

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254931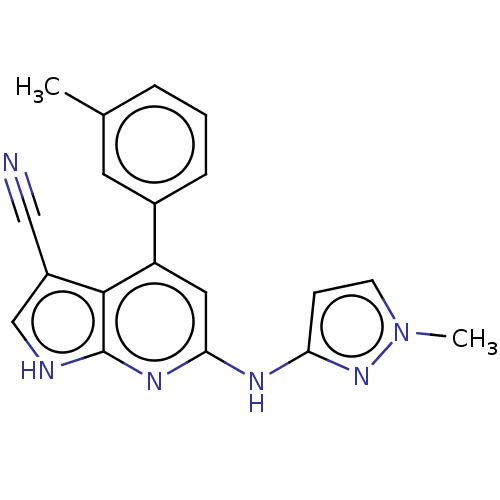 (US9499542, 14 | US9675594, 14) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254931 (US9499542, 14 | US9675594, 14) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrat... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254929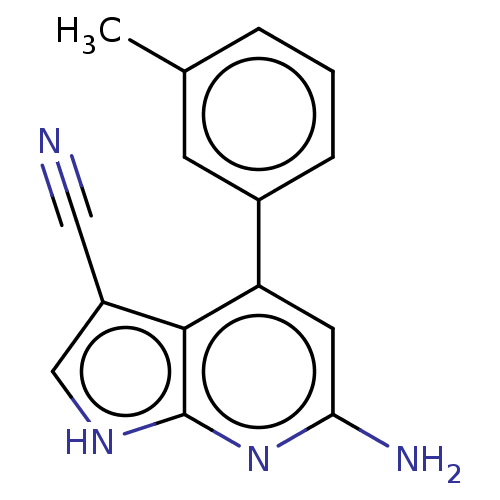 (US9499542, 12 | US9675594, 12) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254929 (US9499542, 12 | US9675594, 12) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrat... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254928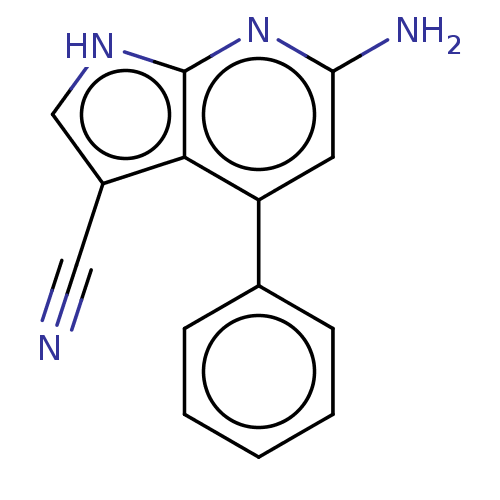 (US9499542, 11 | US9675594, 11) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254919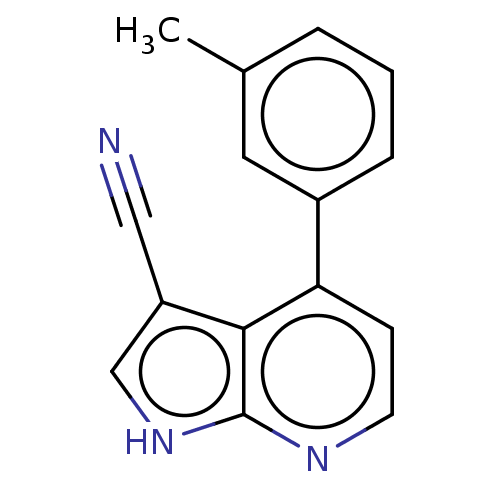 (US9499542, 2 | US9675594, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254918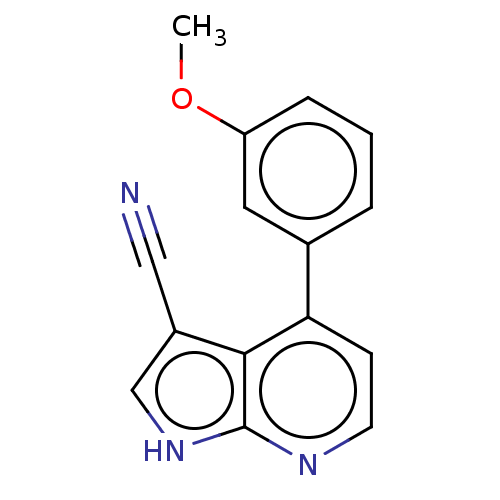 (US9499542, 1 | US9675594, 1) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254918 (US9499542, 1 | US9675594, 1) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type LRRK2 phosphorylation at ser935 transfected in HEK293 cells after 48 hrs by in-cell Western assay | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254928 (US9499542, 11 | US9675594, 11) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrat... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254919 (US9499542, 2 | US9675594, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrat... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50250336 (CHEMBL4068861) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50250338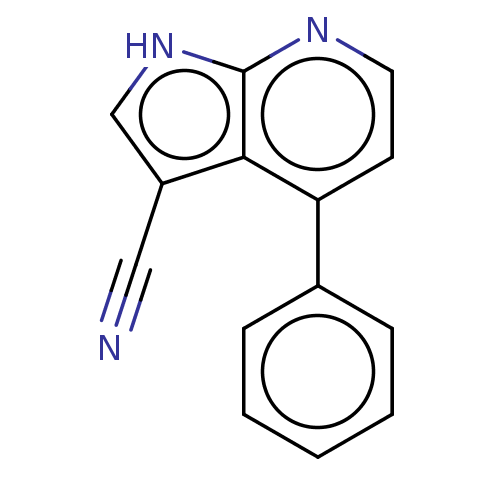 (CHEMBL4077186) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type LRRK2 phosphorylation at ser935 transfected in HEK293 cells after 48 hrs by in-cell Western assay | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50250338 (CHEMBL4077186) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50250344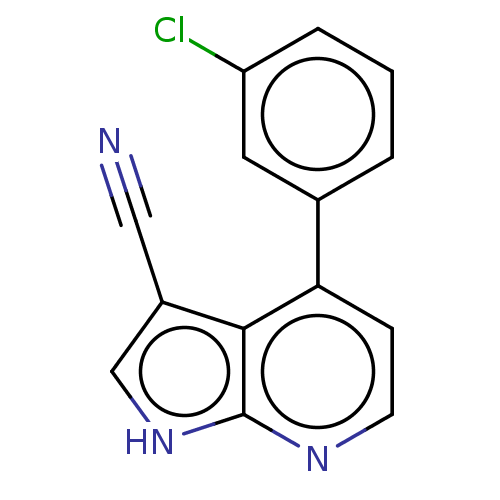 (CHEMBL4088961) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50250336 (CHEMBL4068861) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrat... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254921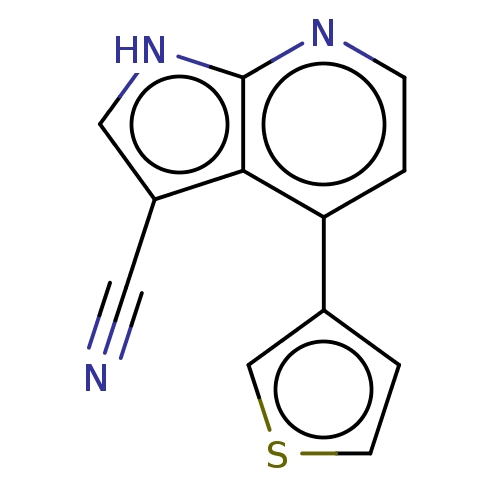 (US9499542, 4 | US9675594, 4) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254921 (US9499542, 4 | US9675594, 4) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrat... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM557699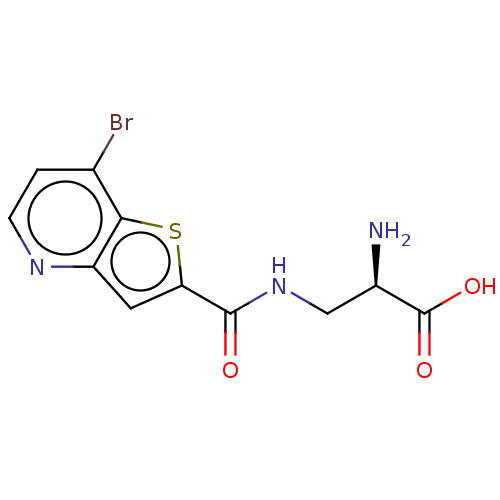 (US11358971, Compound 1h | US11466027, Compound 1l) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V98C93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C/3B (Homo sapiens (Human)) | BDBM557699 (US11358971, Compound 1h | US11466027, Compound 1l) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PK0KCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50250344 (CHEMBL4088961) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrat... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50445041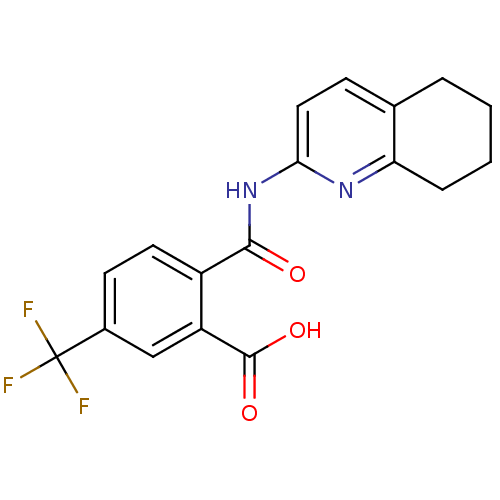 (CHEMBL3098768 | US10195186, Example 48 | US9682967...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 84 | n/a | 88 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159226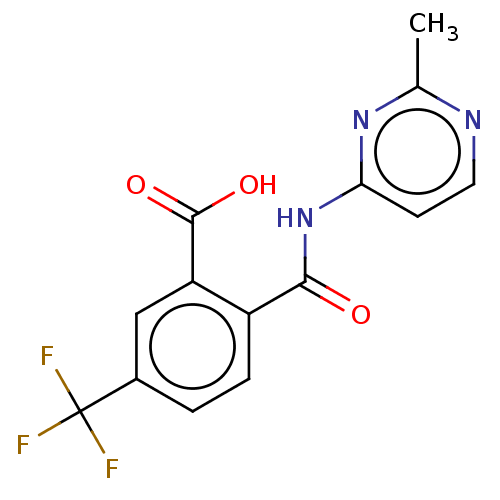 (US10195186, Example 46 | US9682967, 44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50250346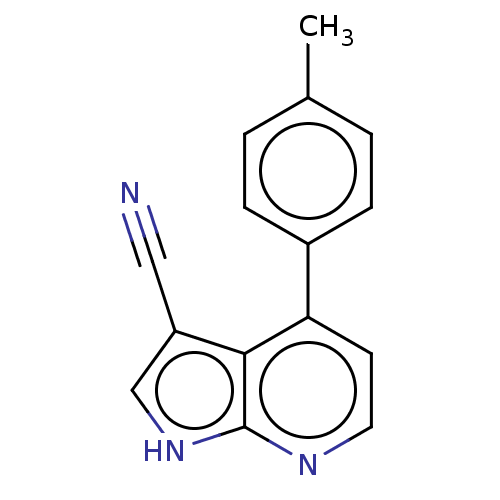 (CHEMBL4075407) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrat... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM557694 (US11358971, Compound 1c | US11466027, Compound 1e) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V98C93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C/3B (Homo sapiens (Human)) | BDBM557694 (US11358971, Compound 1c | US11466027, Compound 1e) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PK0KCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50250346 (CHEMBL4075407) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50469617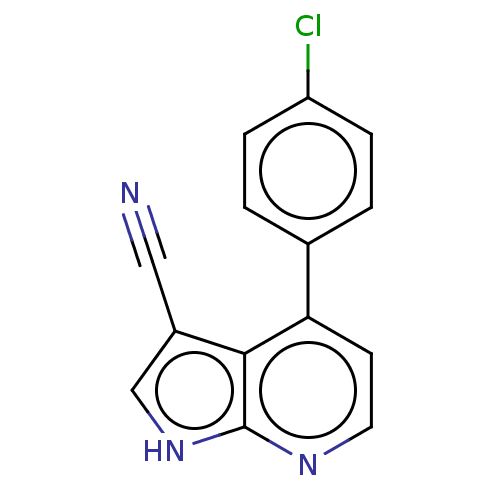 (CHEMBL4066167) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrat... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM557693 (US11358971, Compound 1b | US11466027, Compound 1d) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V98C93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C/3B (Homo sapiens (Human)) | BDBM557693 (US11358971, Compound 1b | US11466027, Compound 1d) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PK0KCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50445042 (CHEMBL3098767 | US10195186, Example 7 | US9682967,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 162 | n/a | 170 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50445042 (CHEMBL3098767 | US10195186, Example 7 | US9682967,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C/3B (Homo sapiens (Human)) | BDBM557667 (US11358971, Compound 1a | US11466027, Compound 1c) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PK0KCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM557667 (US11358971, Compound 1a | US11466027, Compound 1c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V98C93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM557701 (US11358971, Compound 1j | US11466027, Compound 1n) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V98C93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C/3B (Homo sapiens (Human)) | BDBM557701 (US11358971, Compound 1j | US11466027, Compound 1n) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PK0KCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159232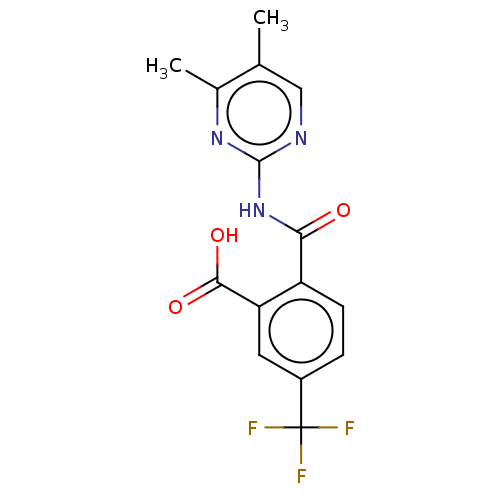 (US10195186, Example 47 | US9682967, 45) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 190 | n/a | 200 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50445055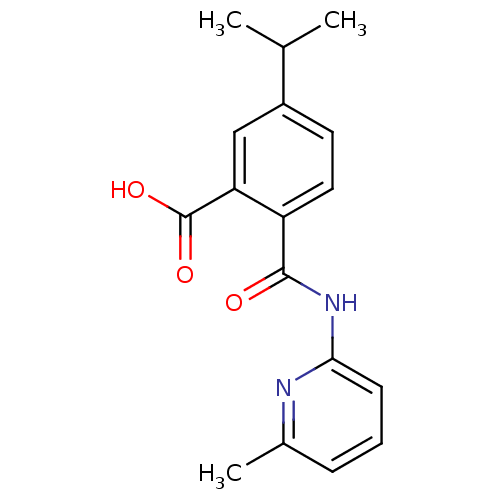 (CHEMBL3098744 | US10195186, Example 45 | US9682967...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Mouse) | BDBM50445054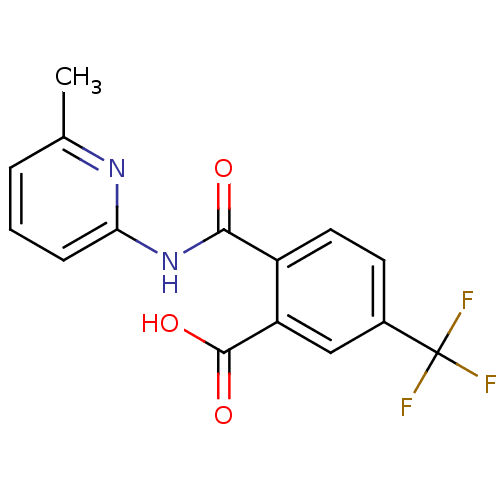 (CHEMBL3098745 | US10195186, Example 1 | US9682967,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | 210 | n/a | 220 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sortilin (Mouse) | BDBM50445054 (CHEMBL3098745 | US10195186, Example 1 | US9682967,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 μl in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM557698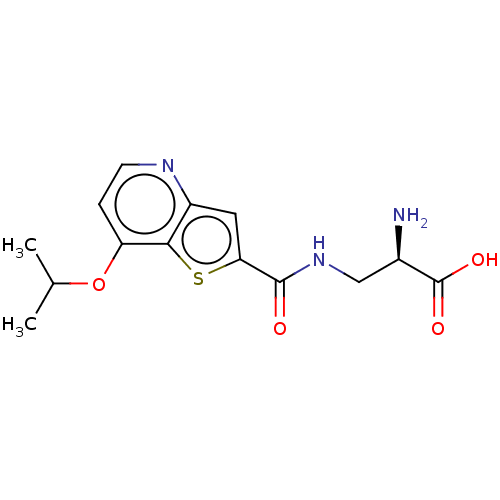 (US11358971, Compound 1g | US11466027, Compound 1k) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V98C93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C/3B (Homo sapiens (Human)) | BDBM576021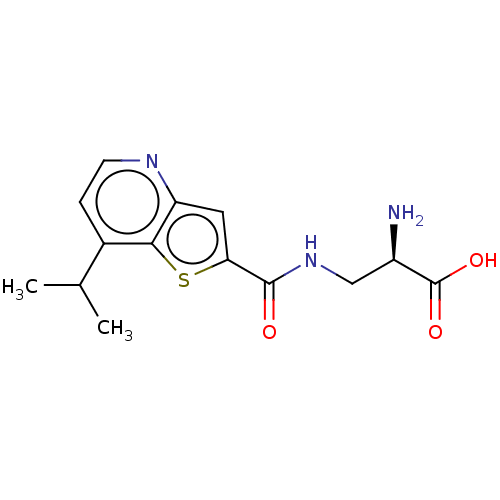 ((R)-2-amino-3-[(7- isopropylthieno[3,2- b]pyridine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PK0KCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C/3B (Homo sapiens (Human)) | BDBM557698 (US11358971, Compound 1g | US11466027, Compound 1k) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PK0KCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50250339 (CHEMBL4092537) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159140 (US10195186, Example 18 | US9682967, 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 229 | n/a | 240 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159140 (US10195186, Example 18 | US9682967, 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM557702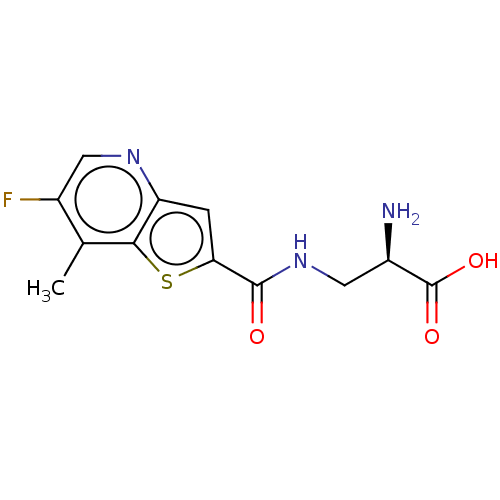 (US11358971, Compound 1k | US11466027, Compound 1o) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V98C93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C/3B (Homo sapiens (Human)) | BDBM557702 (US11358971, Compound 1k | US11466027, Compound 1o) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PK0KCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C/3B (Homo sapiens (Human)) | BDBM576017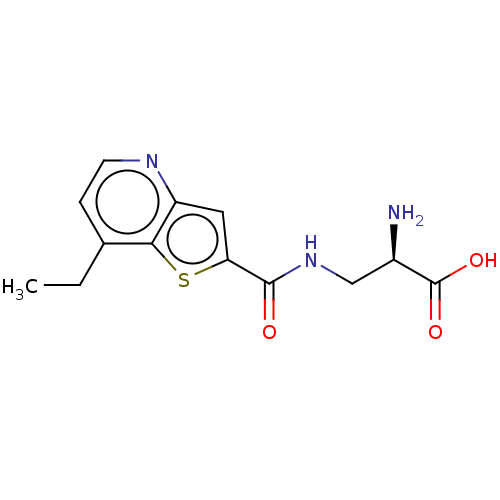 ((R)-2-amino-3-[(7- ethylthieno[3,2-b]pyridine- 2- ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PK0KCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159226 (US10195186, Example 46 | US9682967, 44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 286 | n/a | 300 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159213 (US10195186, Example 44 | US9682967, 42) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 286 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 457 total ) | Next | Last >> |