

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245474 (US9428503, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate preincubated for 30 mins followed by substrate addition and measured after 2 hrs by HTRF as... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50084948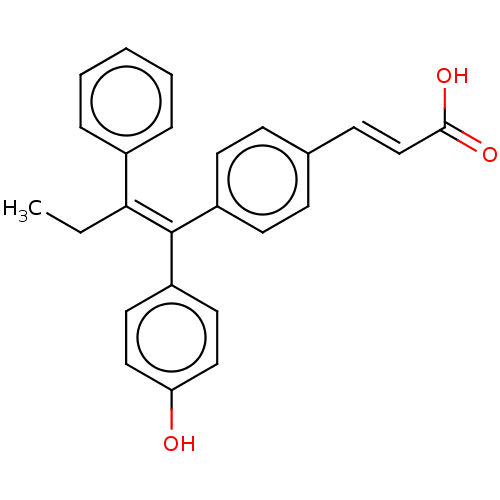 (CHEMBL195515 | GW7604) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to ER alpha (unknown origin) by LanthaScreen TR-FRET competitive binding assay | J Med Chem 58: 3522-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00066 BindingDB Entry DOI: 10.7270/Q2M32XGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245500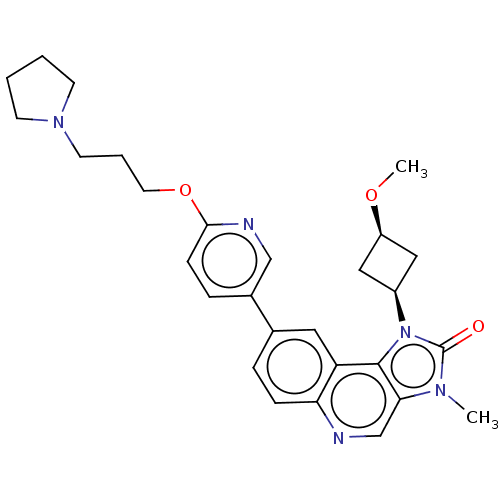 (US9428503, 28) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517560 (CHEMBL4583286) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245505 (US9428503, 33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245478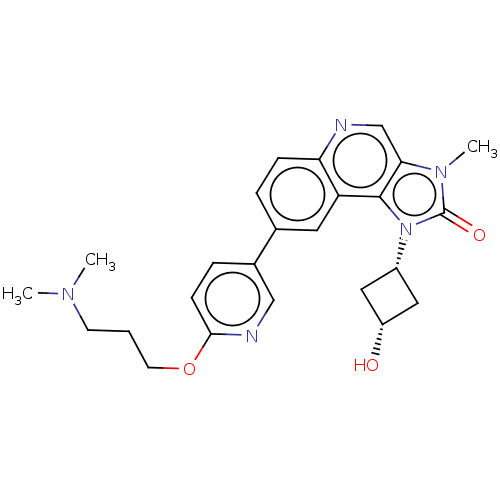 (US9428503, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50153694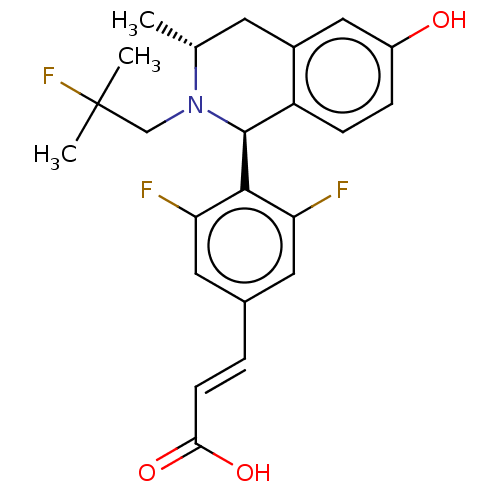 (CHEMBL3774584) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Downregulation of ERalpha in human MCF7 cells incubated for 18 to 22 hrs by immunofluorescence assay | ACS Med Chem Lett 7: 94-9 (2016) Article DOI: 10.1021/acsmedchemlett.5b00413 BindingDB Entry DOI: 10.7270/Q2HD7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245475 (US9428503, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245514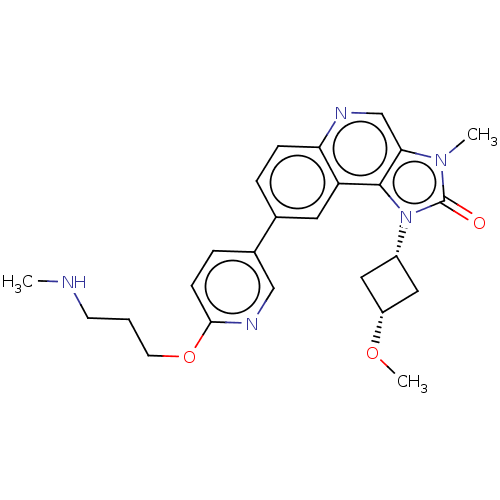 (US9428503, 42) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM288677 (US10087191, Example 94) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells assessed as reduction in ERalpha protein expression after 5 hrs by formaldehyde-staining based a... | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM288677 (US10087191, Example 94) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245510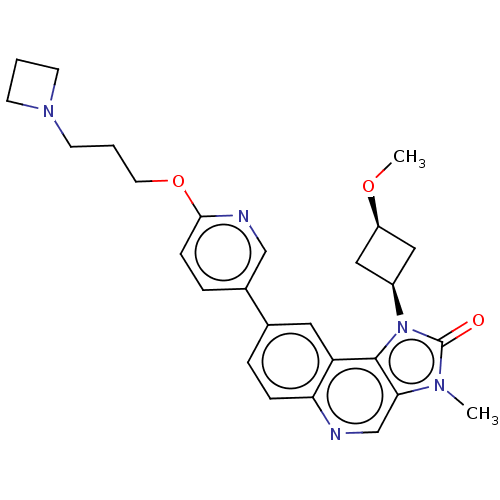 (US9428503, 38) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM288677 (US10087191, Example 94) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to recombinant human GST-tagged ERalpha LBD expressed in baculovirus-infected insect cells by lantha screen assay | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517562 (CHEMBL4586022) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517557 (CHEMBL4436390) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to recombinant human GST-tagged ERalpha LBD expressed in baculovirus-infected insect cells by lantha screen assay | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517560 (CHEMBL4583286) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to recombinant human GST-tagged ERalpha LBD expressed in baculovirus-infected insect cells by lantha screen assay | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517555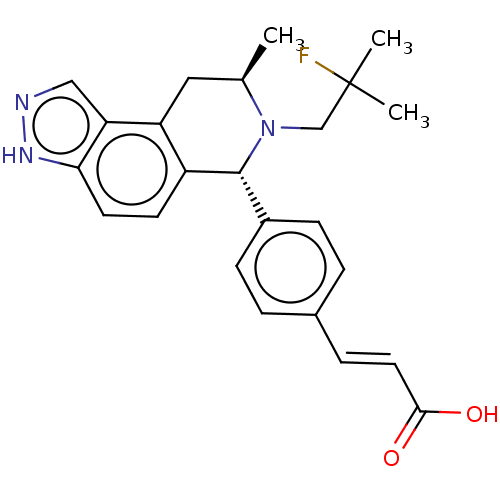 (CHEMBL4515724) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245490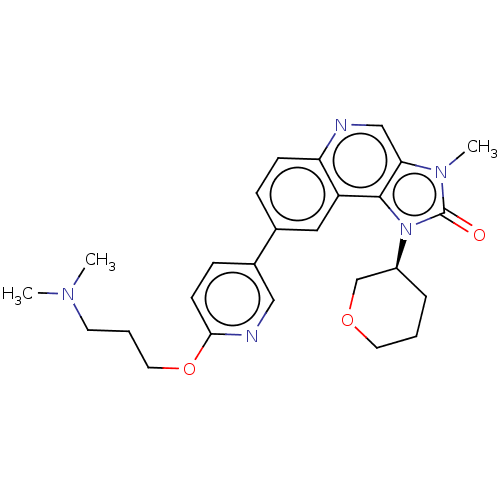 (US9428503, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50153695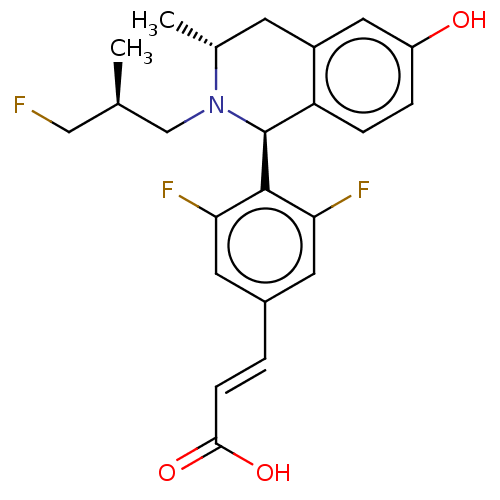 (CHEMBL3775908) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Downregulation of ERalpha in human MCF7 cells incubated for 18 to 22 hrs by immunofluorescence assay | ACS Med Chem Lett 7: 94-9 (2016) Article DOI: 10.1021/acsmedchemlett.5b00413 BindingDB Entry DOI: 10.7270/Q2HD7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517551 (CHEMBL4436187) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to recombinant human GST-tagged ERalpha LBD expressed in baculovirus-infected insect cells by lantha screen assay | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517559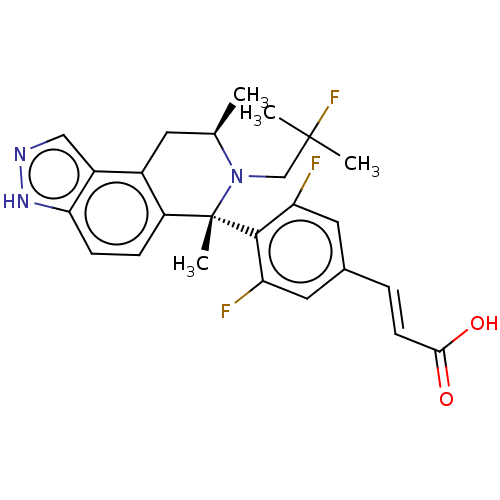 (CHEMBL4584370) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to recombinant human GST-tagged ERalpha LBD expressed in baculovirus-infected insect cells by lantha screen assay | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517551 (CHEMBL4436187) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50153696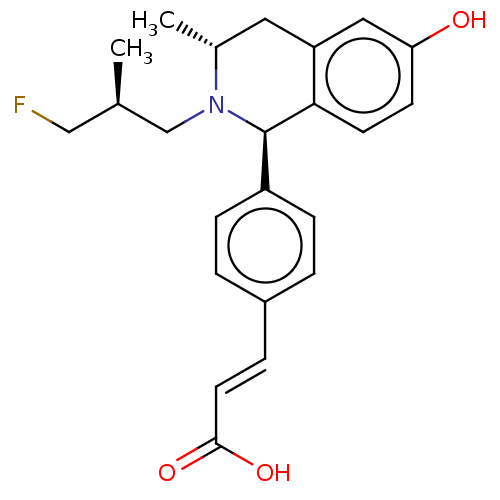 (CHEMBL3774690) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Downregulation of ERalpha in human MCF7 cells incubated for 18 to 22 hrs by immunofluorescence assay | ACS Med Chem Lett 7: 94-9 (2016) Article DOI: 10.1021/acsmedchemlett.5b00413 BindingDB Entry DOI: 10.7270/Q2HD7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50084973 (CHEMBL3427401) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to ER alpha (unknown origin) by LanthaScreen TR-FRET competitive binding assay | J Med Chem 58: 3522-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00066 BindingDB Entry DOI: 10.7270/Q2M32XGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50084974 (CHEMBL3427402) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to ER alpha (unknown origin) by LanthaScreen TR-FRET competitive binding assay | J Med Chem 58: 3522-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00066 BindingDB Entry DOI: 10.7270/Q2M32XGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517562 (CHEMBL4586022) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to recombinant human GST-tagged ERalpha LBD expressed in baculovirus-infected insect cells by lantha screen assay | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245474 (US9428503, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50593696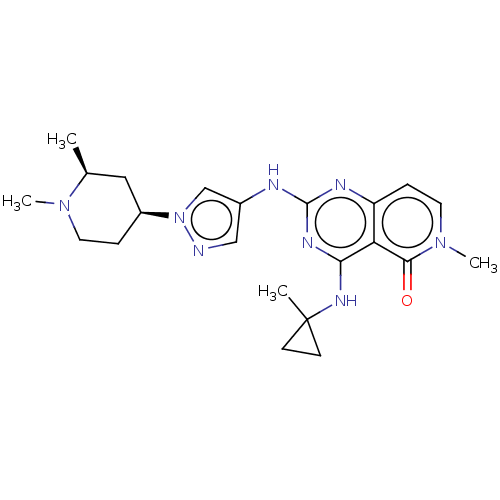 (CHEMBL5200601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116729 BindingDB Entry DOI: 10.7270/Q22V2M4V | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50612188 (CHEMBL5283628) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50593694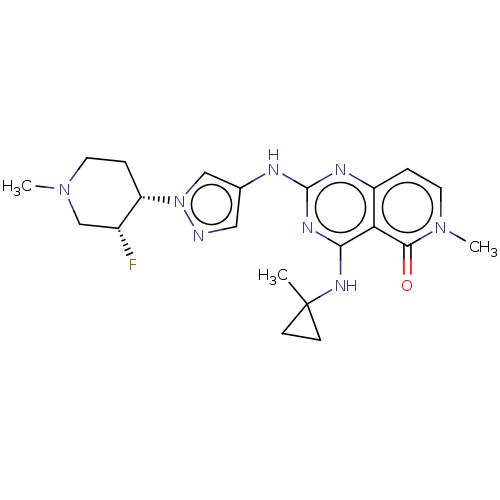 (CHEMBL5193253) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116729 BindingDB Entry DOI: 10.7270/Q22V2M4V | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50459018 (CHEMBL4166405) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50612201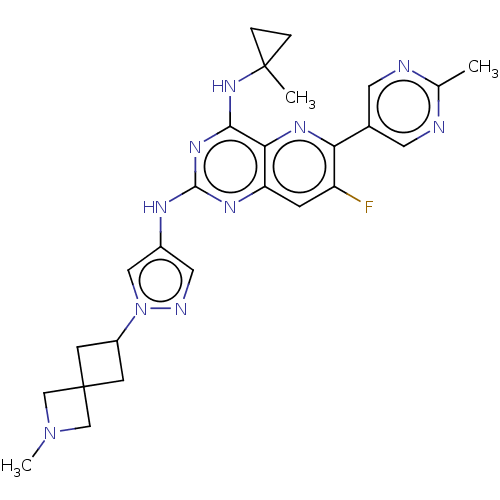 (CHEMBL5270030) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517559 (CHEMBL4584370) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517563 (CHEMBL4541930) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517564 (CHEMBL4536148) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50153661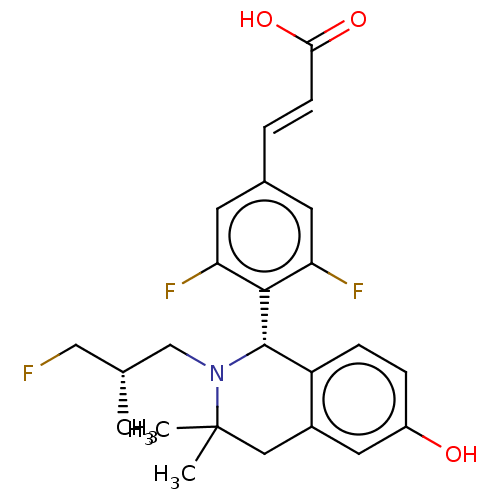 (CHEMBL3774510) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluormone ES2 from GST-tagged recombinant human ERalpha LBD after 1 hr by Lanthascreen TR-FRET assay | ACS Med Chem Lett 7: 94-9 (2016) Article DOI: 10.1021/acsmedchemlett.5b00413 BindingDB Entry DOI: 10.7270/Q2HD7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245491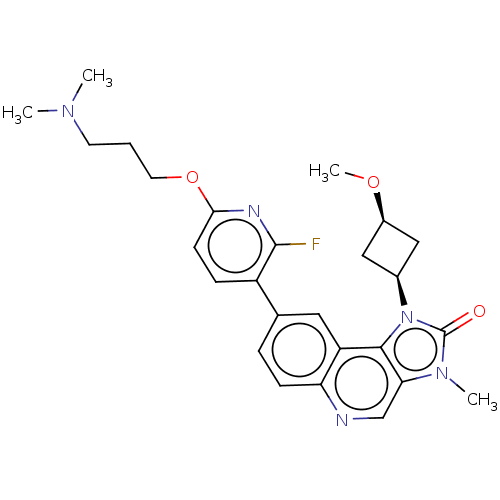 (US9428503, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245481 (US9428503, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517558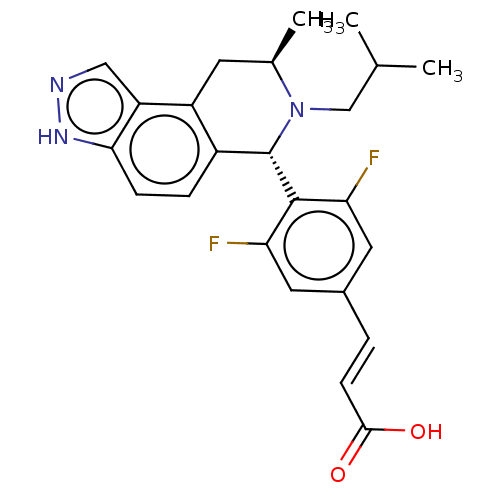 (CHEMBL4462367) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517557 (CHEMBL4436390) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM288677 (US10087191, Example 94) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells assessed as reduction in ERalpha protein expression after 5 hrs in absence of 0.25 uM tamoxifen ... | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50593693 (CHEMBL5201376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116729 BindingDB Entry DOI: 10.7270/Q22V2M4V | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535970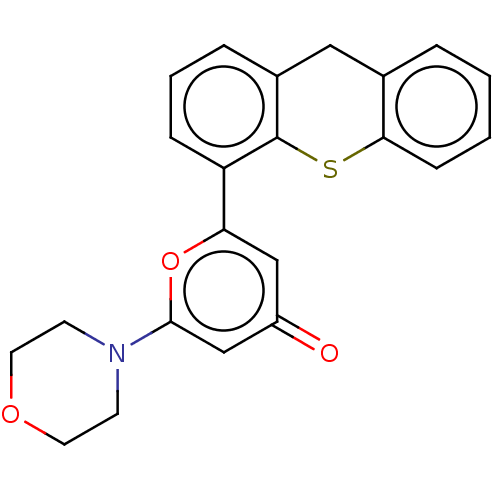 (CHEMBL4569967) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245489 (US9428503, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50153708 (CHEMBL3774777) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluormone ES2 from GST-tagged recombinant human ERalpha LBD after 1 hr by Lanthascreen TR-FRET assay | ACS Med Chem Lett 7: 94-9 (2016) Article DOI: 10.1021/acsmedchemlett.5b00413 BindingDB Entry DOI: 10.7270/Q2HD7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50153694 (CHEMBL3774584) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluormone ES2 from GST-tagged recombinant human ERalpha LBD after 1 hr by Lanthascreen TR-FRET assay | ACS Med Chem Lett 7: 94-9 (2016) Article DOI: 10.1021/acsmedchemlett.5b00413 BindingDB Entry DOI: 10.7270/Q2HD7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50612193 (CHEMBL5290090) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50084971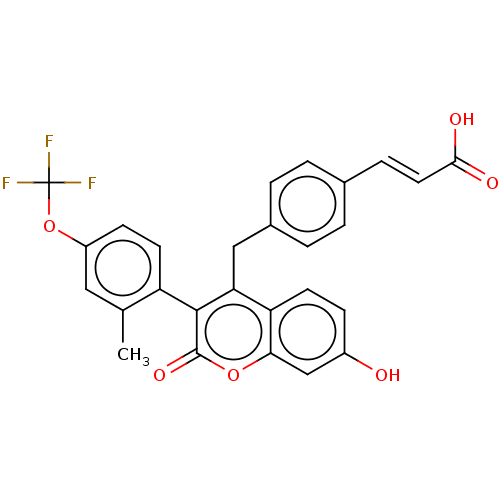 (CHEMBL3427399) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to ER alpha (unknown origin) by LanthaScreen TR-FRET competitive binding assay | J Med Chem 58: 3522-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00066 BindingDB Entry DOI: 10.7270/Q2M32XGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM119230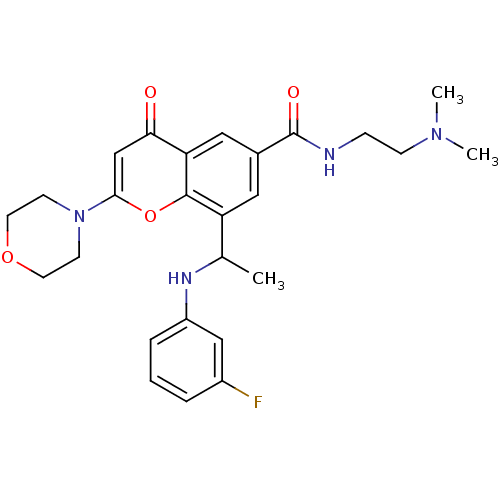 (US8673906, 1.01 | US9718800, 1.01) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
ASTRAZENECA AB US Patent | Assay Description Compounds in 100% DMSO were added to assay plates by acoustic dispensing. PI3Kβ was added in a Tris buffer (50 mM Tris pH7.4, 0.05% CHAPS, 2.1 m... | US Patent US9718800 (2017) BindingDB Entry DOI: 10.7270/Q2P2714C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM288677 (US10087191, Example 94) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at ERalpha in human MCF7 cells assessed as inhibition of estradiol-driven PR response by microplate cytometry | J Med Chem 62: 1593-1608 (2019) Article DOI: 10.1021/acs.jmedchem.8b01837 BindingDB Entry DOI: 10.7270/Q2NZ8C16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1726 total ) | Next | Last >> |