

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50210424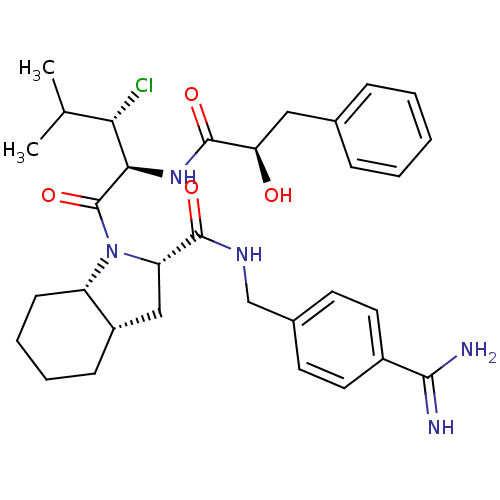 ((2S,3aS,7aS)-1-[3-chloro-2-((S)-(2S,3R)-2-hydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 17: 3480-5 (2007) Article DOI: 10.1016/j.bmcl.2007.03.075 BindingDB Entry DOI: 10.7270/Q25M65FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210431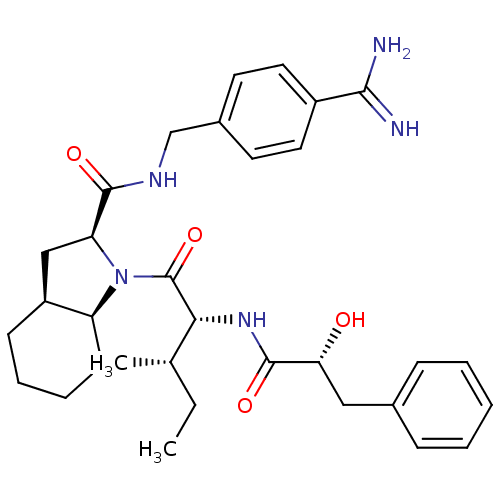 ((2S,3aS,7aS)-1-[2-((R)-(2S,3R)-2-hydroxy-3-phenyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 17: 3480-5 (2007) Article DOI: 10.1016/j.bmcl.2007.03.075 BindingDB Entry DOI: 10.7270/Q25M65FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210427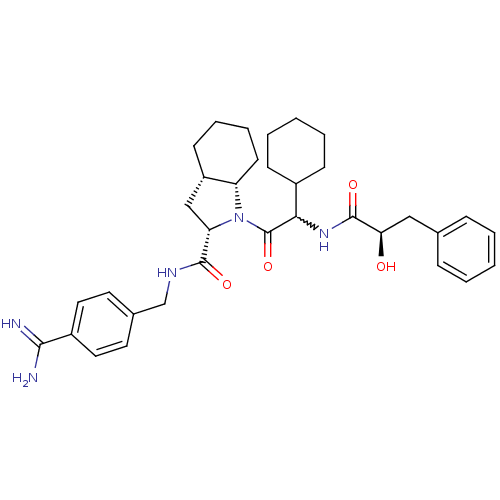 ((2S,3aS,7aS)-1-[2-cyclohexyl-2-((R)-2-hydroxy-3-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 17: 3480-5 (2007) Article DOI: 10.1016/j.bmcl.2007.03.075 BindingDB Entry DOI: 10.7270/Q25M65FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210435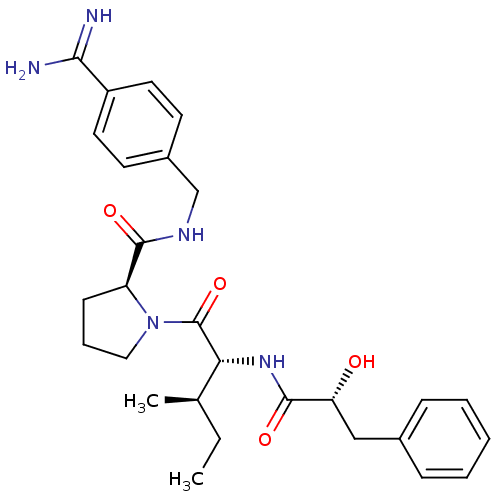 ((S)-1-[(2R,3R)-2-((R)-2-hydroxy-3-phenyl-propionyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 17: 3480-5 (2007) Article DOI: 10.1016/j.bmcl.2007.03.075 BindingDB Entry DOI: 10.7270/Q25M65FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210428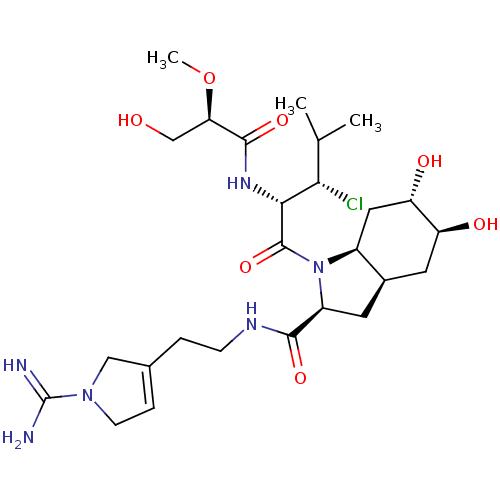 ((2S,3aR,5S,6S,7aS)-1-{(S)-3-chloro-2-[(S)-3-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 17: 3480-5 (2007) Article DOI: 10.1016/j.bmcl.2007.03.075 BindingDB Entry DOI: 10.7270/Q25M65FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013820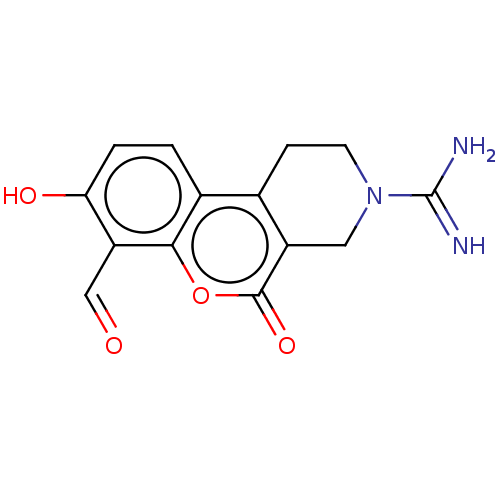 (CHEMBL3265274 | US10323013, Compound 34) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013820 (CHEMBL3265274 | US10323013, Compound 34) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210423 ((2S,3aS,7aS)-1-[(S)-2-((R)-2-hydroxy-propionylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 17: 3480-5 (2007) Article DOI: 10.1016/j.bmcl.2007.03.075 BindingDB Entry DOI: 10.7270/Q25M65FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210432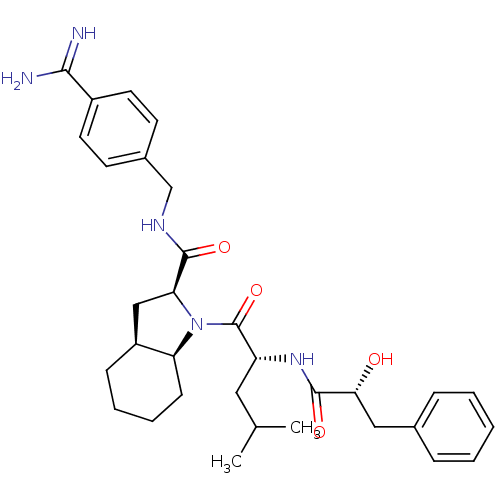 ((2S,3aS,7aS)-1-[(R)-2-((R)-2-hydroxy-3-phenyl-prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 17: 3480-5 (2007) Article DOI: 10.1016/j.bmcl.2007.03.075 BindingDB Entry DOI: 10.7270/Q25M65FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210425 ((2S,3aS,7aS)-1-((S)-2-(R)-amino-3-methyl-pentanoyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 17: 3480-5 (2007) Article DOI: 10.1016/j.bmcl.2007.03.075 BindingDB Entry DOI: 10.7270/Q25M65FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013802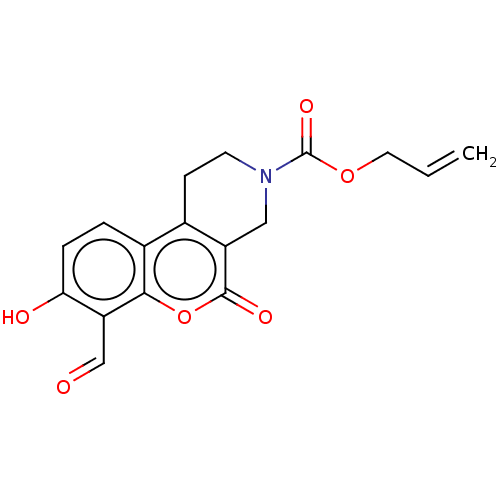 (CHEMBL3265261 | US10323013, Compound 21b) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013802 (CHEMBL3265261 | US10323013, Compound 21b) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013817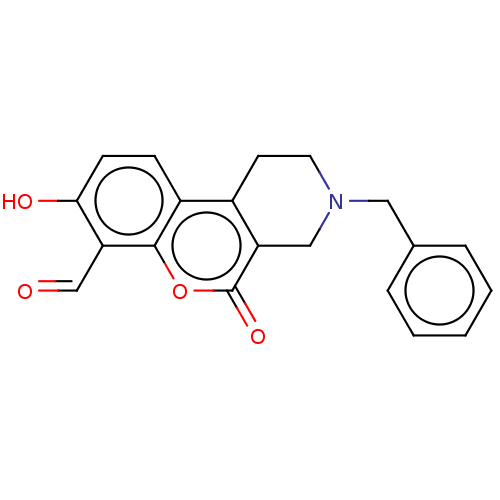 (CHEMBL3265271 | US10323013, Compound 31) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013817 (CHEMBL3265271 | US10323013, Compound 31) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013831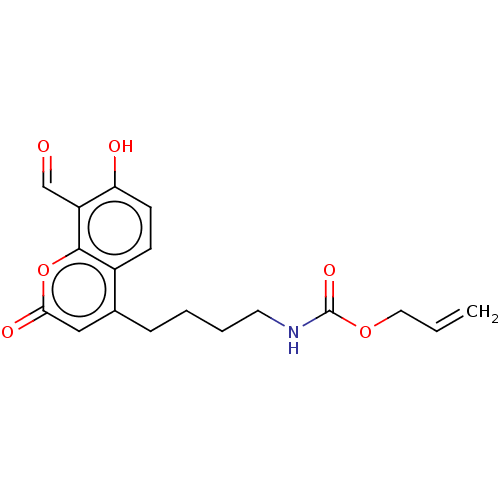 (CHEMBL3265285) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013832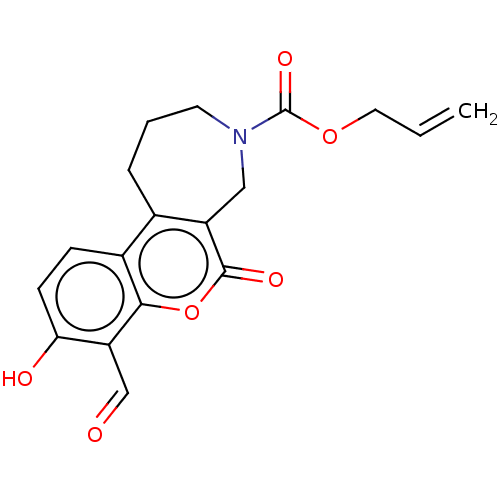 (CHEMBL3265286) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210429 ((2S,3aS,7aS)-1-[(R)-2-((R)-2-hydroxy-3-phenyl-prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 17: 3480-5 (2007) Article DOI: 10.1016/j.bmcl.2007.03.075 BindingDB Entry DOI: 10.7270/Q25M65FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013833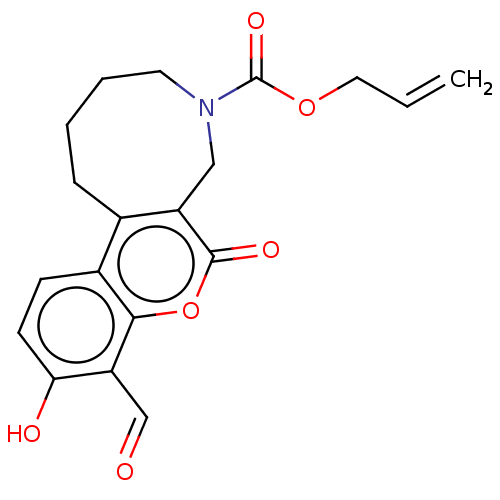 (CHEMBL3265287) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210433 (CHEMBL397123 | {(S)-1-[(2S,3aS,7aS)-2-(4-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 171 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 17: 3480-5 (2007) Article DOI: 10.1016/j.bmcl.2007.03.075 BindingDB Entry DOI: 10.7270/Q25M65FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013830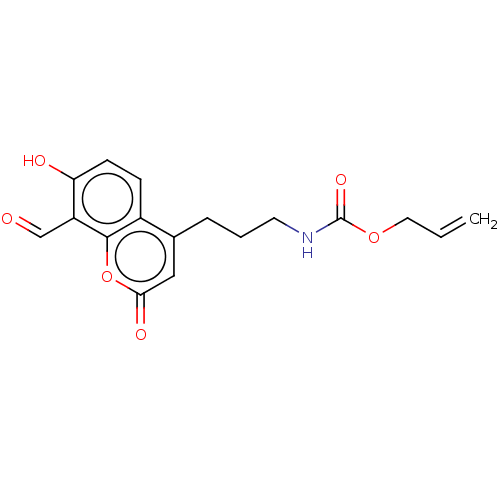 (CHEMBL3265284) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013814 (CHEMBL3265270 | US10323013, Compound 30) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013814 (CHEMBL3265270 | US10323013, Compound 30) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013795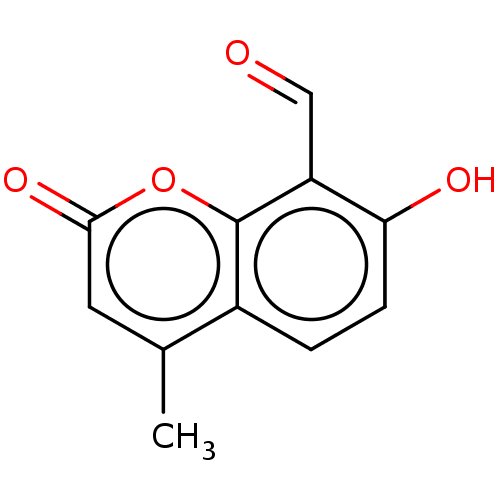 (CHEMBL272357) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210426 ((2S,3aS,6R,7aS)-6-hydroxy-1-[(R)-2-((R)-2-hydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 17: 3480-5 (2007) Article DOI: 10.1016/j.bmcl.2007.03.075 BindingDB Entry DOI: 10.7270/Q25M65FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013819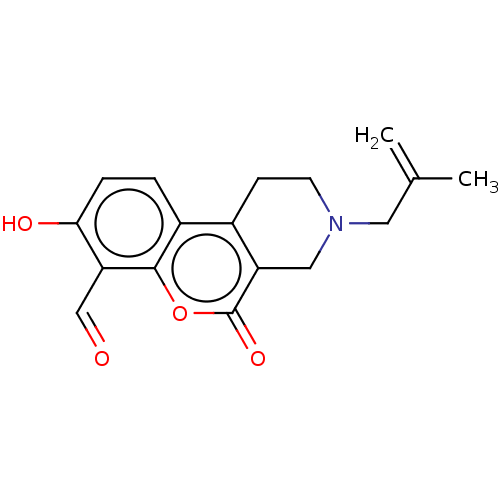 (CHEMBL3265273 | US10323013, Compound 33) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 255 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013819 (CHEMBL3265273 | US10323013, Compound 33) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 255 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013829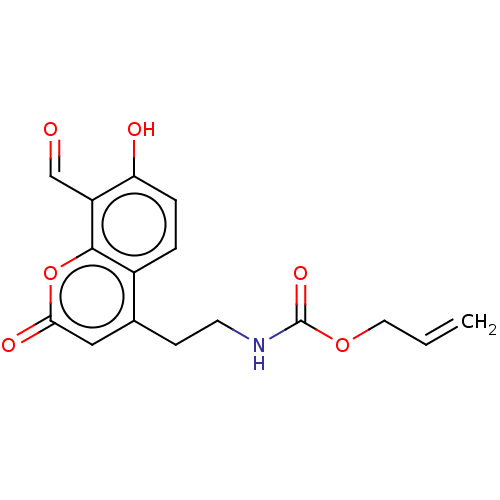 (CHEMBL3265283) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 296 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013818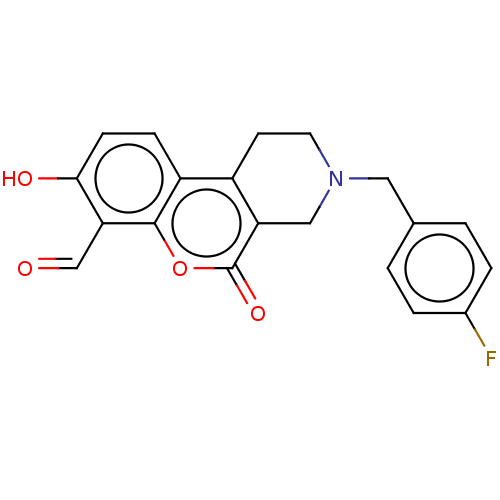 (CHEMBL3265272 | US10323013, Compound 32) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 303 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013818 (CHEMBL3265272 | US10323013, Compound 32) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 303 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210430 ((2S,3aS,6R,7aS)-1-[3-chloro-2-((S)-(2S,3R)-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 17: 3480-5 (2007) Article DOI: 10.1016/j.bmcl.2007.03.075 BindingDB Entry DOI: 10.7270/Q25M65FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013813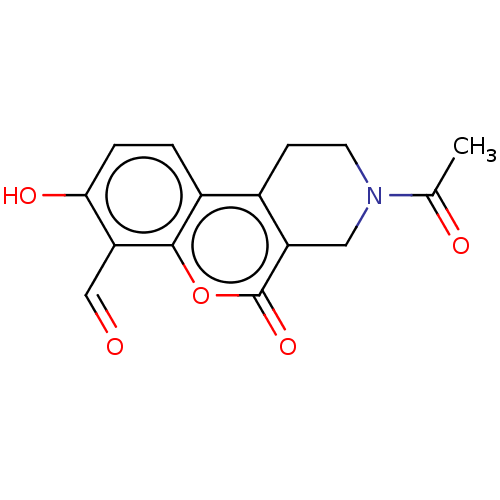 (CHEMBL3265269 | US10323013, Compound 29) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013813 (CHEMBL3265269 | US10323013, Compound 29) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210436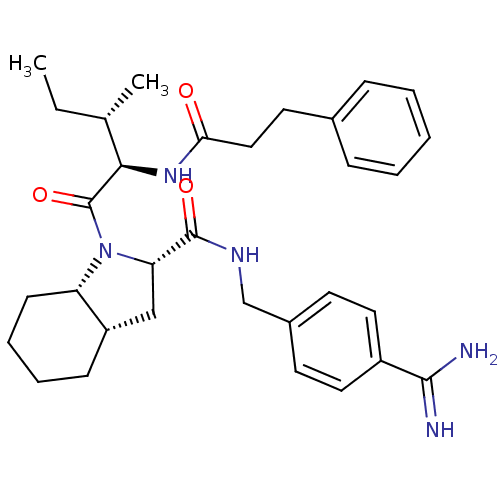 ((2S,3aS,7aS)-1-[(S)-3-methyl-2-((R)-3-phenyl-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 334 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 17: 3480-5 (2007) Article DOI: 10.1016/j.bmcl.2007.03.075 BindingDB Entry DOI: 10.7270/Q25M65FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013828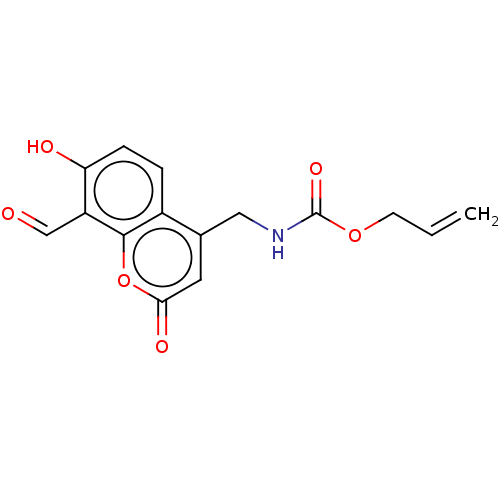 (CHEMBL3265282) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 472 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210434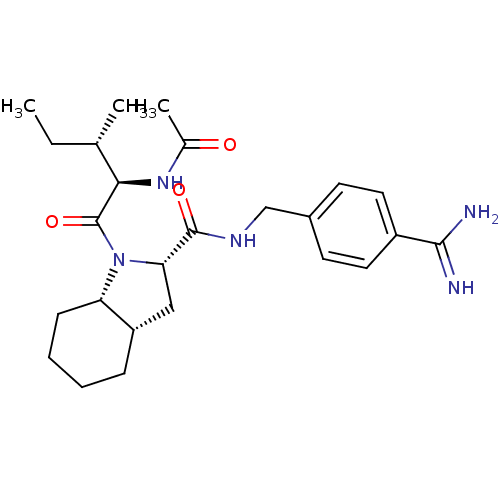 ((2S,3aS,7aS)-1-[(S)-2-((R)-acetylamino)-3-methyl-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 786 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 17: 3480-5 (2007) Article DOI: 10.1016/j.bmcl.2007.03.075 BindingDB Entry DOI: 10.7270/Q25M65FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013812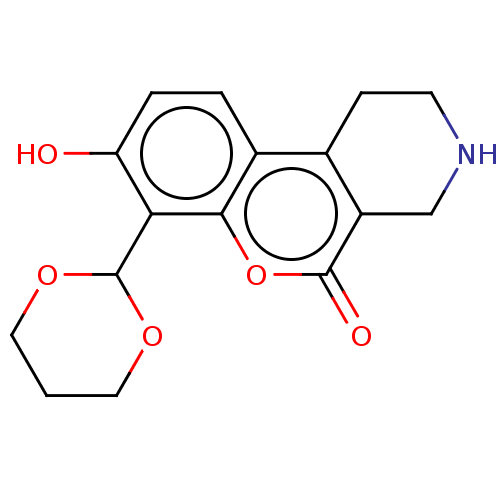 (CHEMBL3265268 | US10323013, Compound 28) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013812 (CHEMBL3265268 | US10323013, Compound 28) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210437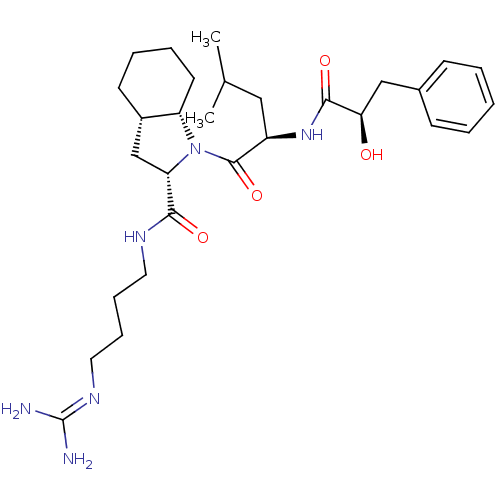 ((2S,3aS,7aS)-1-[(R)-2-((R)-2-hydroxy-3-phenyl-prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 17: 3480-5 (2007) Article DOI: 10.1016/j.bmcl.2007.03.075 BindingDB Entry DOI: 10.7270/Q25M65FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM416659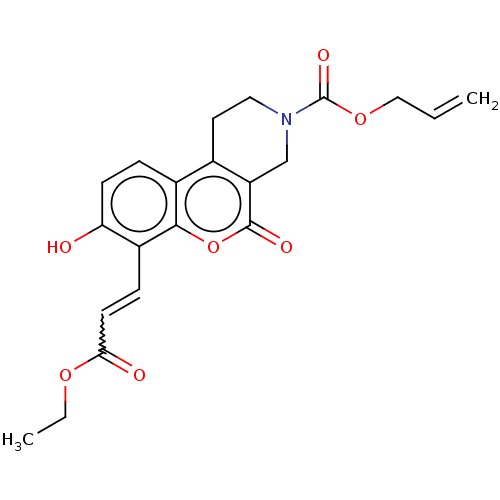 (US10323013, Compound 37 | allyl 7-(3-ethoxy-3-oxop...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013823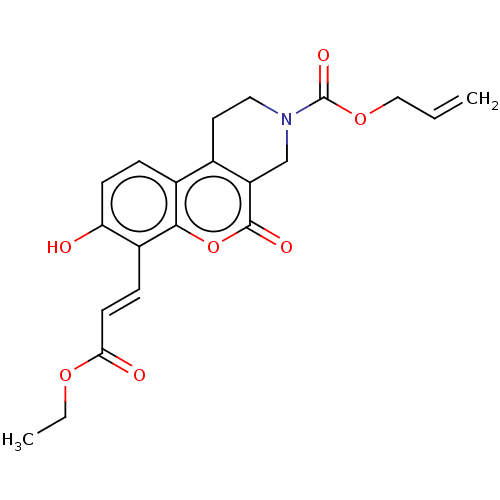 (CHEMBL3265277) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013807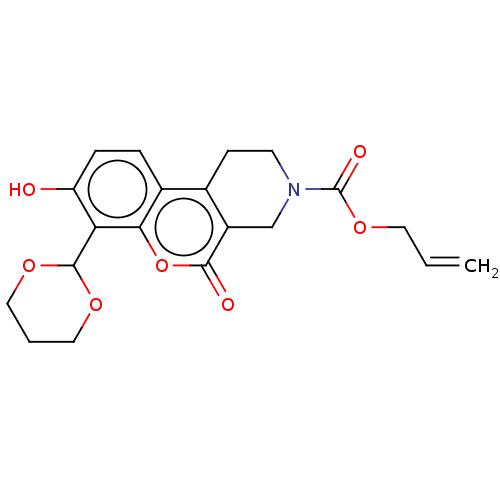 (CHEMBL3265264 | US10323013, Compound 24) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013807 (CHEMBL3265264 | US10323013, Compound 24) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50358345 (CHEMBL1922695) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of His-tagged ATP-binding domain of AKT1 assessed as GRPRTSSFAEG crosstide phosphorylation using [33P]ATP | Bioorg Med Chem Lett 21: 7166-9 (2011) Article DOI: 10.1016/j.bmcl.2011.09.079 BindingDB Entry DOI: 10.7270/Q2GX4C0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013825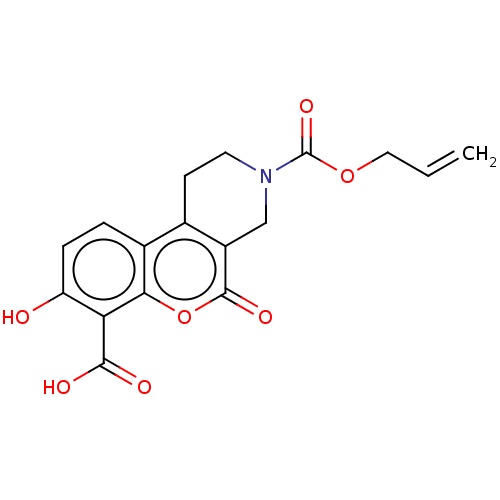 (CHEMBL3265279 | US10323013, Compound 40) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013825 (CHEMBL3265279 | US10323013, Compound 40) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210438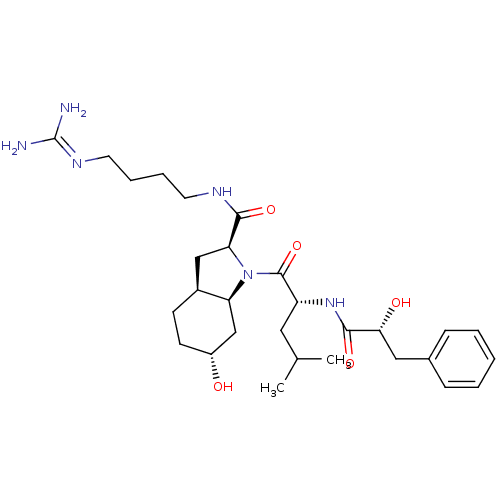 ((2S,3aS,6R,7aS)-6-hydroxy-1-[(R)-2-((R)-2-hydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 17: 3480-5 (2007) Article DOI: 10.1016/j.bmcl.2007.03.075 BindingDB Entry DOI: 10.7270/Q25M65FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013826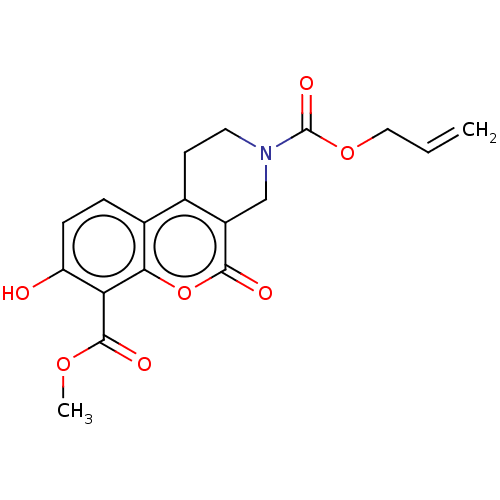 (CHEMBL3265280) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM416662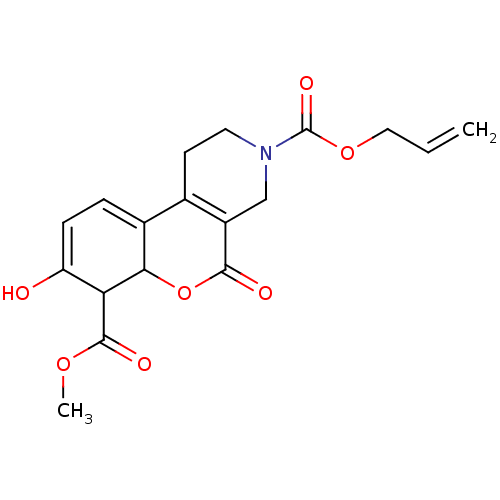 (3-allyl 7-methyl 8-hydroxy-5-oxo-4,5-dihydro-1H-ch...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013794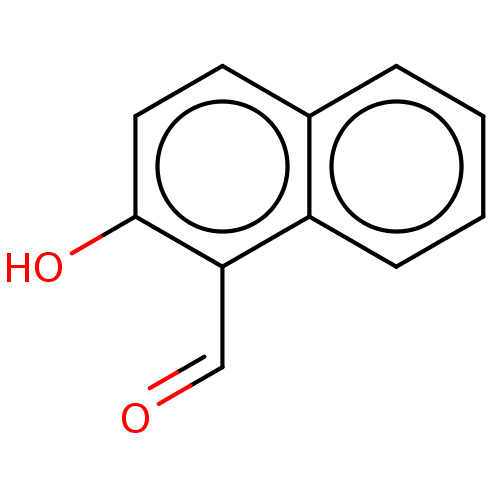 (CHEMBL3265255) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013793 (CHEMBL3192687) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant puritin-His-tagged IRE-1 RNase expressed in SF21 cells using XBP-1 RNA stem loop as substrate incubated for 30 mins p... | J Med Chem 57: 4289-301 (2014) Article DOI: 10.1021/jm5002452 BindingDB Entry DOI: 10.7270/Q23R0VDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 82 total ) | Next | Last >> |