

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM620450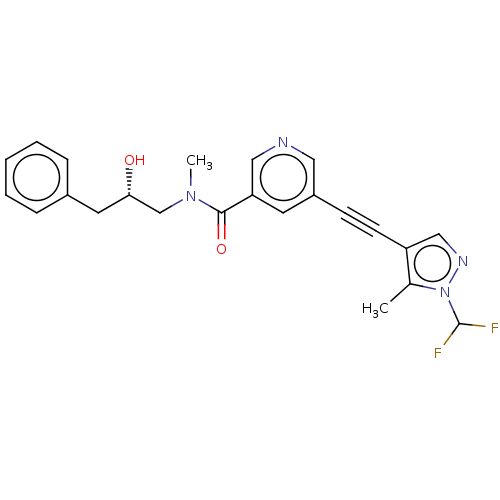 ((S)-5-((1-(difluoromethyl)-5-methyl-1H-pyrazol-4-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM620443 (US11767310, Example 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM620438 (US11767310, Example 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM620434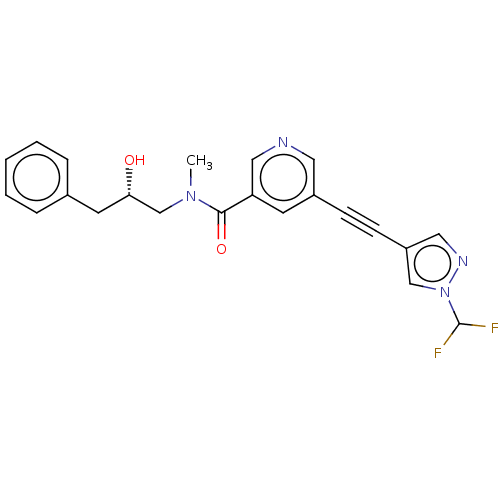 (US11767310, Example 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM620440 (US11767310, Example 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM620439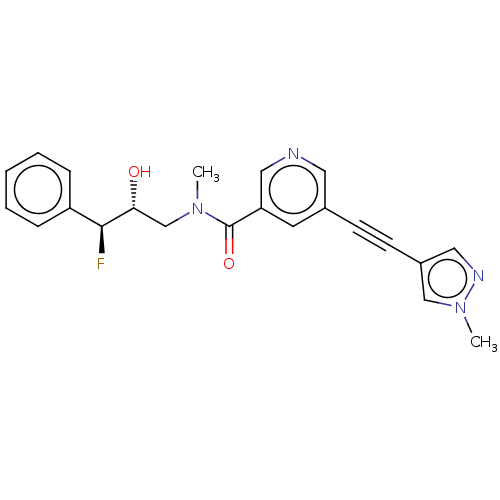 (US11767310, Example 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM620446 (US11767310, Example 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50323351 (6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-isobutyl-6,7-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 | Bioorg Med Chem Lett 23: 5471-83 (2013) Article DOI: 10.1016/j.bmcl.2013.08.003 BindingDB Entry DOI: 10.7270/Q2R49S6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM620433 (US11767310, Example 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50323353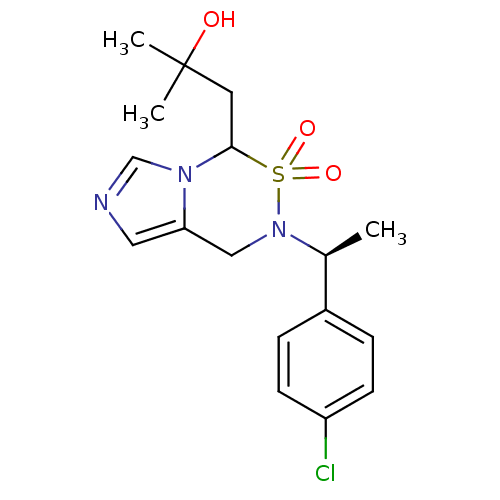 (1-{6-[(S)-1-(4-Chloro-phenyl)-ethyl]-5,5-dioxo-4,5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 | Bioorg Med Chem Lett 23: 5471-83 (2013) Article DOI: 10.1016/j.bmcl.2013.08.003 BindingDB Entry DOI: 10.7270/Q2R49S6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM620444 (US11767310, Example 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM620437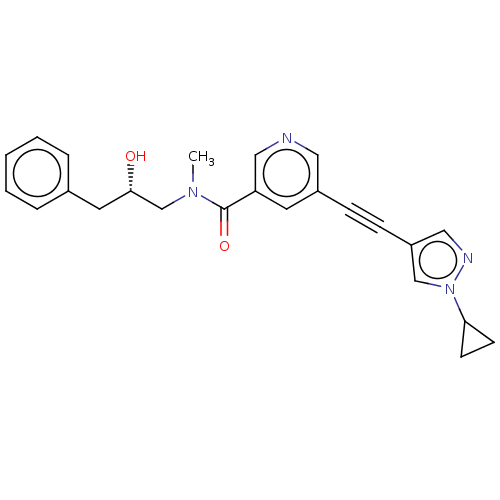 (US11767310, Example 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM620441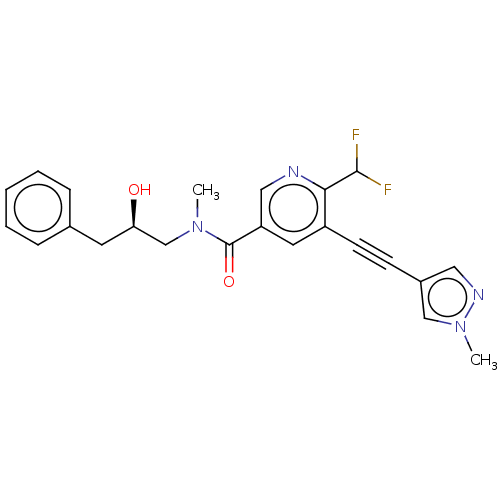 (US11767310, Example 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50155144 (4-(3-Phenyl-1H-indol-2-ylmethylene)-5-pyrazin-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2(KDR) without DTT(dithiothreitol) | Bioorg Med Chem Lett 14: 5503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.09.007 BindingDB Entry DOI: 10.7270/Q2NK3DJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM620436 (US11767310, Example 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM620449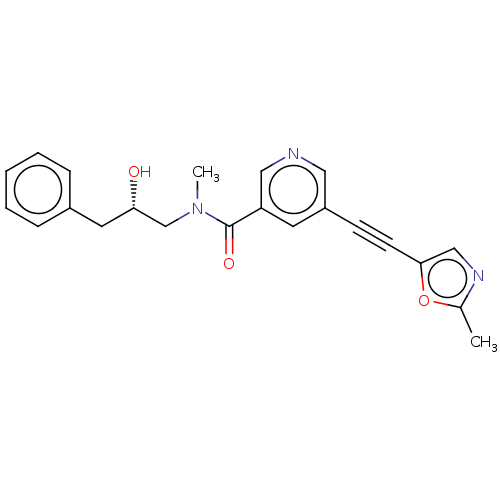 (US11767310, Example 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM620435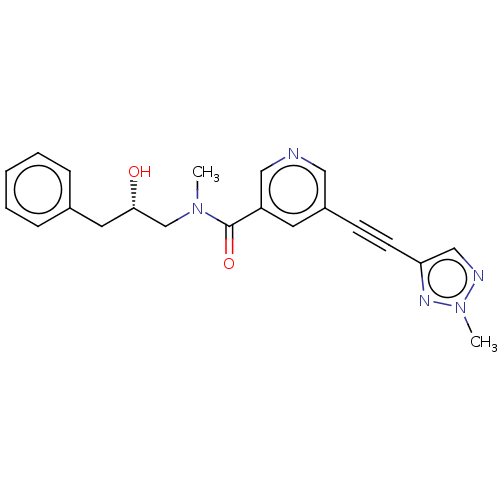 (US11767310, Example 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM620442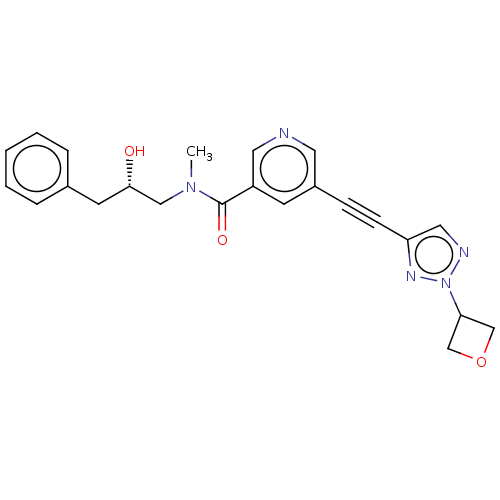 (US11767310, Example 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50155149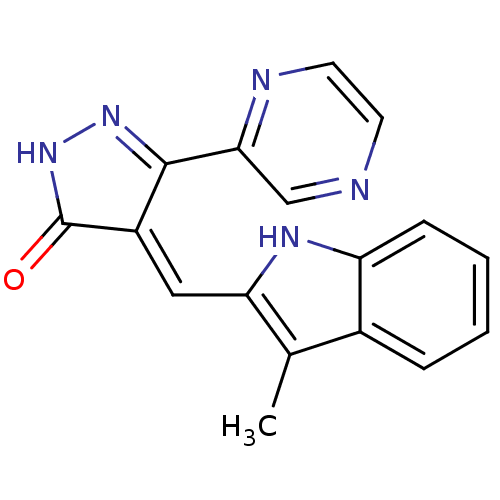 (4-(3-Methyl-1H-indol-2-ylmethylene)-5-pyrazin-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2(KDR) without DTT(dithiothreitol) | Bioorg Med Chem Lett 14: 5503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.09.007 BindingDB Entry DOI: 10.7270/Q2NK3DJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50155148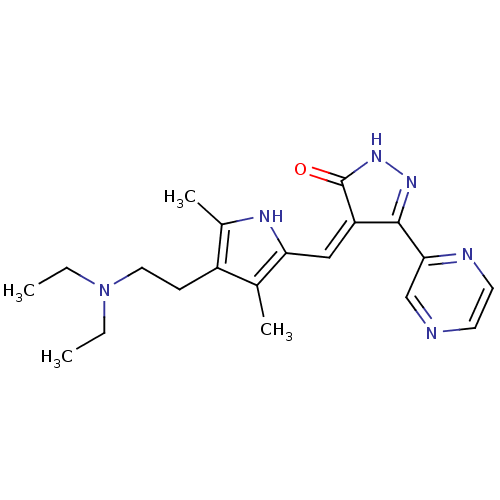 ((Z)-4-((4-(2-(diethylamino)ethyl)-3,5-dimethyl-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2(KDR) with DTT(dithiothreitol) | Bioorg Med Chem Lett 14: 5503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.09.007 BindingDB Entry DOI: 10.7270/Q2NK3DJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM620445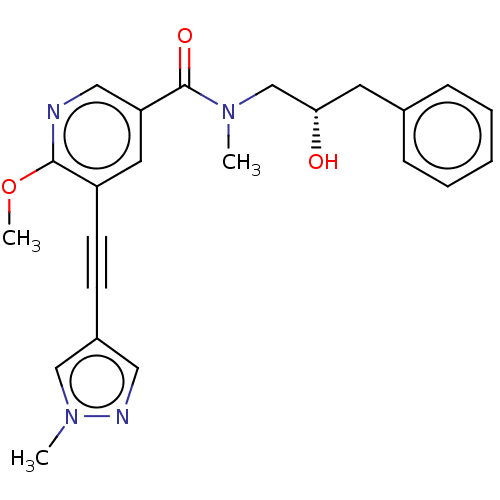 (US11767310, Example 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292711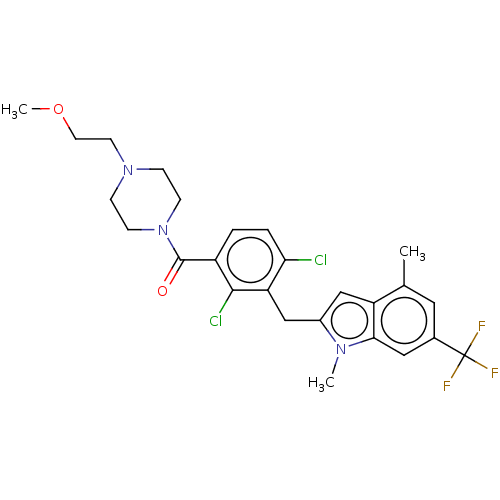 (US10106501, Example A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292717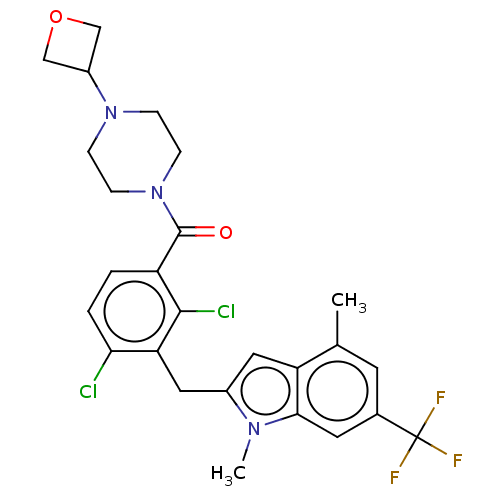 (US10106501, Example A1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292718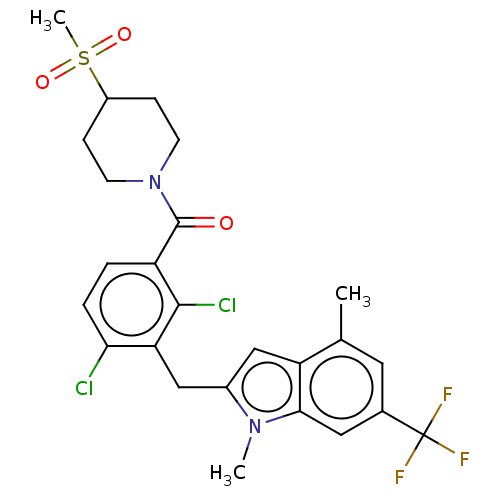 (US10106501, Example A1-11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292719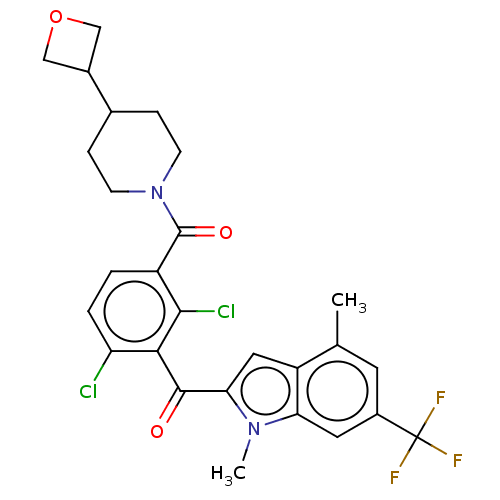 (US10106501, Example AP-6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292720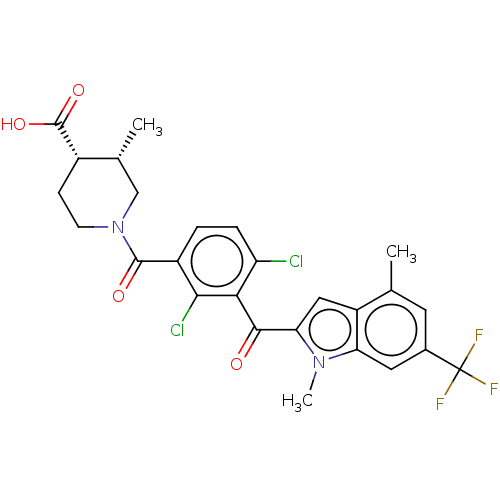 (US10106501, Example AQ-3 | US10106501, Example AQ-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292720 (US10106501, Example AQ-3 | US10106501, Example AQ-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292722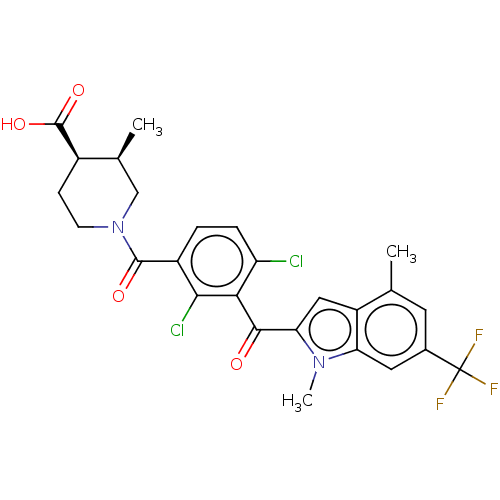 (US10106501, Example AQ-5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292723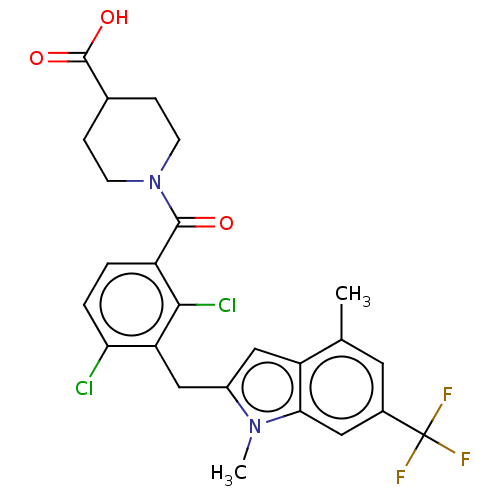 (US10106501, Example B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292724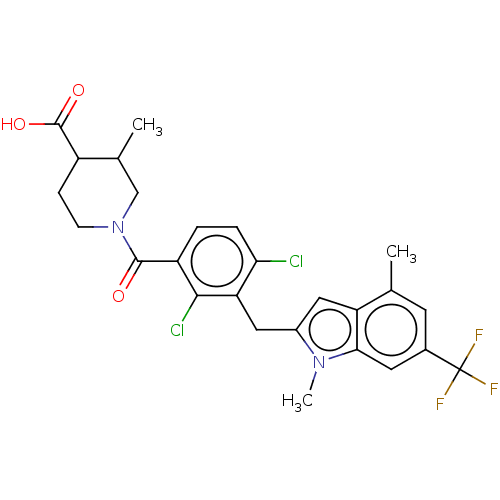 (US10106501, Example B-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292725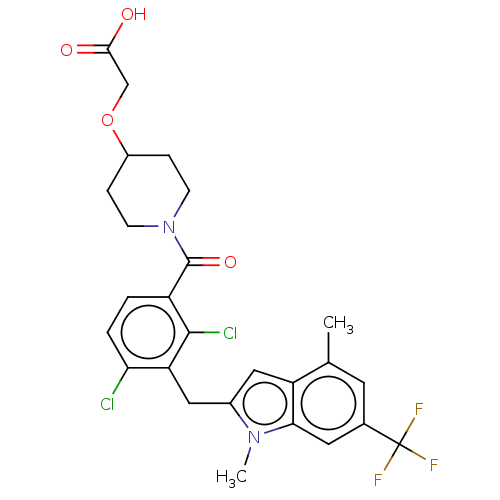 (US10106501, Example B-2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50155151 (4-((1-methyl-1H-indol-3-yl)methylene)-3-(pyrazin-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2(KDR) without DTT(dithiothreitol) | Bioorg Med Chem Lett 14: 5503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.09.007 BindingDB Entry DOI: 10.7270/Q2NK3DJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292729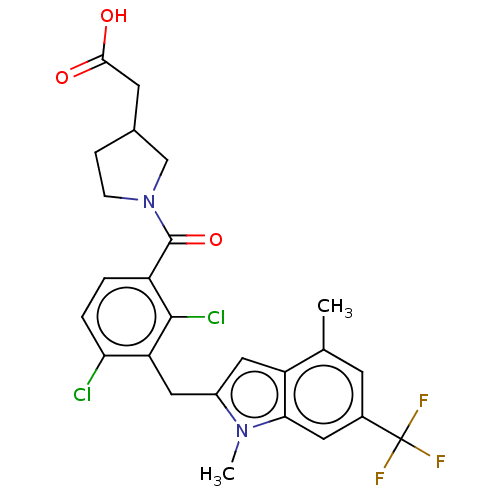 (US10106501, Example B-6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292730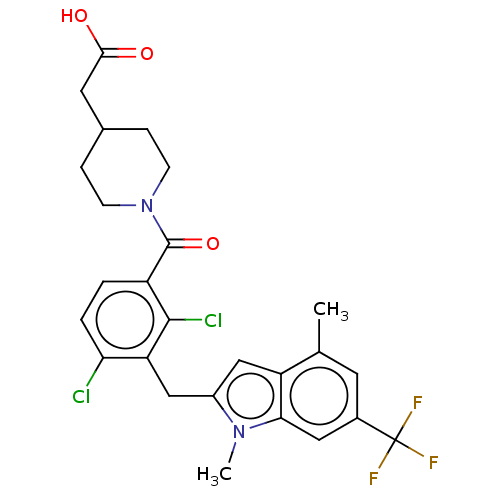 (US10106501, Example B-7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292731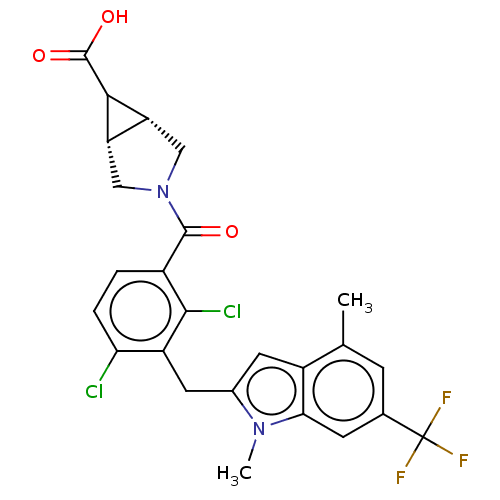 (US10106501, Example B-8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292732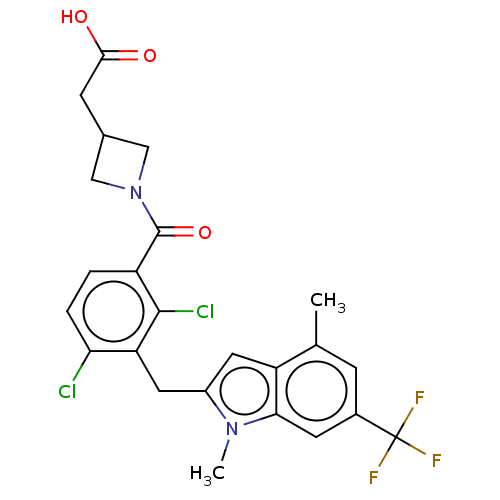 (US10106501, Example B-9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292740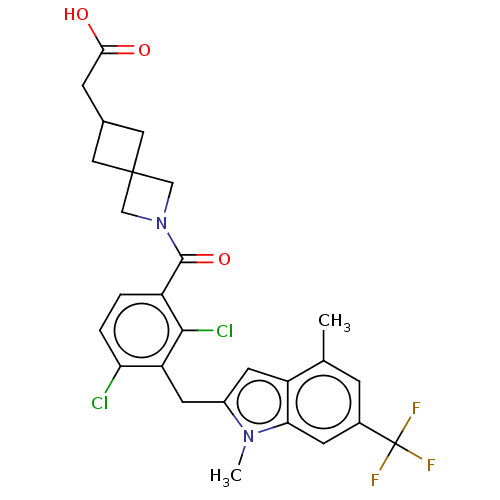 (US10106501, Example B-17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292741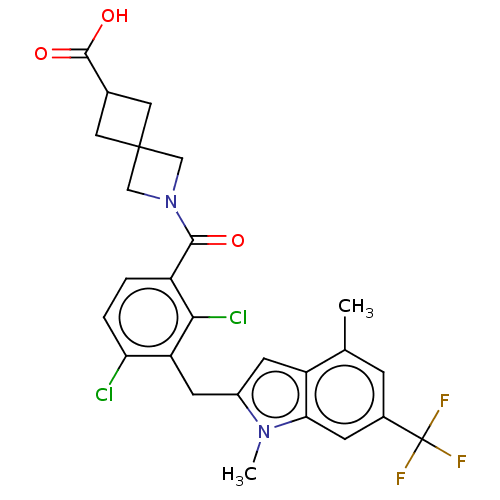 (US10106501, Example B-18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292742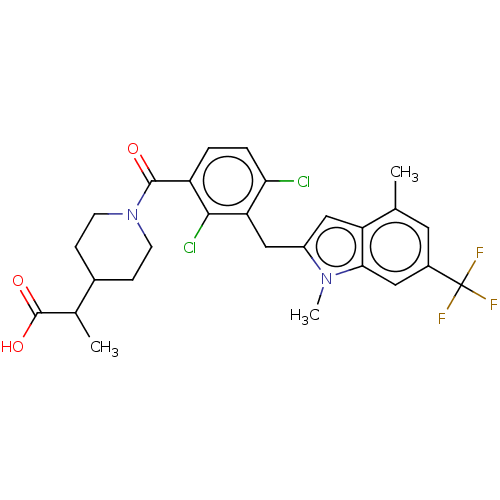 (US10106501, Example B-19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292743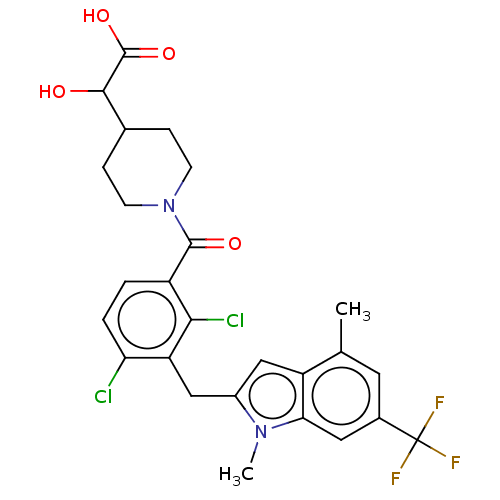 (US10106501, Example B-20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292744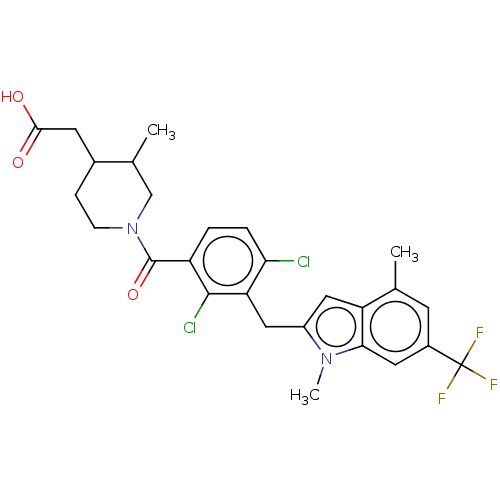 (US10106501, Example B-21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292745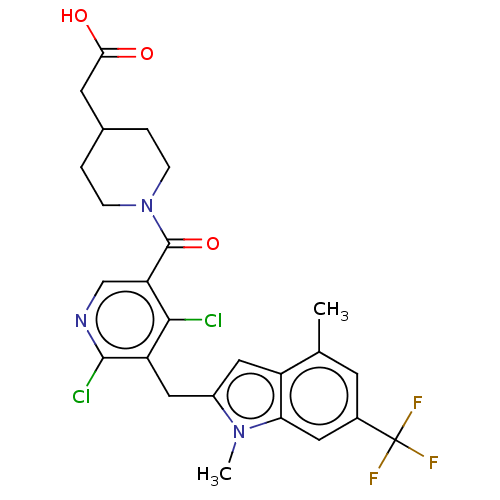 (US10106501, Example B-22) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292746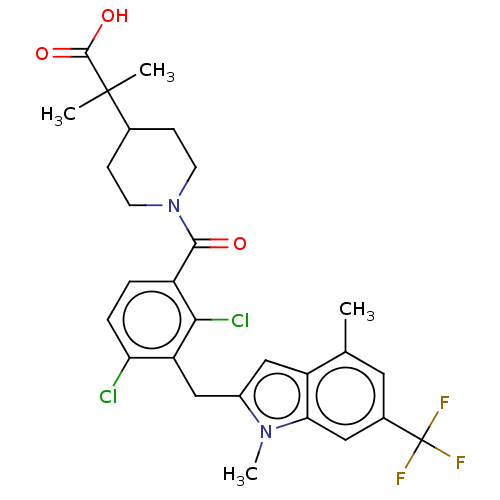 (US10106501, Example B-23) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292747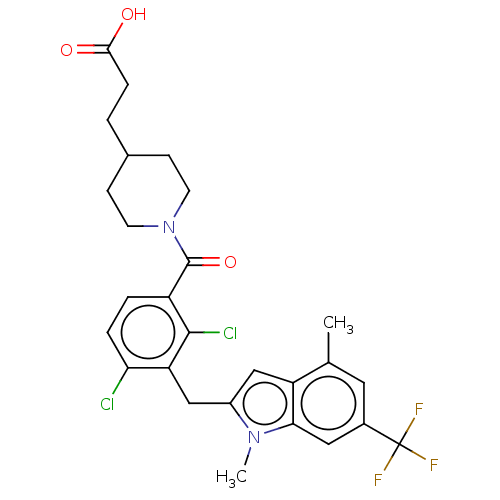 (US10106501, Example B-24) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292748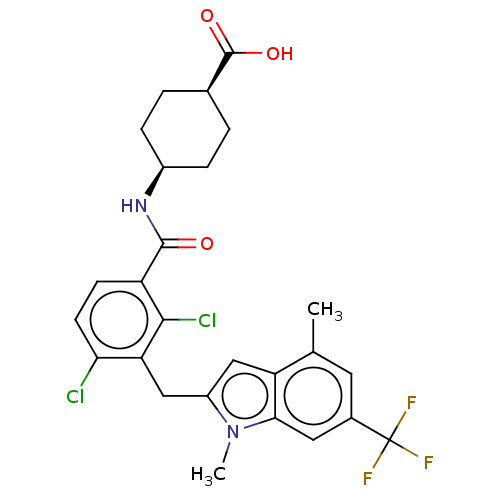 (US10106501, Example B-25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292749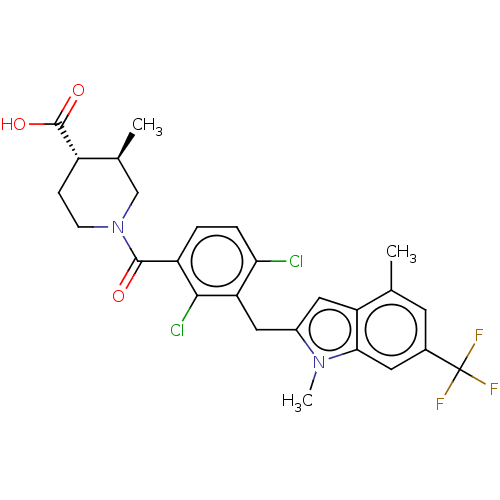 (US10106501, Example B-26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292750 (US10106501, Example GH) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292755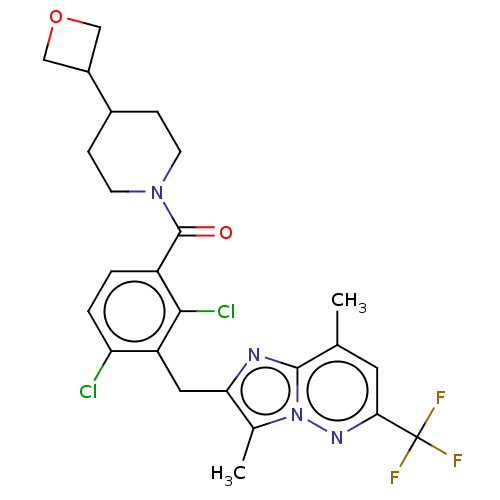 (US10106501, Example CZ-2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292757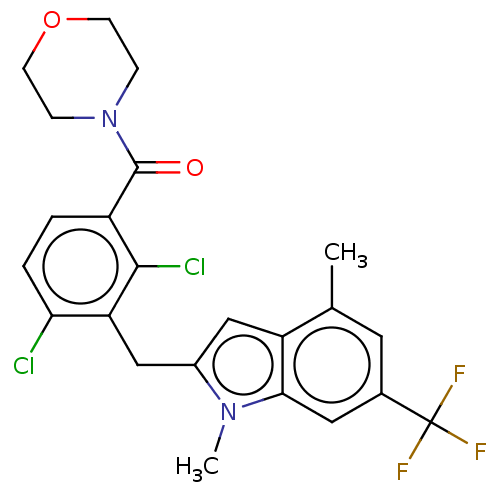 (US10106501, Example D) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM292758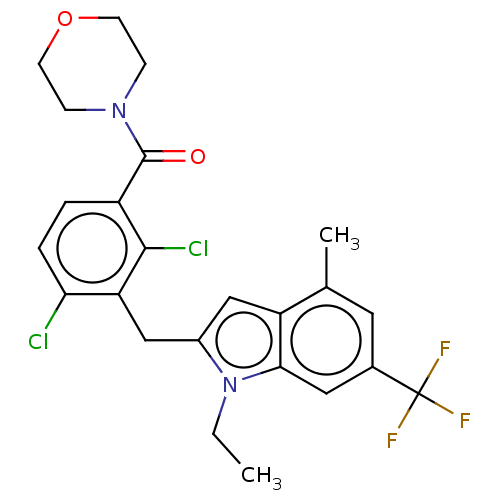 (US10106501, Example D-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The primary screen was performed by transient transactivation assays. These cell-based assays were carried out using Cos-7 cells transfected with a c... | US Patent US10106501 (2018) BindingDB Entry DOI: 10.7270/Q2G44SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 526 total ) | Next | Last >> |