 Found 794 hits with Last Name = 'cussac' and Initial = 'd'
Found 794 hits with Last Name = 'cussac' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM22867
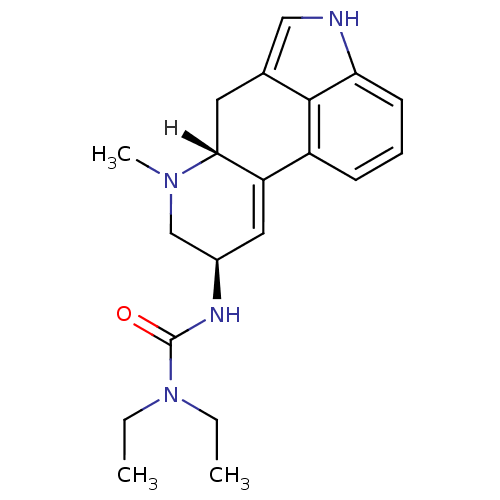
(1,1-diethyl-3-[(8beta)-6-methyl-9,10-didehydroergo...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)NC(=O)N(CC)CC)c34 |r,c:12| Show InChI InChI=1S/C20H26N4O/c1-4-24(5-2)20(25)22-14-10-16-15-7-6-8-17-19(15)13(11-21-17)9-18(16)23(3)12-14/h6-8,10-11,14,18,21H,4-5,9,12H2,1-3H3,(H,22,25)/t14-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 791-804 (2002)
Article DOI: 10.1124/jpet.102.039867
BindingDB Entry DOI: 10.7270/Q2MG7N2F |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Homo sapiens (Human)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier, Centre de Recherches de Croissy
Curated by PDSP Ki Database
| |
Synapse 35: 79-95 (2000)
Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X
BindingDB Entry DOI: 10.7270/Q2XK8D4N |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier, Centre de Recherches de Croissy
Curated by PDSP Ki Database
| |
Synapse 35: 79-95 (2000)
Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X
BindingDB Entry DOI: 10.7270/Q2XK8D4N |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier, Centre de Recherches de Croissy
Curated by PDSP Ki Database
| |
Synapse 35: 79-95 (2000)
Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X
BindingDB Entry DOI: 10.7270/Q2XK8D4N |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50417006
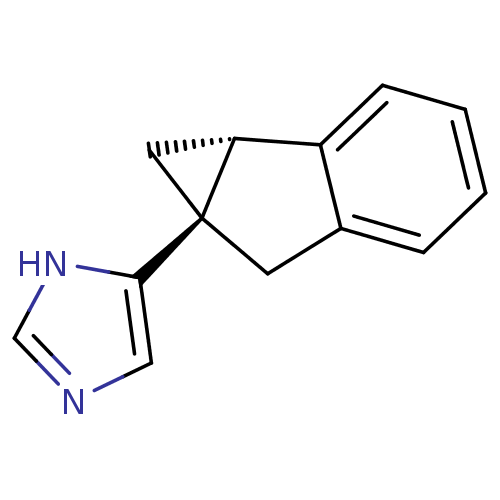
(CHEMBL1256414)Show InChI InChI=1S/C13H12N2/c1-2-4-10-9(3-1)5-13(6-11(10)13)12-7-14-8-15-12/h1-4,7-8,11H,5-6H2,(H,14,15)/t11-,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RX821002 from human adrenergic alpha2A receptor expressed in rat C6 cells after 120 mins by liquid scintillation counting |
J Med Chem 53: 6986-95 (2010)
Article DOI: 10.1021/jm1006269
BindingDB Entry DOI: 10.7270/Q2T154X2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50002173
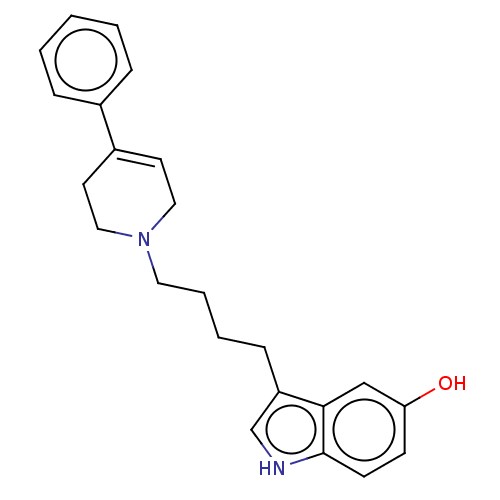
(3-(4-(3,6-dihydro-4-phenyl-1(2H)-pyridinyl)butyl)-...)Show SMILES Oc1ccc2[nH]cc(CCCCN3CCC(=CC3)c3ccccc3)c2c1 |c:15| Show InChI InChI=1S/C23H26N2O/c26-21-9-10-23-22(16-21)20(17-24-23)8-4-5-13-25-14-11-19(12-15-25)18-6-2-1-3-7-18/h1-3,6-7,9-11,16-17,24,26H,4-5,8,12-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 791-804 (2002)
Article DOI: 10.1124/jpet.102.039867
BindingDB Entry DOI: 10.7270/Q2MG7N2F |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM50417006

(CHEMBL1256414)Show InChI InChI=1S/C13H12N2/c1-2-4-10-9(3-1)5-13(6-11(10)13)12-7-14-8-15-12/h1-4,7-8,11H,5-6H2,(H,14,15)/t11-,13+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RX821002 from human adrenergic alpha2B receptor expressed in rat C6 cells after 120 mins by liquid scintillation counting |
J Med Chem 53: 6986-95 (2010)
Article DOI: 10.1021/jm1006269
BindingDB Entry DOI: 10.7270/Q2T154X2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier, Centre de Recherches de Croissy
Curated by PDSP Ki Database
| |
Synapse 35: 79-95 (2000)
Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X
BindingDB Entry DOI: 10.7270/Q2XK8D4N |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50417006

(CHEMBL1256414)Show InChI InChI=1S/C13H12N2/c1-2-4-10-9(3-1)5-13(6-11(10)13)12-7-14-8-15-12/h1-4,7-8,11H,5-6H2,(H,14,15)/t11-,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RX821002 from human adrenergic Alpha-2C receptor expressed in rat C6 cells after 120 mins by liquid scintillation counting |
J Med Chem 53: 6986-95 (2010)
Article DOI: 10.1021/jm1006269
BindingDB Entry DOI: 10.7270/Q2T154X2 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM22867

(1,1-diethyl-3-[(8beta)-6-methyl-9,10-didehydroergo...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)NC(=O)N(CC)CC)c34 |r,c:12| Show InChI InChI=1S/C20H26N4O/c1-4-24(5-2)20(25)22-14-10-16-15-7-6-8-17-19(15)13(11-21-17)9-18(16)23(3)12-14/h6-8,10-11,14,18,21H,4-5,9,12H2,1-3H3,(H,22,25)/t14-,18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 791-804 (2002)
Article DOI: 10.1124/jpet.102.039867
BindingDB Entry DOI: 10.7270/Q2MG7N2F |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50417010
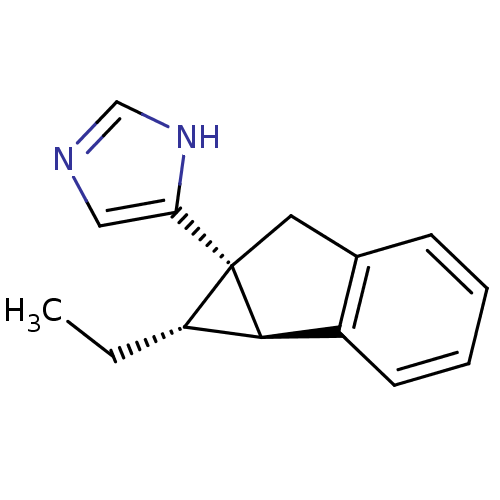
(CHEMBL1255723)Show SMILES CC[C@H]1[C@@H]2c3ccccc3C[C@]12c1cnc[nH]1 |r| Show InChI InChI=1S/C15H16N2/c1-2-12-14-11-6-4-3-5-10(11)7-15(12,14)13-8-16-9-17-13/h3-6,8-9,12,14H,2,7H2,1H3,(H,16,17)/t12-,14-,15+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RX821002 from human adrenergic alpha2A receptor expressed in rat C6 cells after 120 mins by liquid scintillation counting |
J Med Chem 53: 6986-95 (2010)
Article DOI: 10.1021/jm1006269
BindingDB Entry DOI: 10.7270/Q2T154X2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM22867

(1,1-diethyl-3-[(8beta)-6-methyl-9,10-didehydroergo...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)NC(=O)N(CC)CC)c34 |r,c:12| Show InChI InChI=1S/C20H26N4O/c1-4-24(5-2)20(25)22-14-10-16-15-7-6-8-17-19(15)13(11-21-17)9-18(16)23(3)12-14/h6-8,10-11,14,18,21H,4-5,9,12H2,1-3H3,(H,22,25)/t14-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 791-804 (2002)
Article DOI: 10.1124/jpet.102.039867
BindingDB Entry DOI: 10.7270/Q2MG7N2F |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM85709
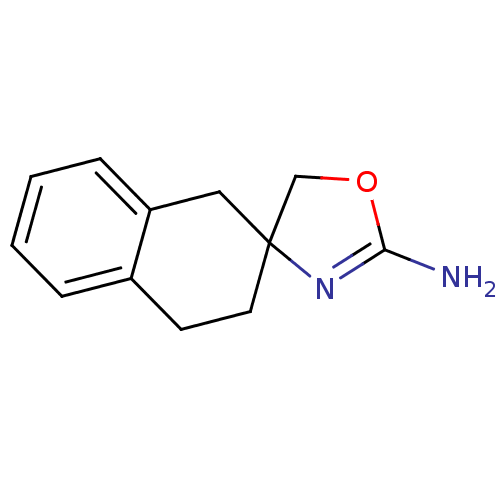
(S18616)Show InChI InChI=1S/C12H14N2O/c13-11-14-12(8-15-11)6-5-9-3-1-2-4-10(9)7-12/h1-4H,5-8H2,(H2,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.159 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Croissy
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1192-205 (2000)
BindingDB Entry DOI: 10.7270/Q28P5Z2C |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50417010

(CHEMBL1255723)Show SMILES CC[C@H]1[C@@H]2c3ccccc3C[C@]12c1cnc[nH]1 |r| Show InChI InChI=1S/C15H16N2/c1-2-12-14-11-6-4-3-5-10(11)7-15(12,14)13-8-16-9-17-13/h3-6,8-9,12,14H,2,7H2,1H3,(H,16,17)/t12-,14-,15+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RX821002 from human adrenergic Alpha-2C receptor expressed in rat C6 cells after 120 mins by liquid scintillation counting |
J Med Chem 53: 6986-95 (2010)
Article DOI: 10.1021/jm1006269
BindingDB Entry DOI: 10.7270/Q2T154X2 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50417019
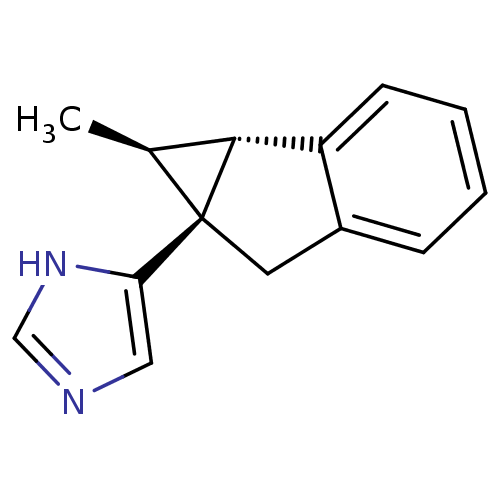
(CHEMBL1256378)Show SMILES C[C@H]1[C@@H]2c3ccccc3C[C@]12c1cnc[nH]1 |r| Show InChI InChI=1S/C14H14N2/c1-9-13-11-5-3-2-4-10(11)6-14(9,13)12-7-15-8-16-12/h2-5,7-9,13H,6H2,1H3,(H,15,16)/t9-,13+,14+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RX821002 from human adrenergic alpha2A receptor expressed in rat C6 cells after 120 mins by liquid scintillation counting |
J Med Chem 53: 6986-95 (2010)
Article DOI: 10.1021/jm1006269
BindingDB Entry DOI: 10.7270/Q2T154X2 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM50417010

(CHEMBL1255723)Show SMILES CC[C@H]1[C@@H]2c3ccccc3C[C@]12c1cnc[nH]1 |r| Show InChI InChI=1S/C15H16N2/c1-2-12-14-11-6-4-3-5-10(11)7-15(12,14)13-8-16-9-17-13/h3-6,8-9,12,14H,2,7H2,1H3,(H,16,17)/t12-,14-,15+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RX821002 from human adrenergic alpha2B receptor expressed in rat C6 cells after 120 mins by liquid scintillation counting |
J Med Chem 53: 6986-95 (2010)
Article DOI: 10.1021/jm1006269
BindingDB Entry DOI: 10.7270/Q2T154X2 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50216892
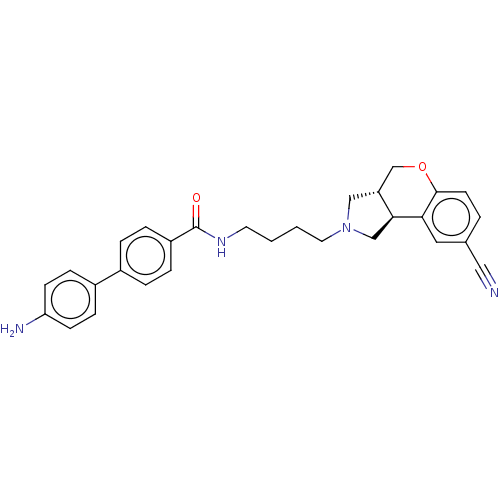
(CHEMBL2112210)Show SMILES [H][C@]12CN(CCCCNC(=O)c3ccc(cc3)-c3ccc(N)cc3)C[C@]1([H])c1cc(ccc1OC2)C#N Show InChI InChI=1S/C29H30N4O2/c30-16-20-3-12-28-26(15-20)27-18-33(17-24(27)19-35-28)14-2-1-13-32-29(34)23-6-4-21(5-7-23)22-8-10-25(31)11-9-22/h3-12,15,24,27H,1-2,13-14,17-19,31H2,(H,32,34)/t24-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Croissy
Curated by ChEMBL
| Assay Description
Ability to displace [125I]iodosulpiride from human dopamine D3 (hD3) receptor transfected into CHO cells. |
Bioorg Med Chem Lett 9: 2059-64 (1999)
BindingDB Entry DOI: 10.7270/Q2WD42R1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85862
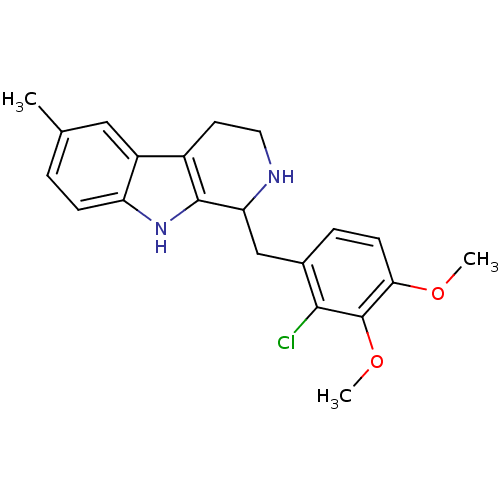
(1-(2-Chloro-3,4-dimethoxybenzyl)-6-methyl-2,3,4,9-...)Show SMILES COc1ccc(CC2NCCc3c2[nH]c2ccc(C)cc32)c(Cl)c1OC Show InChI InChI=1S/C21H23ClN2O2/c1-12-4-6-16-15(10-12)14-8-9-23-17(20(14)24-16)11-13-5-7-18(25-2)21(26-3)19(13)22/h4-7,10,17,23-24H,8-9,11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 365: 242-52 (2002)
Article DOI: 10.1007/s00210-001-0505-y
BindingDB Entry DOI: 10.7270/Q2W37TWZ |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM50417019

(CHEMBL1256378)Show SMILES C[C@H]1[C@@H]2c3ccccc3C[C@]12c1cnc[nH]1 |r| Show InChI InChI=1S/C14H14N2/c1-9-13-11-5-3-2-4-10(11)6-14(9,13)12-7-15-8-16-12/h2-5,7-9,13H,6H2,1H3,(H,15,16)/t9-,13+,14+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RX821002 from human adrenergic alpha2B receptor expressed in rat C6 cells after 120 mins by liquid scintillation counting |
J Med Chem 53: 6986-95 (2010)
Article DOI: 10.1021/jm1006269
BindingDB Entry DOI: 10.7270/Q2T154X2 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50417020
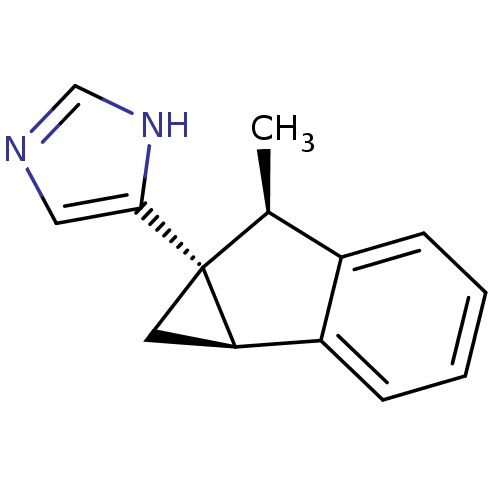
(CHEMBL1256609)Show SMILES C[C@H]1c2ccccc2[C@H]2C[C@@]12c1cnc[nH]1 |r| Show InChI InChI=1S/C14H14N2/c1-9-10-4-2-3-5-11(10)12-6-14(9,12)13-7-15-8-16-13/h2-5,7-9,12H,6H2,1H3,(H,15,16)/t9-,12+,14+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.219 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RX821002 from human adrenergic alpha2A receptor expressed in rat C6 cells after 120 mins by liquid scintillation counting |
J Med Chem 53: 6986-95 (2010)
Article DOI: 10.1021/jm1006269
BindingDB Entry DOI: 10.7270/Q2T154X2 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier, Centre de Recherches de Croissy
Curated by PDSP Ki Database
| |
Synapse 35: 79-95 (2000)
Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X
BindingDB Entry DOI: 10.7270/Q2XK8D4N |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM85672
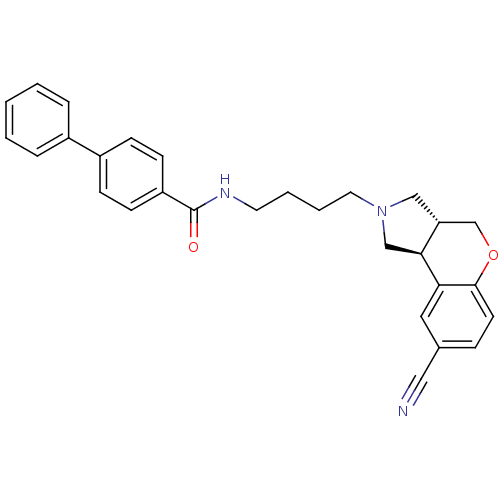
(S33084)Show SMILES O=C(NCCCCN1C[C@@H]2COc3ccc(cc3[C@H]2C1)C#N)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C29H29N3O2/c30-17-21-8-13-28-26(16-21)27-19-32(18-25(27)20-34-28)15-5-4-14-31-29(33)24-11-9-23(10-12-24)22-6-2-1-3-7-22/h1-3,6-13,16,25,27H,4-5,14-15,18-20H2,(H,31,33)/t25-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Croissy
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 293: 1048-62 (2000)
BindingDB Entry DOI: 10.7270/Q2ZC81DX |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]
(Rattus norvegicus (rat)) | BDBM85709

(S18616)Show InChI InChI=1S/C12H14N2O/c13-11-14-12(8-15-11)6-5-9-3-1-2-4-10(9)7-12/h1-4H,5-8H2,(H2,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Croissy
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1192-205 (2000)
BindingDB Entry DOI: 10.7270/Q28P5Z2C |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM85709

(S18616)Show InChI InChI=1S/C12H14N2O/c13-11-14-12(8-15-11)6-5-9-3-1-2-4-10(9)7-12/h1-4H,5-8H2,(H2,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Croissy
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1192-205 (2000)
BindingDB Entry DOI: 10.7270/Q28P5Z2C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM85672

(S33084)Show SMILES O=C(NCCCCN1C[C@@H]2COc3ccc(cc3[C@H]2C1)C#N)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C29H29N3O2/c30-17-21-8-13-28-26(16-21)27-19-32(18-25(27)20-34-28)15-5-4-14-31-29(33)24-11-9-23(10-12-24)22-6-2-1-3-7-22/h1-3,6-13,16,25,27H,4-5,14-15,18-20H2,(H,31,33)/t25-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 309: 903-20 (2004)
Article DOI: 10.1124/jpet.103.062398
BindingDB Entry DOI: 10.7270/Q29S1PM2 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Croissy-sur-Seine Paris
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 361: 569-72 (2000)
Article DOI: 10.1007/s002100000217
BindingDB Entry DOI: 10.7270/Q2GB22M2 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM22867

(1,1-diethyl-3-[(8beta)-6-methyl-9,10-didehydroergo...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)NC(=O)N(CC)CC)c34 |r,c:12| Show InChI InChI=1S/C20H26N4O/c1-4-24(5-2)20(25)22-14-10-16-15-7-6-8-17-19(15)13(11-21-17)9-18(16)23(3)12-14/h6-8,10-11,14,18,21H,4-5,9,12H2,1-3H3,(H,22,25)/t14-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 791-804 (2002)
Article DOI: 10.1124/jpet.102.039867
BindingDB Entry DOI: 10.7270/Q2MG7N2F |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Croissy-sur-Seine Paris
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 361: 569-72 (2000)
Article DOI: 10.1007/s002100000217
BindingDB Entry DOI: 10.7270/Q2GB22M2 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50417019

(CHEMBL1256378)Show SMILES C[C@H]1[C@@H]2c3ccccc3C[C@]12c1cnc[nH]1 |r| Show InChI InChI=1S/C14H14N2/c1-9-13-11-5-3-2-4-10(11)6-14(9,13)12-7-15-8-16-12/h2-5,7-9,13H,6H2,1H3,(H,15,16)/t9-,13+,14+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RX821002 from human adrenergic Alpha-2C receptor expressed in rat C6 cells after 120 mins by liquid scintillation counting |
J Med Chem 53: 6986-95 (2010)
Article DOI: 10.1021/jm1006269
BindingDB Entry DOI: 10.7270/Q2T154X2 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50017519
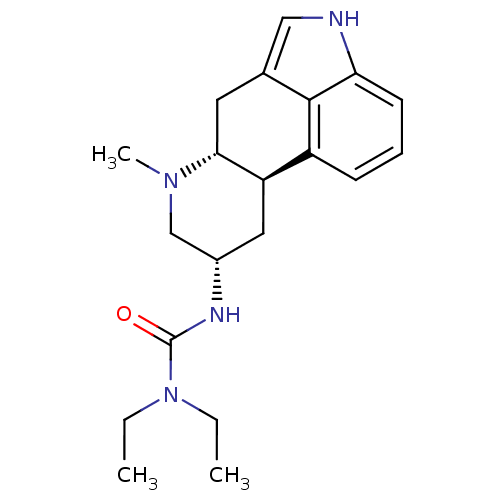
(1,1-Diethyl-3-(7-methyl-4,6,6a,7,8,9,10,10a-octahy...)Show SMILES CCN(CC)C(=O)N[C@H]1C[C@H]2[C@@H](Cc3c[nH]c4cccc2c34)N(C)C1 Show InChI InChI=1S/C20H28N4O/c1-4-24(5-2)20(25)22-14-10-16-15-7-6-8-17-19(15)13(11-21-17)9-18(16)23(3)12-14/h6-8,11,14,16,18,21H,4-5,9-10,12H2,1-3H3,(H,22,25)/t14-,16+,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 791-804 (2002)
Article DOI: 10.1124/jpet.102.039867
BindingDB Entry DOI: 10.7270/Q2MG7N2F |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM85672

(S33084)Show SMILES O=C(NCCCCN1C[C@@H]2COc3ccc(cc3[C@H]2C1)C#N)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C29H29N3O2/c30-17-21-8-13-28-26(16-21)27-19-32(18-25(27)20-34-28)15-5-4-14-31-29(33)24-11-9-23(10-12-24)22-6-2-1-3-7-22/h1-3,6-13,16,25,27H,4-5,14-15,18-20H2,(H,31,33)/t25-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Croissy-sur-Seine Paris
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 361: 569-72 (2000)
Article DOI: 10.1007/s002100000217
BindingDB Entry DOI: 10.7270/Q2GB22M2 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM85709

(S18616)Show InChI InChI=1S/C12H14N2O/c13-11-14-12(8-15-11)6-5-9-3-1-2-4-10(9)7-12/h1-4H,5-8H2,(H2,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Croissy
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1192-205 (2000)
BindingDB Entry DOI: 10.7270/Q28P5Z2C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM85672

(S33084)Show SMILES O=C(NCCCCN1C[C@@H]2COc3ccc(cc3[C@H]2C1)C#N)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C29H29N3O2/c30-17-21-8-13-28-26(16-21)27-19-32(18-25(27)20-34-28)15-5-4-14-31-29(33)24-11-9-23(10-12-24)22-6-2-1-3-7-22/h1-3,6-13,16,25,27H,4-5,14-15,18-20H2,(H,31,33)/t25-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Croissy
Curated by ChEMBL
| Assay Description
Ability to displace [125I]iodosulpiride from human dopamine D3 (hD3) receptor transfected into CHO cells. |
Bioorg Med Chem Lett 9: 2059-64 (1999)
BindingDB Entry DOI: 10.7270/Q2WD42R1 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM22867

(1,1-diethyl-3-[(8beta)-6-methyl-9,10-didehydroergo...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)NC(=O)N(CC)CC)c34 |r,c:12| Show InChI InChI=1S/C20H26N4O/c1-4-24(5-2)20(25)22-14-10-16-15-7-6-8-17-19(15)13(11-21-17)9-18(16)23(3)12-14/h6-8,10-11,14,18,21H,4-5,9,12H2,1-3H3,(H,22,25)/t14-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 791-804 (2002)
Article DOI: 10.1124/jpet.102.039867
BindingDB Entry DOI: 10.7270/Q2MG7N2F |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM85709

(S18616)Show InChI InChI=1S/C12H14N2O/c13-11-14-12(8-15-11)6-5-9-3-1-2-4-10(9)7-12/h1-4H,5-8H2,(H2,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.346 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Croissy
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1192-205 (2000)
BindingDB Entry DOI: 10.7270/Q28P5Z2C |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM50417009
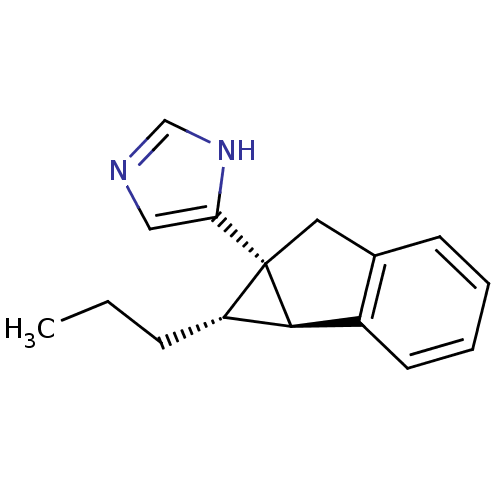
(CHEMBL1255724)Show SMILES CCC[C@H]1[C@@H]2c3ccccc3C[C@]12c1cnc[nH]1 |r| Show InChI InChI=1S/C16H18N2/c1-2-5-13-15-12-7-4-3-6-11(12)8-16(13,15)14-9-17-10-18-14/h3-4,6-7,9-10,13,15H,2,5,8H2,1H3,(H,17,18)/t13-,15-,16+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RX821002 from human adrenergic alpha2B receptor expressed in rat C6 cells after 120 mins by liquid scintillation counting |
J Med Chem 53: 6986-95 (2010)
Article DOI: 10.1021/jm1006269
BindingDB Entry DOI: 10.7270/Q2T154X2 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM85672

(S33084)Show SMILES O=C(NCCCCN1C[C@@H]2COc3ccc(cc3[C@H]2C1)C#N)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C29H29N3O2/c30-17-21-8-13-28-26(16-21)27-19-32(18-25(27)20-34-28)15-5-4-14-31-29(33)24-11-9-23(10-12-24)22-6-2-1-3-7-22/h1-3,6-13,16,25,27H,4-5,14-15,18-20H2,(H,31,33)/t25-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Croissy-sur-Seine Paris
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 361: 569-72 (2000)
Article DOI: 10.1007/s002100000217
BindingDB Entry DOI: 10.7270/Q2GB22M2 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM85672

(S33084)Show SMILES O=C(NCCCCN1C[C@@H]2COc3ccc(cc3[C@H]2C1)C#N)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C29H29N3O2/c30-17-21-8-13-28-26(16-21)27-19-32(18-25(27)20-34-28)15-5-4-14-31-29(33)24-11-9-23(10-12-24)22-6-2-1-3-7-22/h1-3,6-13,16,25,27H,4-5,14-15,18-20H2,(H,31,33)/t25-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Croissy
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 293: 1048-62 (2000)
BindingDB Entry DOI: 10.7270/Q2ZC81DX |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50417009

(CHEMBL1255724)Show SMILES CCC[C@H]1[C@@H]2c3ccccc3C[C@]12c1cnc[nH]1 |r| Show InChI InChI=1S/C16H18N2/c1-2-5-13-15-12-7-4-3-6-11(12)8-16(13,15)14-9-17-10-18-14/h3-4,6-7,9-10,13,15H,2,5,8H2,1H3,(H,17,18)/t13-,15-,16+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RX821002 from human adrenergic alpha2A receptor expressed in rat C6 cells after 120 mins by liquid scintillation counting |
J Med Chem 53: 6986-95 (2010)
Article DOI: 10.1021/jm1006269
BindingDB Entry DOI: 10.7270/Q2T154X2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 309: 903-20 (2004)
Article DOI: 10.1124/jpet.103.062398
BindingDB Entry DOI: 10.7270/Q29S1PM2 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50417009

(CHEMBL1255724)Show SMILES CCC[C@H]1[C@@H]2c3ccccc3C[C@]12c1cnc[nH]1 |r| Show InChI InChI=1S/C16H18N2/c1-2-5-13-15-12-7-4-3-6-11(12)8-16(13,15)14-9-17-10-18-14/h3-4,6-7,9-10,13,15H,2,5,8H2,1H3,(H,17,18)/t13-,15-,16+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RX821002 from human adrenergic Alpha-2C receptor expressed in rat C6 cells after 120 mins by liquid scintillation counting |
J Med Chem 53: 6986-95 (2010)
Article DOI: 10.1021/jm1006269
BindingDB Entry DOI: 10.7270/Q2T154X2 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50417008
(CHEMBL1255771)Show InChI InChI=1S/C14H14N2/c1-13-8-14(13,12-7-15-9-16-12)6-10-4-2-3-5-11(10)13/h2-5,7,9H,6,8H2,1H3,(H,15,16)/t13-,14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RX821002 from human adrenergic alpha2A receptor expressed in rat C6 cells after 120 mins by liquid scintillation counting |
J Med Chem 53: 6986-95 (2010)
Article DOI: 10.1021/jm1006269
BindingDB Entry DOI: 10.7270/Q2T154X2 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM50017519

(1,1-Diethyl-3-(7-methyl-4,6,6a,7,8,9,10,10a-octahy...)Show SMILES CCN(CC)C(=O)N[C@H]1C[C@H]2[C@@H](Cc3c[nH]c4cccc2c34)N(C)C1 Show InChI InChI=1S/C20H28N4O/c1-4-24(5-2)20(25)22-14-10-16-15-7-6-8-17-19(15)13(11-21-17)9-18(16)23(3)12-14/h6-8,11,14,16,18,21H,4-5,9-10,12H2,1-3H3,(H,22,25)/t14-,16+,18+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 791-804 (2002)
Article DOI: 10.1124/jpet.102.039867
BindingDB Entry DOI: 10.7270/Q2MG7N2F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM30704
((phenylmethyl) N-[[(6aR,9S,10aR)-4,7-dimethyl-6,6a...)Show SMILES CN1C[C@H](CNC(=O)OCc2ccccc2)C[C@H]2[C@H]1Cc1cn(C)c3cccc2c13 Show InChI InChI=1S/C25H29N3O2/c1-27-14-18(13-26-25(29)30-16-17-7-4-3-5-8-17)11-21-20-9-6-10-22-24(20)19(12-23(21)27)15-28(22)2/h3-10,15,18,21,23H,11-14,16H2,1-2H3,(H,26,29)/t18-,21+,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 365: 242-52 (2002)
Article DOI: 10.1007/s00210-001-0505-y
BindingDB Entry DOI: 10.7270/Q2W37TWZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85097
(CAS_181632-25-7 | CHEMBL14563 | SB 242084)Show SMILES Cc1cc2CCN(C(=O)Nc3ccc(Oc4cccnc4C)nc3)c2cc1Cl Show InChI InChI=1S/C21H19ClN4O2/c1-13-10-15-7-9-26(18(15)11-17(13)22)21(27)25-16-5-6-20(24-12-16)28-19-4-3-8-23-14(19)2/h3-6,8,10-12H,7,9H2,1-2H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 365: 242-52 (2002)
Article DOI: 10.1007/s00210-001-0505-y
BindingDB Entry DOI: 10.7270/Q2W37TWZ |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM85595
(CAS_105182-45-4 | Fluparoxan | NSC_72036)Show InChI InChI=1S/C10H10FNO2/c11-6-2-1-3-7-10(6)14-9-5-12-4-8(9)13-7/h1-3,8-9,12H,4-5H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier, Centre de Recherches de Croissy
Curated by PDSP Ki Database
| |
Synapse 35: 79-95 (2000)
Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X
BindingDB Entry DOI: 10.7270/Q2XK8D4N |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50417013

(CHEMBL1256610)Show SMILES CC[C@H]1c2ccccc2[C@H]2C[C@@]12c1cnc[nH]1 |r| Show InChI InChI=1S/C15H16N2/c1-2-12-10-5-3-4-6-11(10)13-7-15(12,13)14-8-16-9-17-14/h3-6,8-9,12-13H,2,7H2,1H3,(H,16,17)/t12-,13+,15+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RX821002 from human adrenergic alpha2A receptor expressed in rat C6 cells after 120 mins by liquid scintillation counting |
J Med Chem 53: 6986-95 (2010)
Article DOI: 10.1021/jm1006269
BindingDB Entry DOI: 10.7270/Q2T154X2 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50417020
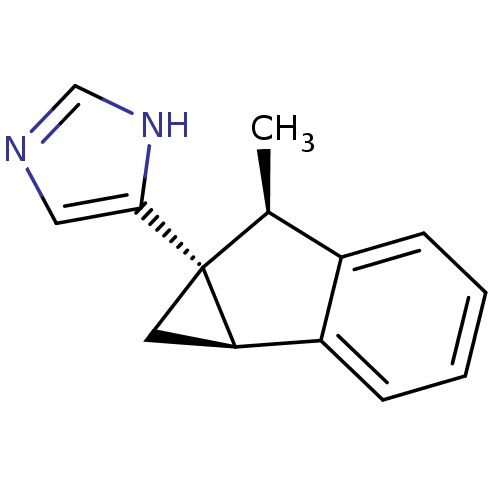
(CHEMBL1256609)Show SMILES C[C@H]1c2ccccc2[C@H]2C[C@@]12c1cnc[nH]1 |r| Show InChI InChI=1S/C14H14N2/c1-9-10-4-2-3-5-11(10)12-6-14(9,12)13-7-15-8-16-13/h2-5,7-9,12H,6H2,1H3,(H,15,16)/t9-,12+,14+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RX821002 from human adrenergic Alpha-2C receptor expressed in rat C6 cells after 120 mins by liquid scintillation counting |
J Med Chem 53: 6986-95 (2010)
Article DOI: 10.1021/jm1006269
BindingDB Entry DOI: 10.7270/Q2T154X2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85528
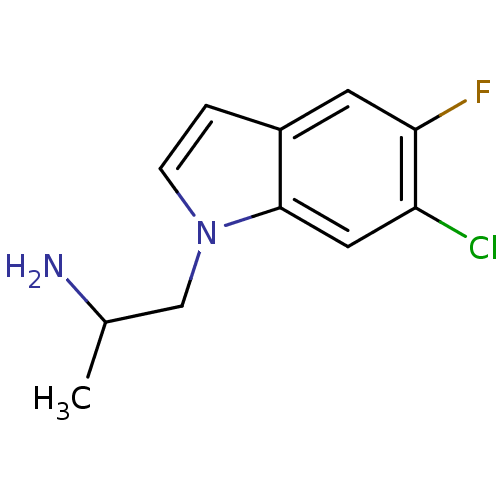
(CAS_3045226 | NSC_3045226 | Ro 60-0175)Show InChI InChI=1S/C11H12ClFN2/c1-7(14)6-15-3-2-8-4-10(13)9(12)5-11(8)15/h2-5,7H,6,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 365: 242-52 (2002)
Article DOI: 10.1007/s00210-001-0505-y
BindingDB Entry DOI: 10.7270/Q2W37TWZ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM85709

(S18616)Show InChI InChI=1S/C12H14N2O/c13-11-14-12(8-15-11)6-5-9-3-1-2-4-10(9)7-12/h1-4H,5-8H2,(H2,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Croissy
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1192-205 (2000)
BindingDB Entry DOI: 10.7270/Q28P5Z2C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data