 23: 5471-83 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.003
BindingDB Entry DOI: 10.7270/Q2R49S6F |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM620444

(US11767310, Example 12)Show SMILES CN(C[C@@H](O)Cc1cccc(F)c1)C(=O)c1cncc(c1)C#Cc1cnn(C)c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM620437
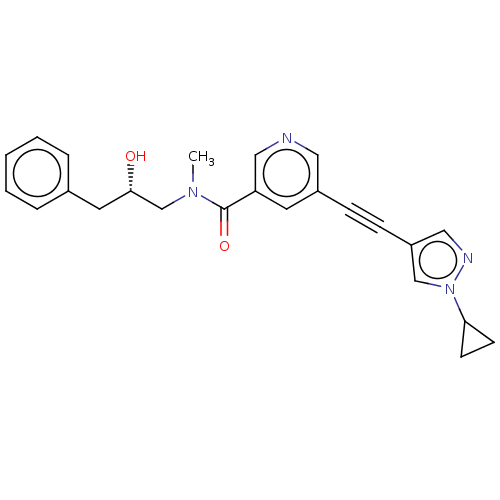
(US11767310, Example 5)Show SMILES CN(C[C@@H](O)Cc1ccccc1)C(=O)c1cncc(c1)C#Cc1cnn(c1)C1CC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM620441
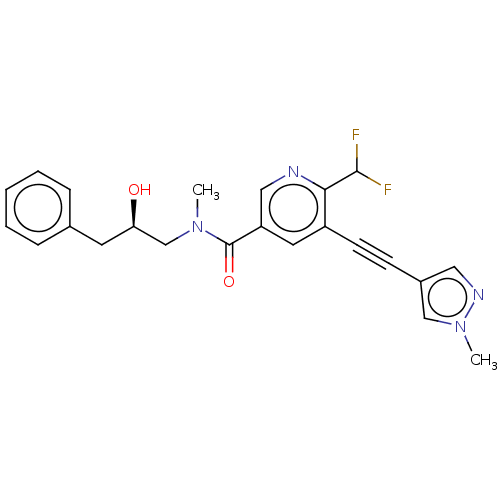
(US11767310, Example 9)Show SMILES CN(C[C@H](O)Cc1ccccc1)C(=O)c1cnc(C(F)F)c(c1)C#Cc1cnn(C)c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 8
(Homo sapiens (Human)) | BDBM28043
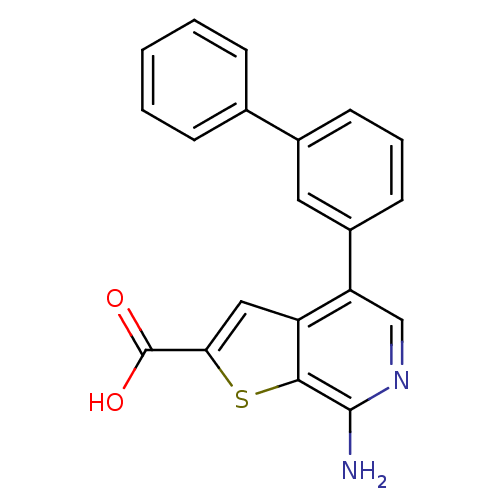
(7-amino-4-(3-phenylphenyl)thieno[2,3-c]pyridine-2-...)Show SMILES Nc1ncc(-c2cccc(c2)-c2ccccc2)c2cc(sc12)C(O)=O Show InChI InChI=1S/C20H14N2O2S/c21-19-18-15(10-17(25-18)20(23)24)16(11-22-19)14-8-4-7-13(9-14)12-5-2-1-3-6-12/h1-11H,(H2,21,22)(H,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The COT direct HTRF assay was carried out using biotin-MEK1 as a substrate. The reaction was carried out in black 96 half-well plates. At designated... |
Bioorg Med Chem Lett 19: 1722-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.088
BindingDB Entry DOI: 10.7270/Q2VH5M4P |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50155144

(4-(3-Phenyl-1H-indol-2-ylmethylene)-5-pyrazin-2-yl...)Show SMILES O=C1NN=C(\C1=C\c1[nH]c2ccccc2c1-c1ccccc1)c1cnccn1 |c:3| Show InChI InChI=1S/C22H15N5O/c28-22-16(21(26-27-22)19-13-23-10-11-24-19)12-18-20(14-6-2-1-3-7-14)15-8-4-5-9-17(15)25-18/h1-13,25H,(H,27,28)/b16-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of Vascular endothelial growth factor receptor 2(KDR) without DTT(dithiothreitol) |
Bioorg Med Chem Lett 14: 5503-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.007
BindingDB Entry DOI: 10.7270/Q2NK3DJN |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM620436

(US11767310, Example 4)Show SMILES CN(C[C@@H](O)Cc1ccccc1)C(=O)c1cncc(c1)C#Cc1cnn(c1)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM620449
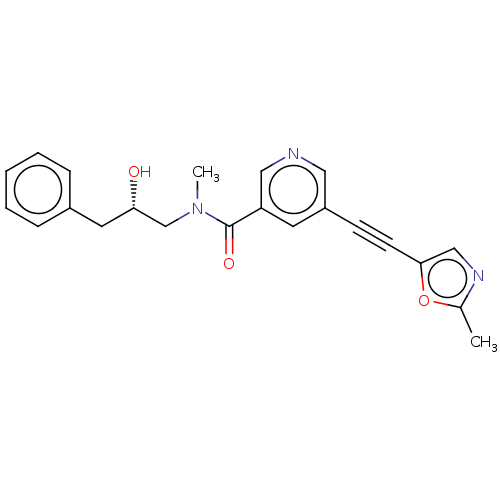
(US11767310, Example 17)Show SMILES CN(C[C@@H](O)Cc1ccccc1)C(=O)c1cncc(c1)C#Cc1cnc(C)o1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM620435
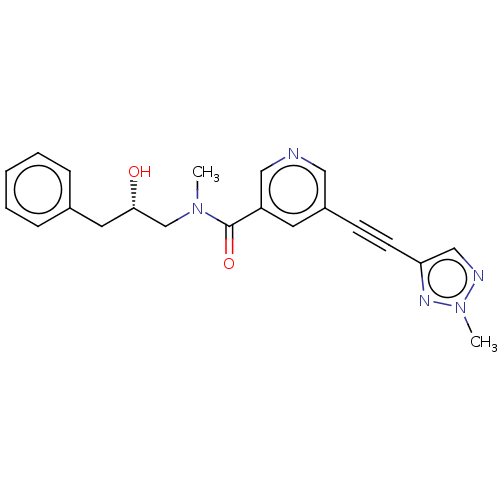
(US11767310, Example 3)Show SMILES CN(C[C@@H](O)Cc1ccccc1)C(=O)c1cncc(c1)C#Cc1cnn(C)n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 8
(Homo sapiens (Human)) | BDBM50264865

((4'-(2-(2H-tetrazol-5-yl)thieno[2,3-c]pyridin-4-yl...)Show SMILES OCc1ccc(cc1)-c1ccc(Oc2cncc3sc(cc23)-c2nnn[nH]2)cc1 Show InChI InChI=1S/C21H15N5O2S/c27-12-13-1-3-14(4-2-13)15-5-7-16(8-6-15)28-18-10-22-11-20-17(18)9-19(29-20)21-23-25-26-24-21/h1-11,27H,12H2,(H,23,24,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COT by HTRF-based assay |
Bioorg Med Chem Lett 18: 4952-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.037
BindingDB Entry DOI: 10.7270/Q23J3CTH |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM620442
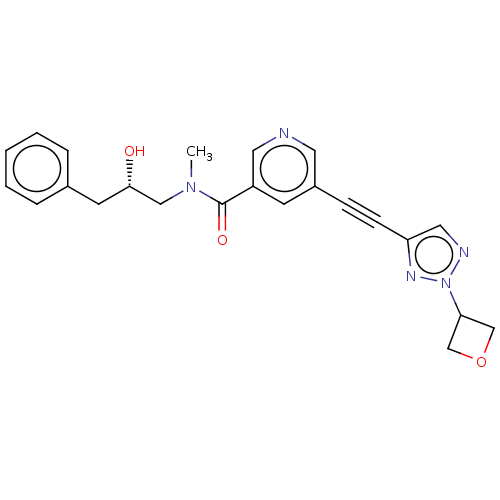
(US11767310, Example 10)Show SMILES CN(C[C@@H](O)Cc1ccccc1)C(=O)c1cncc(c1)C#Cc1cnn(n1)C1COC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 8
(Homo sapiens (Human)) | BDBM50264773
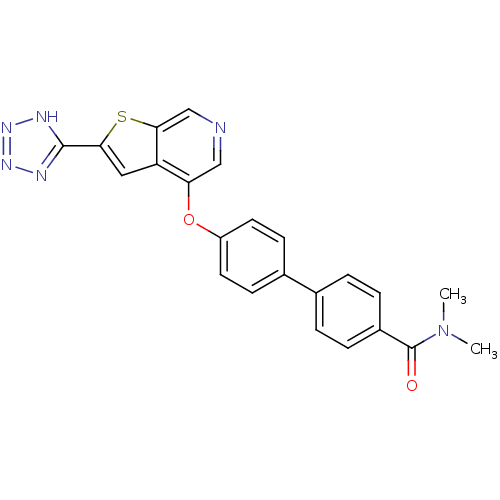
(4'-(2-(2H-tetrazol-5-yl)thieno[2,3-c]pyridin-4-ylo...)Show SMILES CN(C)C(=O)c1ccc(cc1)-c1ccc(Oc2cncc3sc(cc23)-c2nnn[nH]2)cc1 Show InChI InChI=1S/C23H18N6O2S/c1-29(2)23(30)16-5-3-14(4-6-16)15-7-9-17(10-8-15)31-19-12-24-13-21-18(19)11-20(32-21)22-25-27-28-26-22/h3-13H,1-2H3,(H,25,26,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COT by HTRF-based assay |
Bioorg Med Chem Lett 18: 4952-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.037
BindingDB Entry DOI: 10.7270/Q23J3CTH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 8
(Homo sapiens (Human)) | BDBM28037
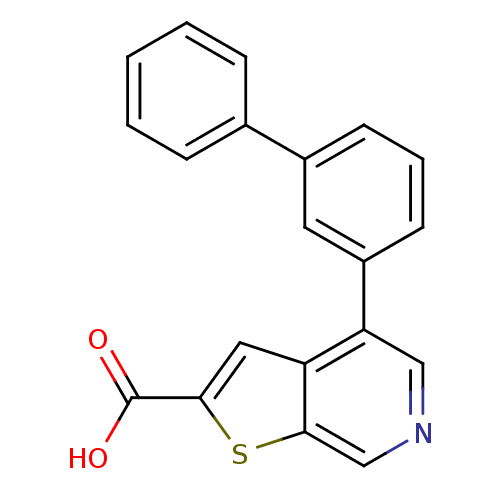
(4-(3-phenylphenyl)thieno[2,3-c]pyridine-2-carboxyl...)Show InChI InChI=1S/C20H13NO2S/c22-20(23)18-10-16-17(11-21-12-19(16)24-18)15-8-4-7-14(9-15)13-5-2-1-3-6-13/h1-12H,(H,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The COT direct HTRF assay was carried out using biotin-MEK1 as a substrate. The reaction was carried out in black 96 half-well plates. At designated... |
Bioorg Med Chem Lett 19: 1722-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.088
BindingDB Entry DOI: 10.7270/Q2VH5M4P |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50155149
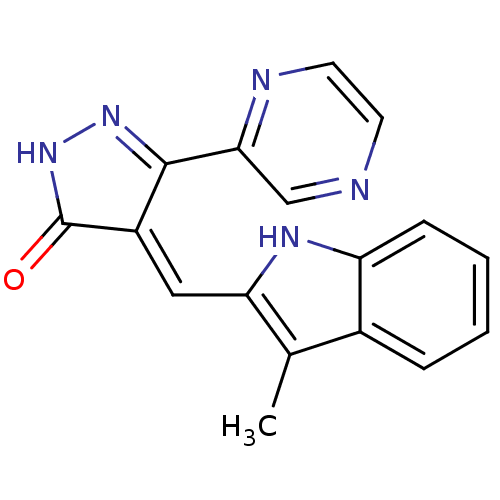
(4-(3-Methyl-1H-indol-2-ylmethylene)-5-pyrazin-2-yl...)Show SMILES Cc1c(\C=C2/C(=O)NN=C2c2cnccn2)[nH]c2ccccc12 |c:8| Show InChI InChI=1S/C17H13N5O/c1-10-11-4-2-3-5-13(11)20-14(10)8-12-16(21-22-17(12)23)15-9-18-6-7-19-15/h2-9,20H,1H3,(H,22,23)/b12-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of Vascular endothelial growth factor receptor 2(KDR) without DTT(dithiothreitol) |
Bioorg Med Chem Lett 14: 5503-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.007
BindingDB Entry DOI: 10.7270/Q2NK3DJN |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50155148
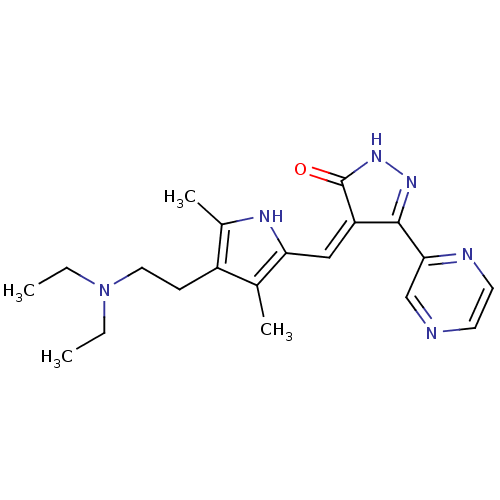
((Z)-4-((4-(2-(diethylamino)ethyl)-3,5-dimethyl-1H-...)Show SMILES CCN(CC)CCc1c(C)[nH]c(\C=C2/C(=O)NN=C2c2cnccn2)c1C |c:17| Show InChI InChI=1S/C20H26N6O/c1-5-26(6-2)10-7-15-13(3)17(23-14(15)4)11-16-19(24-25-20(16)27)18-12-21-8-9-22-18/h8-9,11-12,23H,5-7,10H2,1-4H3,(H,25,27)/b16-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of Vascular endothelial growth factor receptor 2(KDR) with DTT(dithiothreitol) |
Bioorg Med Chem Lett 14: 5503-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.007
BindingDB Entry DOI: 10.7270/Q2NK3DJN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 8
(Homo sapiens (Human)) | BDBM28039
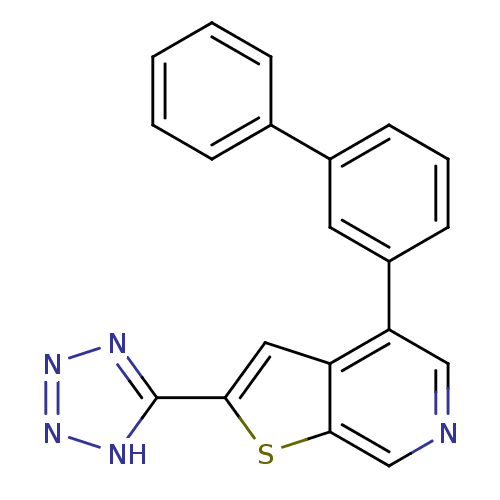
(5-[4-(3-phenylphenyl)thieno[2,3-c]pyridin-2-yl]-1H...)Show SMILES c1c(sc2cncc(-c3cccc(c3)-c3ccccc3)c12)-c1nnn[nH]1 Show InChI InChI=1S/C20H13N5S/c1-2-5-13(6-3-1)14-7-4-8-15(9-14)17-11-21-12-19-16(17)10-18(26-19)20-22-24-25-23-20/h1-12H,(H,22,23,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The COT direct HTRF assay was carried out using biotin-MEK1 as a substrate. The reaction was carried out in black 96 half-well plates. At designated... |
Bioorg Med Chem Lett 19: 1722-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.088
BindingDB Entry DOI: 10.7270/Q2VH5M4P |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 8
(Homo sapiens (Human)) | BDBM50265350
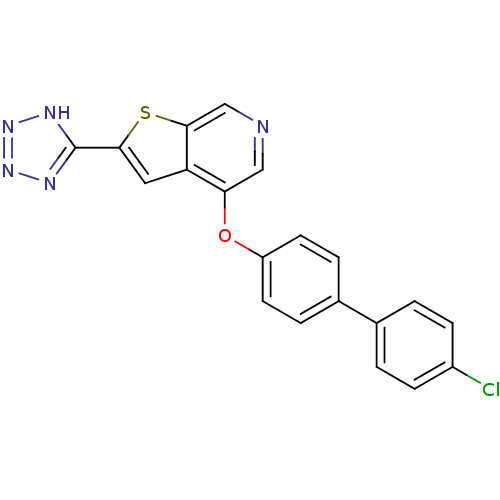
(4-(4'-chlorobiphenyl-4-yloxy)-2-(2H-tetrazol-5-yl)...)Show SMILES Clc1ccc(cc1)-c1ccc(Oc2cncc3sc(cc23)-c2nnn[nH]2)cc1 Show InChI InChI=1S/C20H12ClN5OS/c21-14-5-1-12(2-6-14)13-3-7-15(8-4-13)27-17-10-22-11-19-16(17)9-18(28-19)20-23-25-26-24-20/h1-11H,(H,23,24,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COT by HTRF-based assay |
Bioorg Med Chem Lett 18: 4952-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.037
BindingDB Entry DOI: 10.7270/Q23J3CTH |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM620445
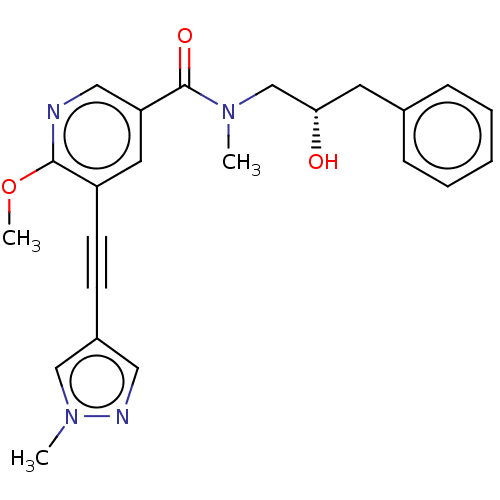
(US11767310, Example 13)Show SMILES COc1ncc(cc1C#Cc1cnn(C)c1)C(=O)N(C)C[C@@H](O)Cc1ccccc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
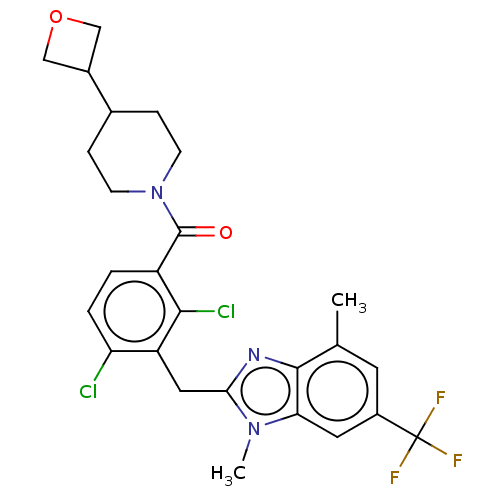
(Homo sapiens (Human)) | BDBM293125
(US10106501, Example BJ-17)Show SMILES Cc1cc(cc2n(C)c(Cc3c(Cl)ccc(C(=O)N4CCC(CC4)C4COC4)c3Cl)nc12)C(F)(F)F Show InChI InChI=1S/C26H26Cl2F3N3O2/c1-14-9-17(26(29,30)31)10-21-24(14)32-22(33(21)2)11-19-20(27)4-3-18(23(19)28)25(35)34-7-5-15(6-8-34)16-12-36-13-16/h3-4,9-10,15-16H,5-8,11-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
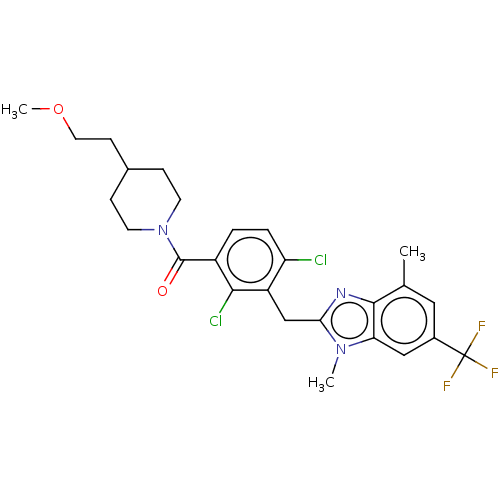
(Homo sapiens (Human)) | BDBM293126
(US10106501, Example BJ-18)Show SMILES COCCC1CCN(CC1)C(=O)c1ccc(Cl)c(Cc2nc3c(C)cc(cc3n2C)C(F)(F)F)c1Cl Show InChI InChI=1S/C26H28Cl2F3N3O2/c1-15-12-17(26(29,30)31)13-21-24(15)32-22(33(21)2)14-19-20(27)5-4-18(23(19)28)25(35)34-9-6-16(7-10-34)8-11-36-3/h4-5,12-13,16H,6-11,14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
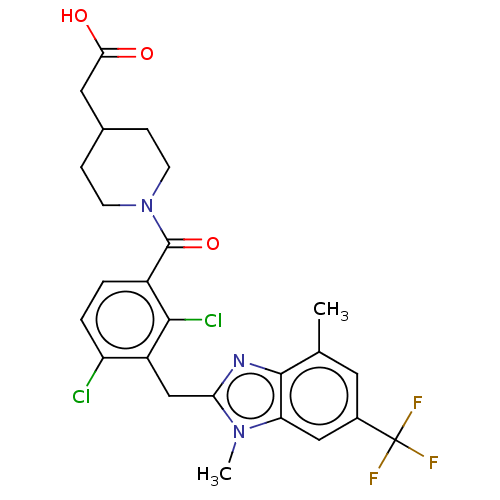
(Homo sapiens (Human)) | BDBM293177
(US10106501, Example BU)Show SMILES Cc1cc(cc2n(C)c(Cc3c(Cl)ccc(C(=O)N4CCC(CC(O)=O)CC4)c3Cl)nc12)C(F)(F)F Show InChI InChI=1S/C25H24Cl2F3N3O3/c1-13-9-15(25(28,29)30)11-19-23(13)31-20(32(19)2)12-17-18(26)4-3-16(22(17)27)24(36)33-7-5-14(6-8-33)10-21(34)35/h3-4,9,11,14H,5-8,10,12H2,1-2H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293207
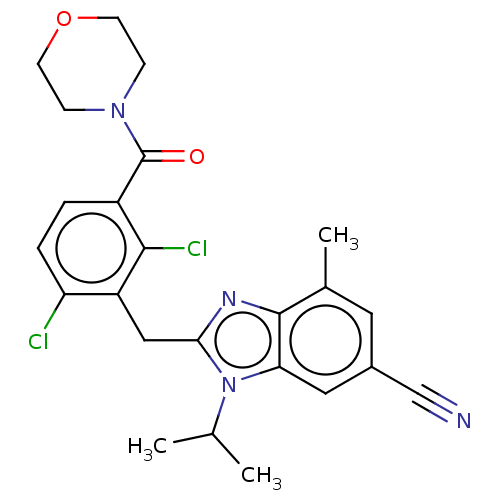
(US10106501, Example CH)Show SMILES CC(C)n1c(Cc2c(Cl)ccc(C(=O)N3CCOCC3)c2Cl)nc2c(C)cc(cc12)C#N Show InChI InChI=1S/C24H24Cl2N4O2/c1-14(2)30-20-11-16(13-27)10-15(3)23(20)28-21(30)12-18-19(25)5-4-17(22(18)26)24(31)29-6-8-32-9-7-29/h4-5,10-11,14H,6-9,12H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293211
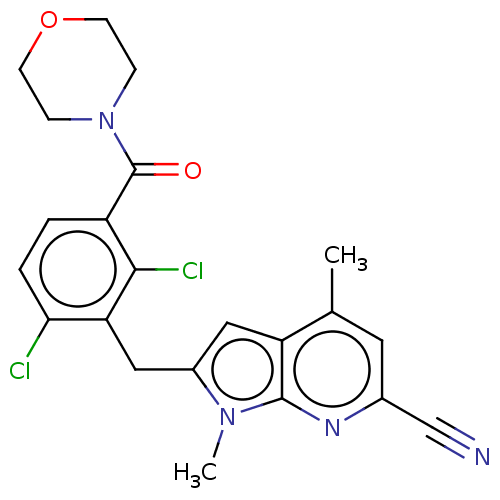
(US10106501, Example CL)Show SMILES Cc1cc(nc2n(C)c(Cc3c(Cl)ccc(C(=O)N4CCOCC4)c3Cl)cc12)C#N Show InChI InChI=1S/C22H20Cl2N4O2/c1-13-9-14(12-25)26-21-17(13)10-15(27(21)2)11-18-19(23)4-3-16(20(18)24)22(29)28-5-7-30-8-6-28/h3-4,9-10H,5-8,11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293212
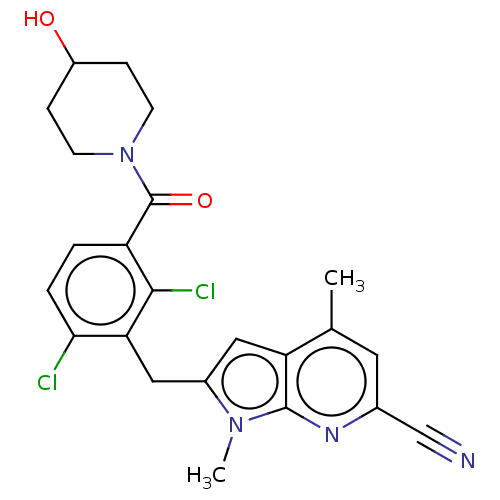
(US10106501, Example CL-1)Show SMILES Cc1cc(nc2n(C)c(Cc3c(Cl)ccc(C(=O)N4CCC(O)CC4)c3Cl)cc12)C#N Show InChI InChI=1S/C23H22Cl2N4O2/c1-13-9-14(12-26)27-22-18(13)10-15(28(22)2)11-19-20(24)4-3-17(21(19)25)23(31)29-7-5-16(30)6-8-29/h3-4,9-10,16,30H,5-8,11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293213
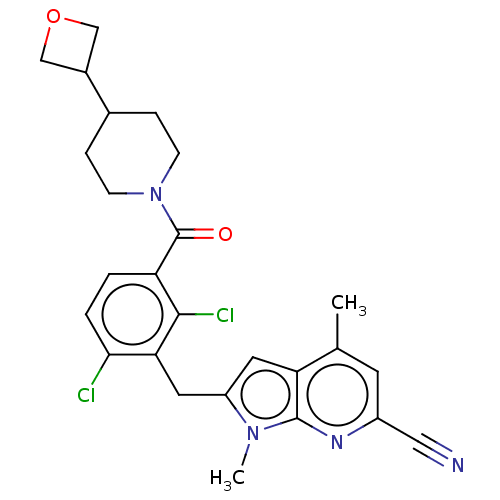
(US10106501, Example CL-2)Show SMILES Cc1cc(nc2n(C)c(Cc3c(Cl)ccc(C(=O)N4CCC(CC4)C4COC4)c3Cl)cc12)C#N Show InChI InChI=1S/C26H26Cl2N4O2/c1-15-9-18(12-29)30-25-21(15)10-19(31(25)2)11-22-23(27)4-3-20(24(22)28)26(33)32-7-5-16(6-8-32)17-13-34-14-17/h3-4,9-10,16-17H,5-8,11,13-14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293214
((2,4-dichloro-3-((1,4-dimethyl-6-(trifluoromethyl)...)Show SMILES Cc1cc(nc2n(C)c(Cc3c(Cl)ccc(C(=O)N4CCOCC4)c3Cl)cc12)C(F)(F)F Show InChI InChI=1S/C22H20Cl2F3N3O2/c1-12-9-18(22(25,26)27)28-20-15(12)10-13(29(20)2)11-16-17(23)4-3-14(19(16)24)21(31)30-5-7-32-8-6-30/h3-4,9-10H,5-8,11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293215
((2,4-dichloro-3-((6-chloro-1,4-dimethyl-1H-pyrrolo...)Show SMILES Cc1cc(Cl)nc2n(C)c(Cc3c(Cl)ccc(C(=O)N4CCOCC4)c3Cl)cc12 Show InChI InChI=1S/C21H20Cl3N3O2/c1-12-9-18(23)25-20-15(12)10-13(26(20)2)11-16-17(22)4-3-14(19(16)24)21(28)27-5-7-29-8-6-27/h3-4,9-10H,5-8,11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293224
(US10106501, Example CT-3)Show SMILES Cc1c(Cc2c(Cl)ccc(C(=O)N3CCC(CC3)C(C)(C)O)c2Cl)nc2c(C)cc(cn12)C(F)(F)F Show InChI InChI=1S/C26H28Cl2F3N3O2/c1-14-11-17(26(29,30)31)13-34-15(2)21(32-23(14)34)12-19-20(27)6-5-18(22(19)28)24(35)33-9-7-16(8-10-33)25(3,4)36/h5-6,11,13,16,36H,7-10,12H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293227
(US10106501, Example CT-6)Show SMILES COC1CCN(CC1)C(=O)c1ccc(Cl)c(Cc2nc3c(C)cc(cn3c2C)C(F)(F)F)c1Cl Show InChI InChI=1S/C24H24Cl2F3N3O2/c1-13-10-15(24(27,28)29)12-32-14(2)20(30-22(13)32)11-18-19(25)5-4-17(21(18)26)23(33)31-8-6-16(34-3)7-9-31/h4-5,10,12,16H,6-9,11H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293228
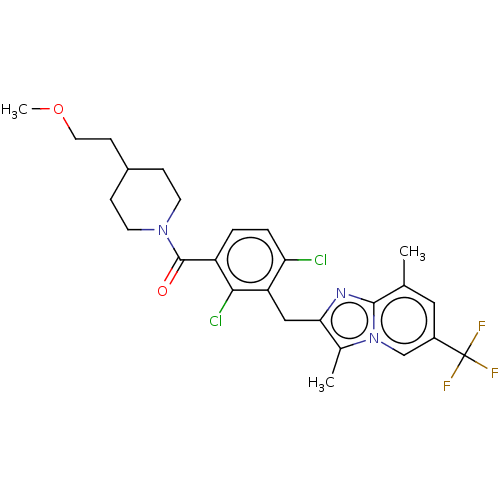
(US10106501, Example CT-7)Show SMILES COCCC1CCN(CC1)C(=O)c1ccc(Cl)c(Cc2nc3c(C)cc(cn3c2C)C(F)(F)F)c1Cl Show InChI InChI=1S/C26H28Cl2F3N3O2/c1-15-12-18(26(29,30)31)14-34-16(2)22(32-24(15)34)13-20-21(27)5-4-19(23(20)28)25(35)33-9-6-17(7-10-33)8-11-36-3/h4-5,12,14,17H,6-11,13H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293229
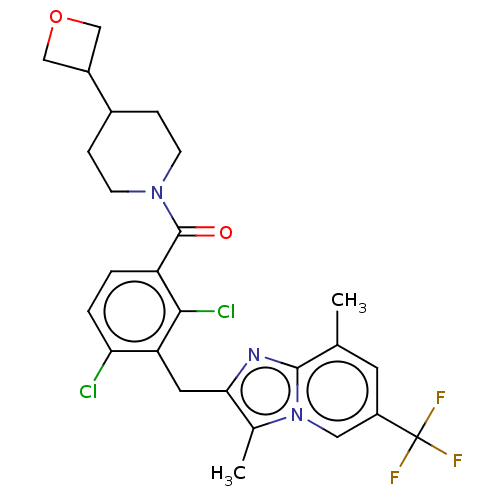
(US10106501, Example CT-8)Show SMILES Cc1c(Cc2c(Cl)ccc(C(=O)N3CCC(CC3)C3COC3)c2Cl)nc2c(C)cc(cn12)C(F)(F)F Show InChI InChI=1S/C26H26Cl2F3N3O2/c1-14-9-18(26(29,30)31)11-34-15(2)22(32-24(14)34)10-20-21(27)4-3-19(23(20)28)25(35)33-7-5-16(6-8-33)17-12-36-13-17/h3-4,9,11,16-17H,5-8,10,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293243
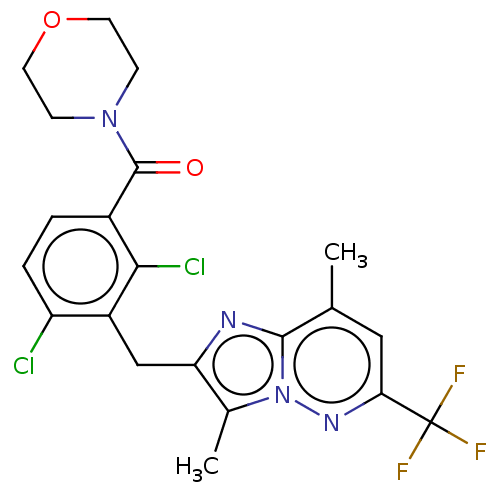
(US10106501, Example CZ)Show SMILES Cc1c(Cc2c(Cl)ccc(C(=O)N3CCOCC3)c2Cl)nc2c(C)cc(nn12)C(F)(F)F Show InChI InChI=1S/C21H19Cl2F3N4O2/c1-11-9-17(21(24,25)26)28-30-12(2)16(27-19(11)30)10-14-15(22)4-3-13(18(14)23)20(31)29-5-7-32-8-6-29/h3-4,9H,5-8,10H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293248
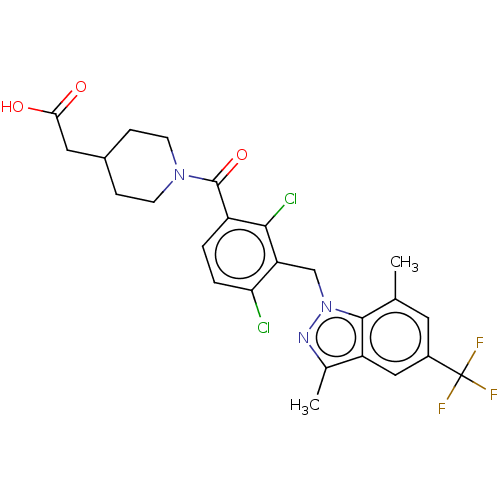
(US10106501, Example DE)Show SMILES Cc1nn(Cc2c(Cl)ccc(C(=O)N3CCC(CC(O)=O)CC3)c2Cl)c2c(C)cc(cc12)C(F)(F)F Show InChI InChI=1S/C25H24Cl2F3N3O3/c1-13-9-16(25(28,29)30)11-18-14(2)31-33(23(13)18)12-19-20(26)4-3-17(22(19)27)24(36)32-7-5-15(6-8-32)10-21(34)35/h3-4,9,11,15H,5-8,10,12H2,1-2H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293250
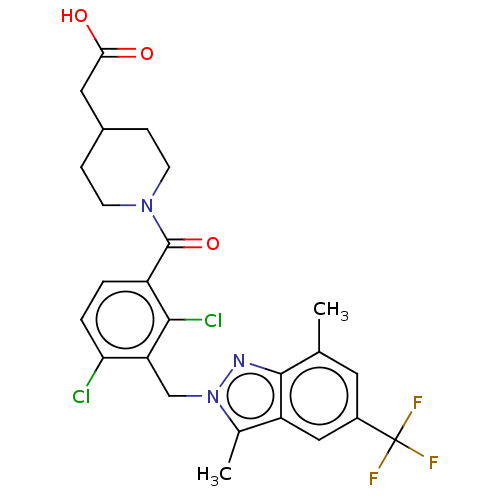
(US10106501, Example DG)Show SMILES Cc1n(Cc2c(Cl)ccc(C(=O)N3CCC(CC(O)=O)CC3)c2Cl)nc2c(C)cc(cc12)C(F)(F)F Show InChI InChI=1S/C25H24Cl2F3N3O3/c1-13-9-16(25(28,29)30)11-18-14(2)33(31-23(13)18)12-19-20(26)4-3-17(22(19)27)24(36)32-7-5-15(6-8-32)10-21(34)35/h3-4,9,11,15H,5-8,10,12H2,1-2H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293251
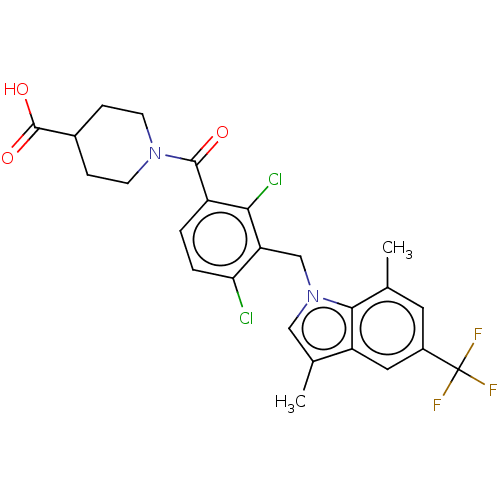
(US10106501, Example DH)Show SMILES Cc1cn(Cc2c(Cl)ccc(C(=O)N3CCC(CC3)C(O)=O)c2Cl)c2c(C)cc(cc12)C(F)(F)F Show InChI InChI=1S/C25H23Cl2F3N2O3/c1-13-9-16(25(28,29)30)10-18-14(2)11-32(22(13)18)12-19-20(26)4-3-17(21(19)27)23(33)31-7-5-15(6-8-31)24(34)35/h3-4,9-11,15H,5-8,12H2,1-2H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293252

(US10106501, Example DI)Show SMILES Cc1cn(Cc2c(Cl)ccc(C(=O)N3CCC(CC(O)=O)CC3)c2Cl)c2c(C)cc(cc12)C(F)(F)F Show InChI InChI=1S/C26H25Cl2F3N2O3/c1-14-9-17(26(29,30)31)11-19-15(2)12-33(24(14)19)13-20-21(27)4-3-18(23(20)28)25(36)32-7-5-16(6-8-32)10-22(34)35/h3-4,9,11-12,16H,5-8,10,13H2,1-2H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293253
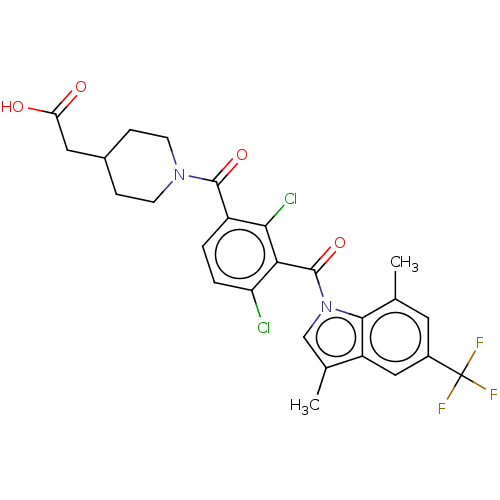
(US10106501, Example DJ)Show SMILES Cc1cn(C(=O)c2c(Cl)ccc(C(=O)N3CCC(CC(O)=O)CC3)c2Cl)c2c(C)cc(cc12)C(F)(F)F Show InChI InChI=1S/C26H23Cl2F3N2O4/c1-13-9-16(26(29,30)31)11-18-14(2)12-33(23(13)18)25(37)21-19(27)4-3-17(22(21)28)24(36)32-7-5-15(6-8-32)10-20(34)35/h3-4,9,11-12,15H,5-8,10H2,1-2H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293255
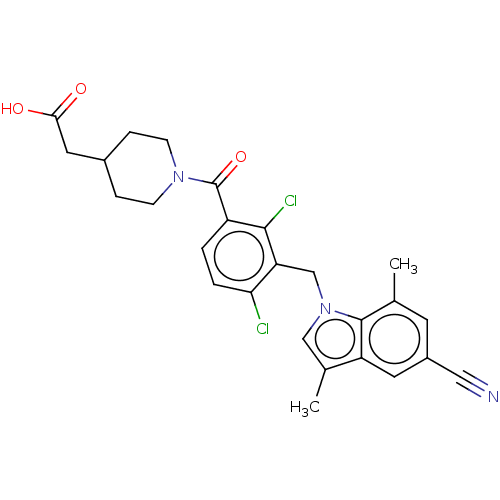
(US10106501, Example DL)Show SMILES Cc1cn(Cc2c(Cl)ccc(C(=O)N3CCC(CC(O)=O)CC3)c2Cl)c2c(C)cc(cc12)C#N Show InChI InChI=1S/C26H25Cl2N3O3/c1-15-9-18(12-29)10-20-16(2)13-31(25(15)20)14-21-22(27)4-3-19(24(21)28)26(34)30-7-5-17(6-8-30)11-23(32)33/h3-4,9-10,13,17H,5-8,11,14H2,1-2H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293257
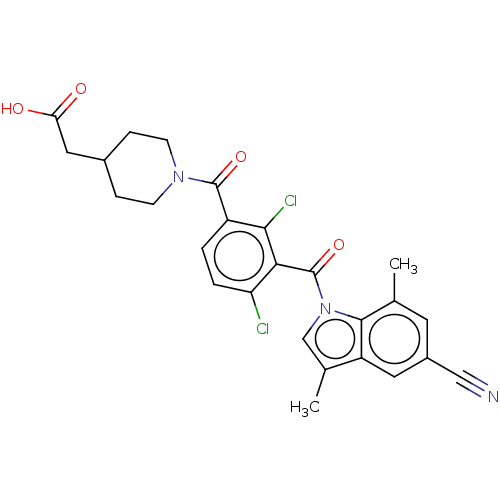
(US10106501, Example DN)Show SMILES Cc1cn(C(=O)c2c(Cl)ccc(C(=O)N3CCC(CC(O)=O)CC3)c2Cl)c2c(C)cc(cc12)C#N Show InChI InChI=1S/C26H23Cl2N3O4/c1-14-9-17(12-29)10-19-15(2)13-31(24(14)19)26(35)22-20(27)4-3-18(23(22)28)25(34)30-7-5-16(6-8-30)11-21(32)33/h3-4,9-10,13,16H,5-8,11H2,1-2H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM293258
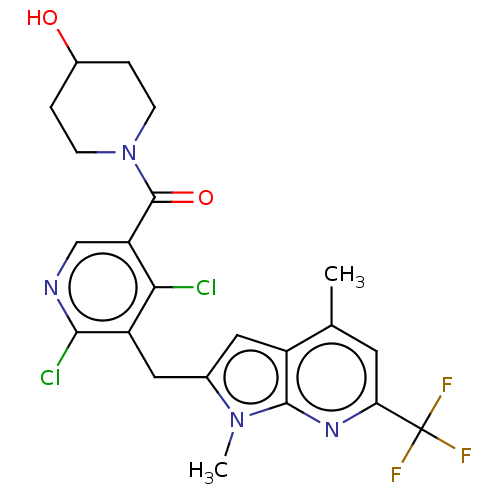
(US10106501, Example DO | US10106501, Example DT)Show SMILES Cc1cc(nc2n(C)c(Cc3c(Cl)ncc(C(=O)N4CCC(O)CC4)c3Cl)cc12)C(F)(F)F Show InChI InChI=1S/C22H21Cl2F3N4O2/c1-11-7-17(22(25,26)27)29-20-14(11)8-12(30(20)2)9-15-18(23)16(10-28-19(15)24)21(33)31-5-3-13(32)4-6-31/h7-8,10,13,32H,3-6,9H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... |
US Patent US10106501 (2018)
BindingDB Entry DOI: 10.7270/Q2G44SBV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data