

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001668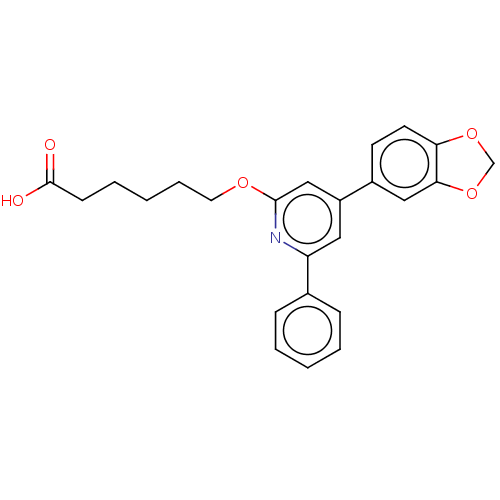 (6-(4-Benzo[1,3]dioxol-5-yl-6-phenyl-pyridin-2-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of LTB4-induced elastase release in human polymorphonuclear leukocytes | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001637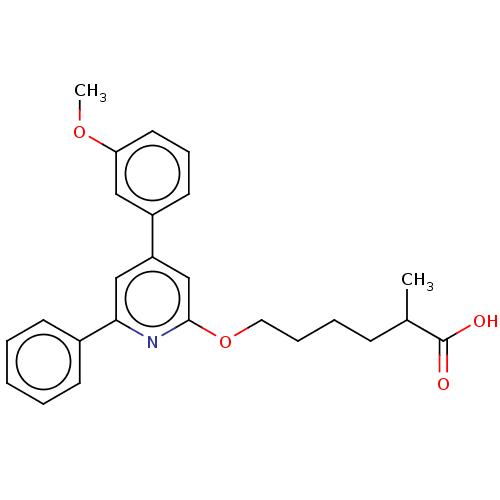 (6-[4-(3-Methoxy-phenyl)-6-phenyl-pyridin-2-yloxy]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Paris Curated by ChEMBL | Assay Description Immunosuppressive activity was measured by inhibition of the IL-2 production in Jurkat cells. | Bioorg Med Chem Lett 13: 4415-9 (2003) BindingDB Entry DOI: 10.7270/Q2183715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001611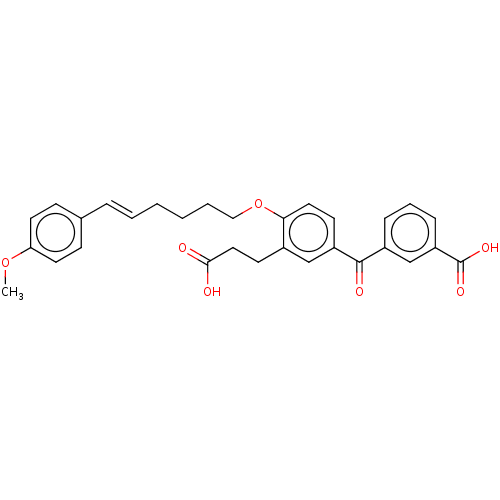 ((E)-3-{3-(2-Carboxy-ethyl)-4-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of LTB4-induced elastase release in human polymorphonuclear leukocytes | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001657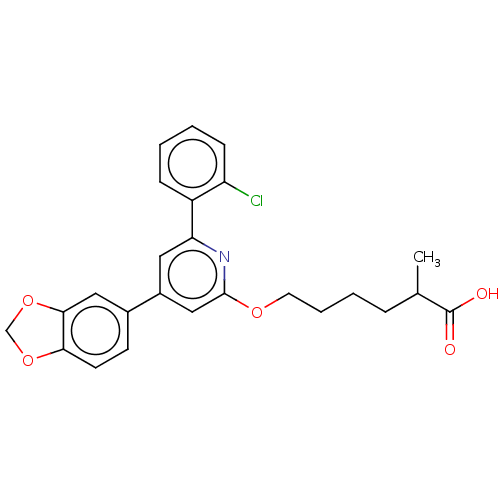 (6-[4-Benzo[1,3]dioxol-5-yl-6-(2-chloro-phenyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of LTB4-induced elastase release in human polymorphonuclear leukocytes | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001667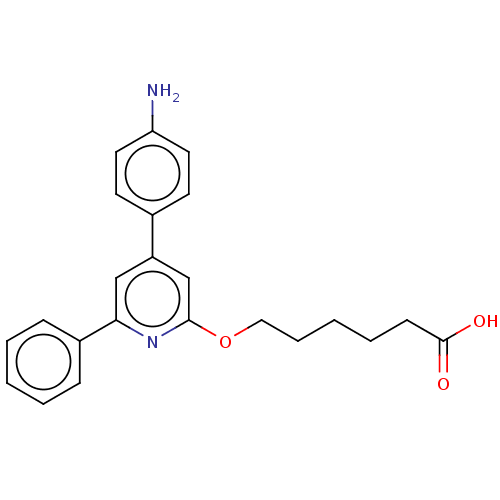 (6-[4-(4-Amino-phenyl)-6-phenyl-pyridin-2-yloxy]-he...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the human polymorphonuclear leukocytes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001611 ((E)-3-{3-(2-Carboxy-ethyl)-4-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the human polymorphonuclear leukocytes | J Med Chem 35: 4306-14 (1992) BindingDB Entry DOI: 10.7270/Q2VH5MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001658 (6-(4,6-Diphenyl-pyridin-2-yloxy)-hexanoic acid | C...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50136490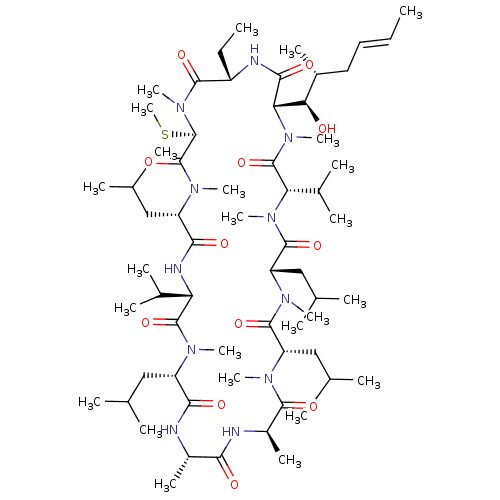 (CHEMBL3038087 | Cyclosporin A analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Paris Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV-1 RT in CEM4 cell line | Bioorg Med Chem Lett 13: 4415-9 (2003) BindingDB Entry DOI: 10.7270/Q2183715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50136490 (CHEMBL3038087 | Cyclosporin A analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Paris Curated by ChEMBL | Assay Description Immunosuppressive activity was measured by inhibition of the IL-2 production in Jurkat cells. | Bioorg Med Chem Lett 13: 4415-9 (2003) BindingDB Entry DOI: 10.7270/Q2183715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001611 ((E)-3-{3-(2-Carboxy-ethyl)-4-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the human polymorphonuclear leukocytes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001611 ((E)-3-{3-(2-Carboxy-ethyl)-4-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the human polymorphonuclear leukocytes | J Med Chem 35: 4306-14 (1992) BindingDB Entry DOI: 10.7270/Q2VH5MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001661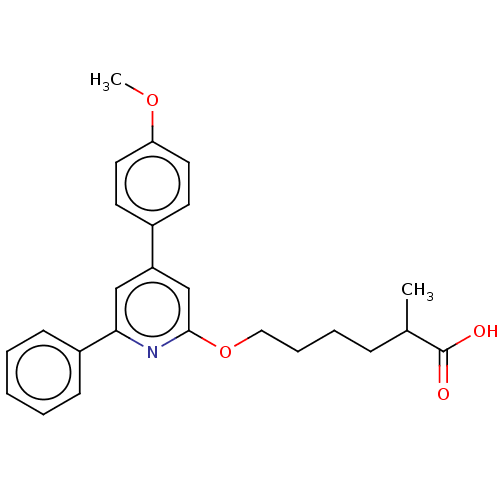 (6-[4-(4-Methoxy-phenyl)-6-phenyl-pyridin-2-yloxy]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001641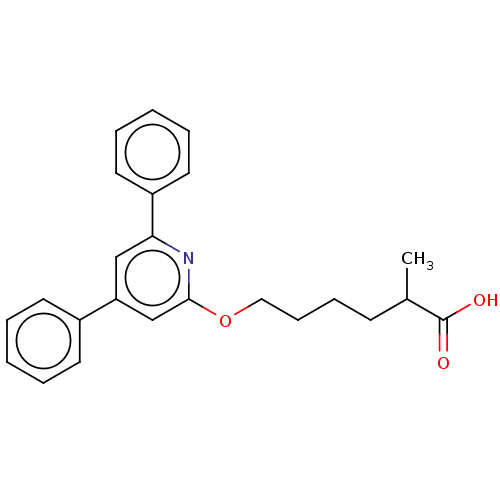 (6-(4,6-Diphenyl-pyridin-2-yloxy)-2-methyl-hexanoic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001645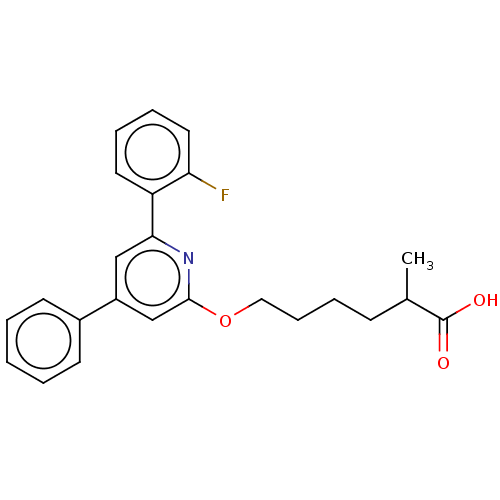 (6-[6-(2-Fluoro-phenyl)-4-phenyl-pyridin-2-yloxy]-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001647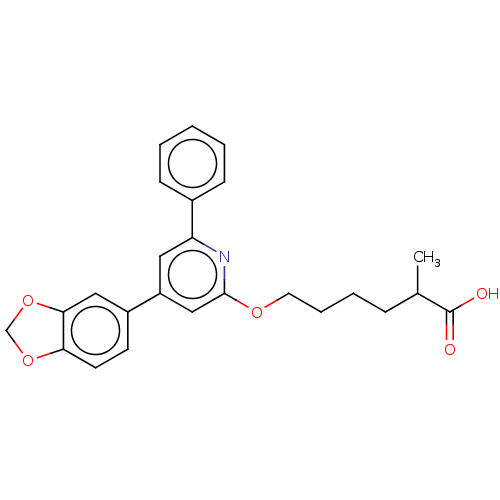 (6-(4-Benzo[1,3]dioxol-5-yl-6-phenyl-pyridin-2-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of LTB4-induced elastase release in human polymorphonuclear leukocytes | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001636 (6-[4-(3-Chloro-phenyl)-6-phenyl-pyridin-2-yloxy]-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001644 (6-(4,6-Diphenyl-pyridin-2-yloxy)-2,2-dimethyl-hexa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001653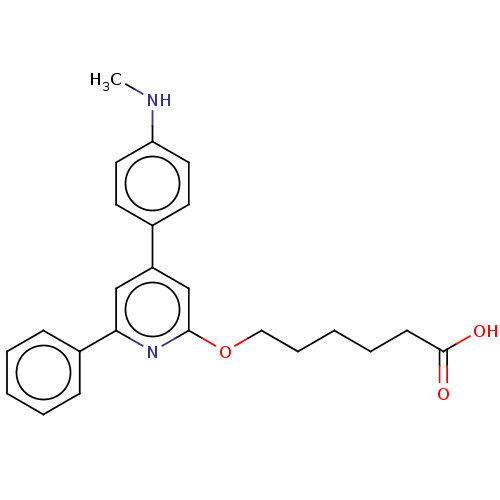 (6-[4-(4-Methylamino-phenyl)-6-phenyl-pyridin-2-ylo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001664 (6-[4-(4-Chloro-phenyl)-6-phenyl-pyridin-2-yloxy]-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the human polymorphonuclear leukocytes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001642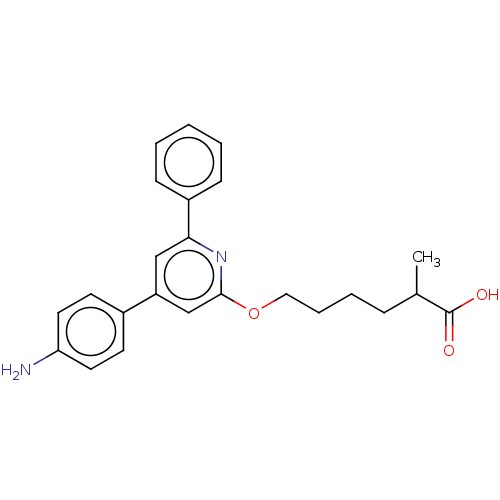 (6-[4-(4-Amino-phenyl)-6-phenyl-pyridin-2-yloxy]-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001668 (6-(4-Benzo[1,3]dioxol-5-yl-6-phenyl-pyridin-2-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor of human polymorphonuclear leukocytes | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001670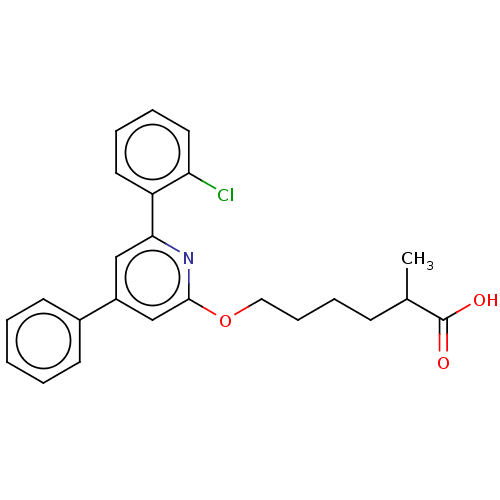 (6-[6-(2-Chloro-phenyl)-4-phenyl-pyridin-2-yloxy]-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001652 (6-[4-(4-Amino-phenyl)-6-phenyl-pyridin-2-yloxy]-2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001647 (6-(4-Benzo[1,3]dioxol-5-yl-6-phenyl-pyridin-2-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the human polymorphonuclear leukocytes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001637 (6-[4-(3-Methoxy-phenyl)-6-phenyl-pyridin-2-yloxy]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001672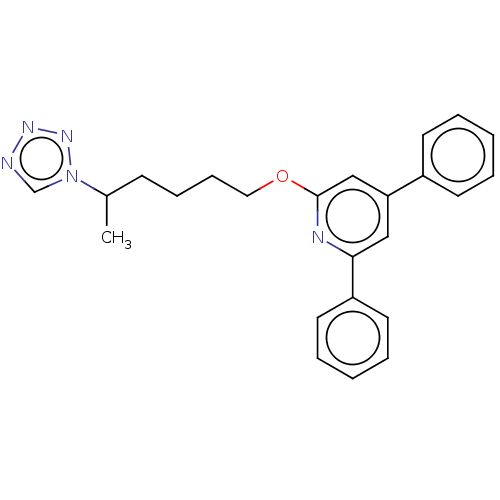 (2,4-Diphenyl-6-(5-tetrazol-1-yl-hexyloxy)-pyridine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001655 (6-(4-Benzo[1,3]dioxol-5-yl-6-phenyl-pyridin-2-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the human polymorphonuclear leukocytes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001672 (2,4-Diphenyl-6-(5-tetrazol-1-yl-hexyloxy)-pyridine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of LTB4-induced elastase release in human polymorphonuclear leukocytes | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001652 (6-[4-(4-Amino-phenyl)-6-phenyl-pyridin-2-yloxy]-2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001649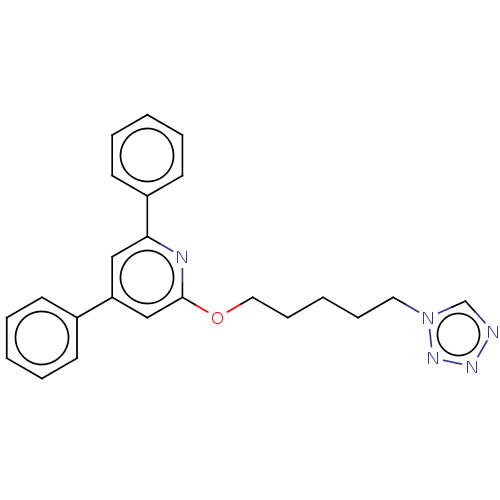 (2,4-Diphenyl-6-(5-tetrazol-1-yl-pentyloxy)-pyridin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001622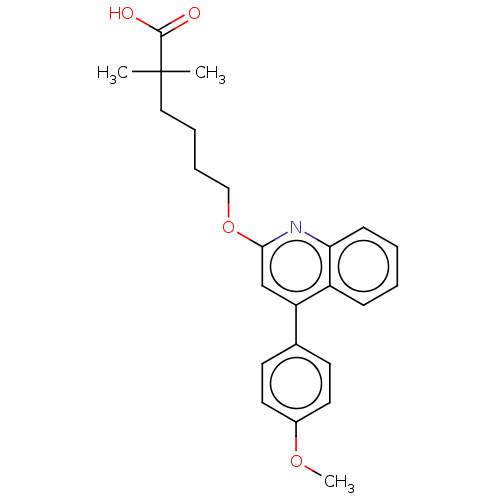 (6-[4-(4-Methoxy-phenyl)-quinolin-2-yloxy]-2,2-dime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes | J Med Chem 35: 4306-14 (1992) BindingDB Entry DOI: 10.7270/Q2VH5MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001651 (6-[4-(2-Fluoro-phenyl)-6-phenyl-pyridin-2-yloxy]-h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001659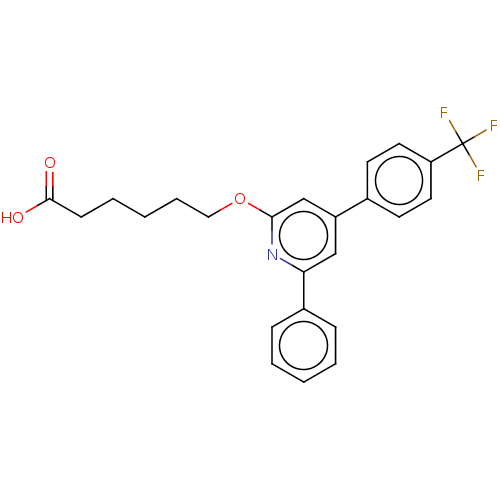 (6-[6-Phenyl-4-(4-trifluoromethyl-phenyl)-pyridin-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001661 (6-[4-(4-Methoxy-phenyl)-6-phenyl-pyridin-2-yloxy]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the human polymorphonuclear leukocytes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001657 (6-[4-Benzo[1,3]dioxol-5-yl-6-(2-chloro-phenyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the human polymorphonuclear leukocytes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001657 (6-[4-Benzo[1,3]dioxol-5-yl-6-(2-chloro-phenyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001660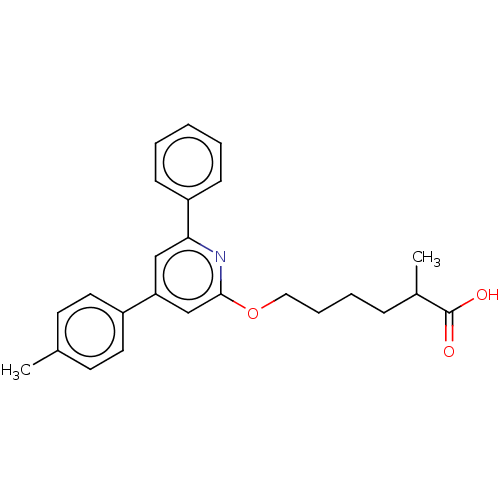 (2-Methyl-6-(6-phenyl-4-p-tolyl-pyridin-2-yloxy)-he...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001650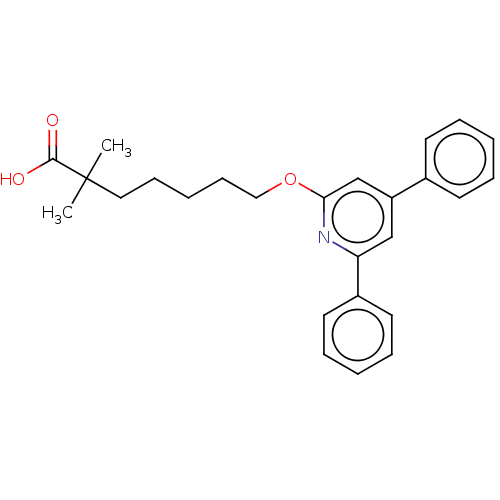 (7-(4,6-Diphenyl-pyridin-2-yloxy)-2,2-dimethyl-hept...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the human polymorphonuclear leukocytes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001619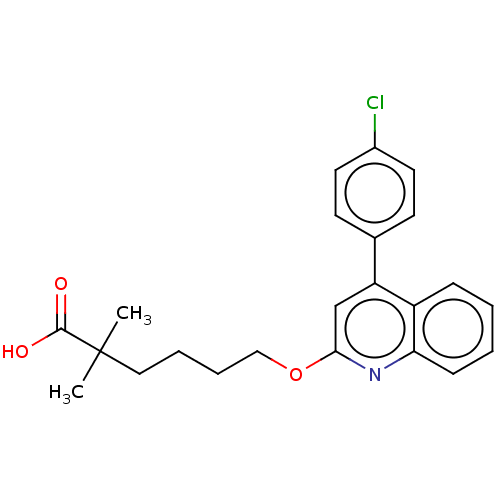 (6-[4-(4-Chloro-phenyl)-quinolin-2-yloxy]-2,2-dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes | J Med Chem 35: 4306-14 (1992) BindingDB Entry DOI: 10.7270/Q2VH5MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001652 (6-[4-(4-Amino-phenyl)-6-phenyl-pyridin-2-yloxy]-2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of LTB4-induced elastase release in human polymorphonuclear leukocytes | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001642 (6-[4-(4-Amino-phenyl)-6-phenyl-pyridin-2-yloxy]-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50136484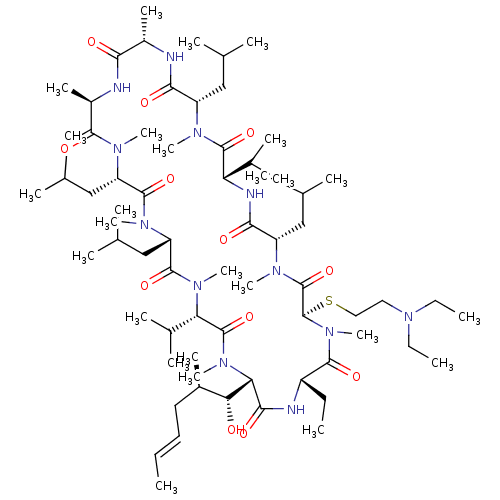 (CHEMBL2407581 | Cyclosporin A analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Paris Curated by ChEMBL | Assay Description Immunosuppressive activity was measured by inhibition of the IL-2 production in Jurkat cells. | Bioorg Med Chem Lett 13: 4415-9 (2003) BindingDB Entry DOI: 10.7270/Q2183715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001669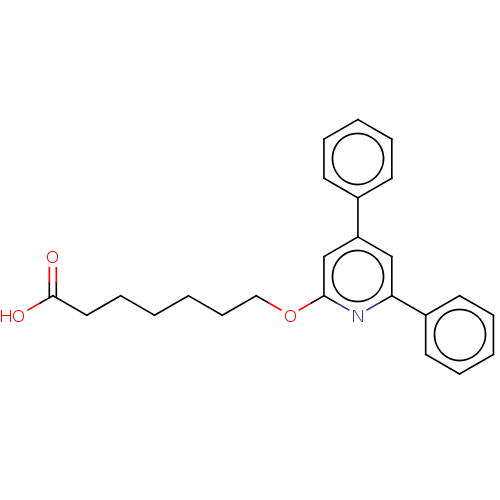 (7-(4,6-Diphenyl-pyridin-2-yloxy)-heptanoic acid | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of LTB4-induced elastase release in human polymorphonuclear leukocytes | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50136484 (CHEMBL2407581 | Cyclosporin A analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Paris Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV-1 RT in CEM4 cell line | Bioorg Med Chem Lett 13: 4415-9 (2003) BindingDB Entry DOI: 10.7270/Q2183715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001645 (6-[6-(2-Fluoro-phenyl)-4-phenyl-pyridin-2-yloxy]-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50136476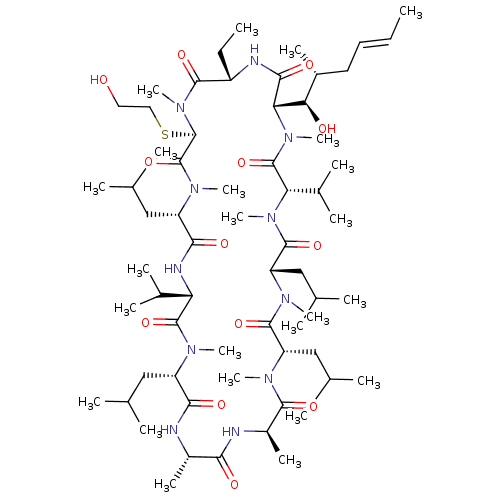 (CHEMBL3038089 | Cyclosporin A analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Paris Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV-1 RT in CEM4 cell line | Bioorg Med Chem Lett 13: 4415-9 (2003) BindingDB Entry DOI: 10.7270/Q2183715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50136476 (CHEMBL3038089 | Cyclosporin A analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Paris Curated by ChEMBL | Assay Description Immunosuppressive activity was measured by inhibition of the IL-2 production in Jurkat cells. | Bioorg Med Chem Lett 13: 4415-9 (2003) BindingDB Entry DOI: 10.7270/Q2183715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50136479 (CHEMBL3038084 | Cyclosporin A analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Paris Curated by ChEMBL | Assay Description Immunosuppressive activity was measured by inhibition of the IL-2 production in Jurkat cells. | Bioorg Med Chem Lett 13: 4415-9 (2003) BindingDB Entry DOI: 10.7270/Q2183715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50136472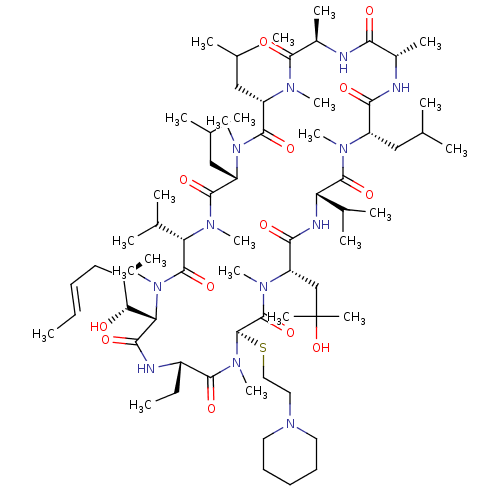 (CHEMBL3038082 | [(40-OH) MeLeu]4-CsA derivatives) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Paris Curated by ChEMBL | Assay Description Immunosuppressive activity was measured by inhibition of the IL-2 production in Jurkat cells. | Bioorg Med Chem Lett 13: 4415-9 (2003) BindingDB Entry DOI: 10.7270/Q2183715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 205 total ) | Next | Last >> |