

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM50442766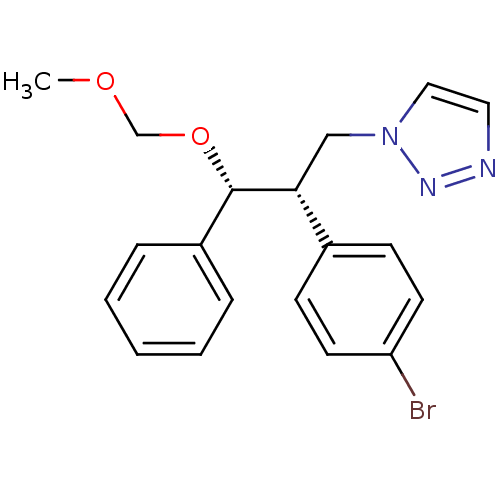 (CHEMBL2443358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay | Bioorg Med Chem Lett 23: 6060-3 (2013) Article DOI: 10.1016/j.bmcl.2013.09.030 BindingDB Entry DOI: 10.7270/Q2QJ7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442776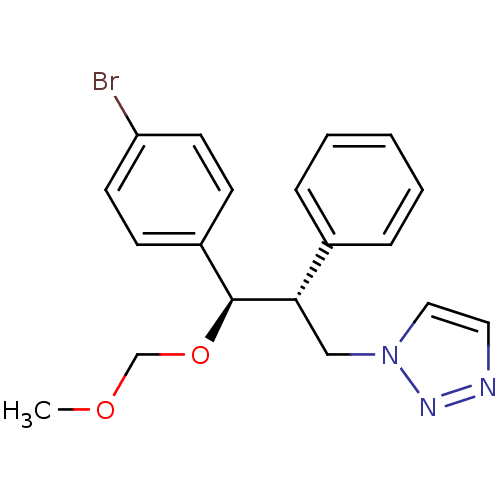 (CHEMBL2443348) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay | Bioorg Med Chem Lett 23: 6060-3 (2013) Article DOI: 10.1016/j.bmcl.2013.09.030 BindingDB Entry DOI: 10.7270/Q2QJ7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50360383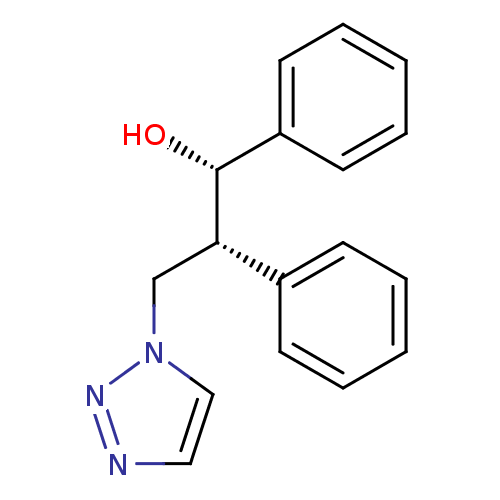 (CHEMBL1933700) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50360381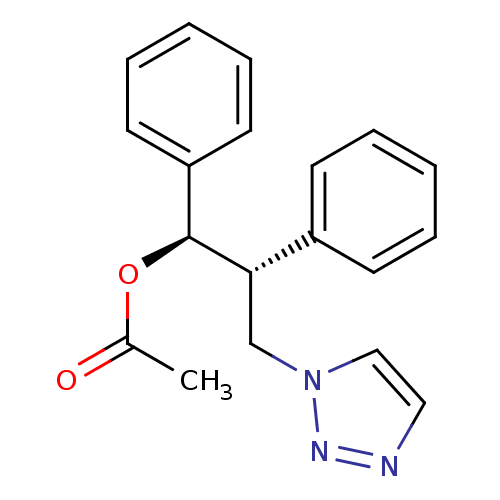 (CHEMBL1933694) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442767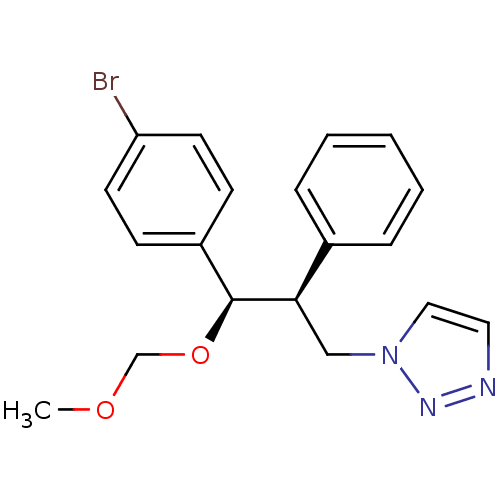 (CHEMBL2443357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay | Bioorg Med Chem Lett 23: 6060-3 (2013) Article DOI: 10.1016/j.bmcl.2013.09.030 BindingDB Entry DOI: 10.7270/Q2QJ7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50360382 (CHEMBL1933699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442777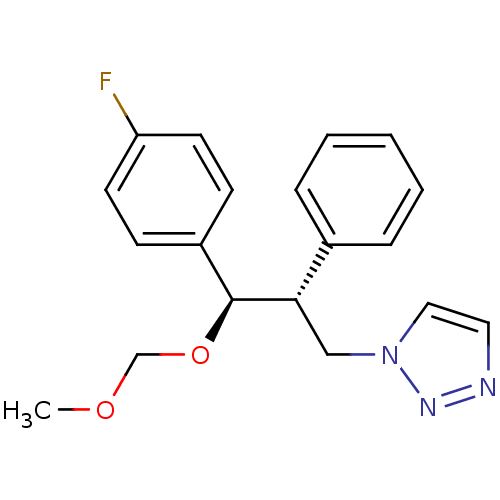 (CHEMBL2443365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay | Bioorg Med Chem Lett 23: 6060-3 (2013) Article DOI: 10.1016/j.bmcl.2013.09.030 BindingDB Entry DOI: 10.7270/Q2QJ7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442770 (CHEMBL2443354) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay | Bioorg Med Chem Lett 23: 6060-3 (2013) Article DOI: 10.1016/j.bmcl.2013.09.030 BindingDB Entry DOI: 10.7270/Q2QJ7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50360380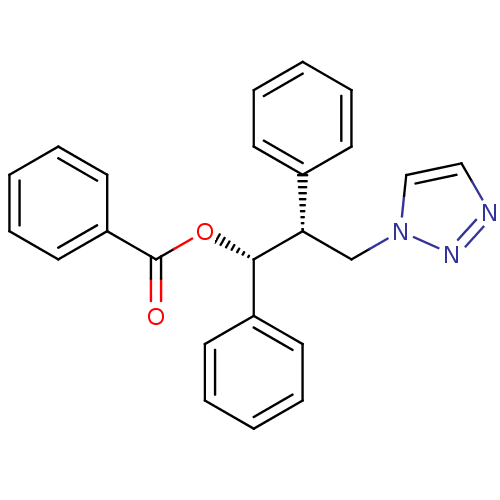 (CHEMBL1933693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442771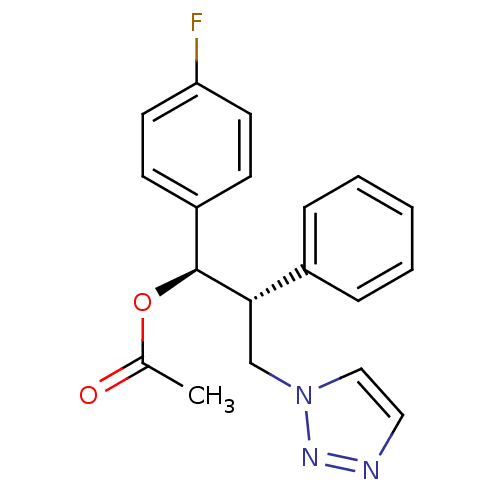 (CHEMBL2443353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay | Bioorg Med Chem Lett 23: 6060-3 (2013) Article DOI: 10.1016/j.bmcl.2013.09.030 BindingDB Entry DOI: 10.7270/Q2QJ7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442775 (CHEMBL2443349) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay | Bioorg Med Chem Lett 23: 6060-3 (2013) Article DOI: 10.1016/j.bmcl.2013.09.030 BindingDB Entry DOI: 10.7270/Q2QJ7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442764 (CHEMBL2443360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay | Bioorg Med Chem Lett 23: 6060-3 (2013) Article DOI: 10.1016/j.bmcl.2013.09.030 BindingDB Entry DOI: 10.7270/Q2QJ7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442768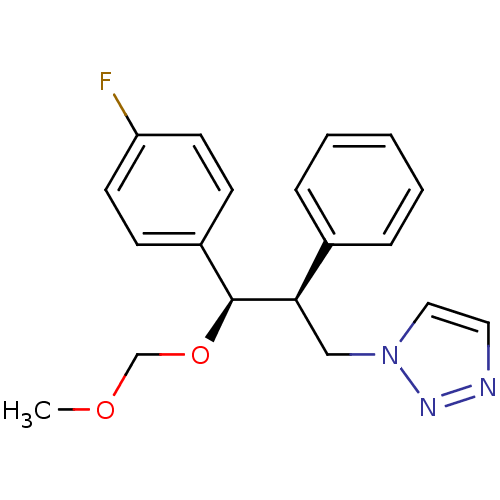 (CHEMBL2443356) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay | Bioorg Med Chem Lett 23: 6060-3 (2013) Article DOI: 10.1016/j.bmcl.2013.09.030 BindingDB Entry DOI: 10.7270/Q2QJ7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442773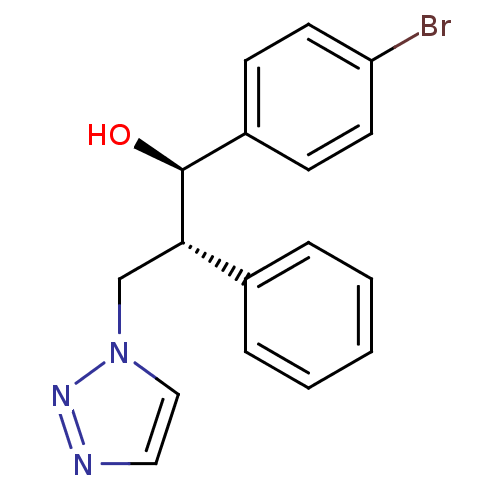 (CHEMBL2443351) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay | Bioorg Med Chem Lett 23: 6060-3 (2013) Article DOI: 10.1016/j.bmcl.2013.09.030 BindingDB Entry DOI: 10.7270/Q2QJ7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442765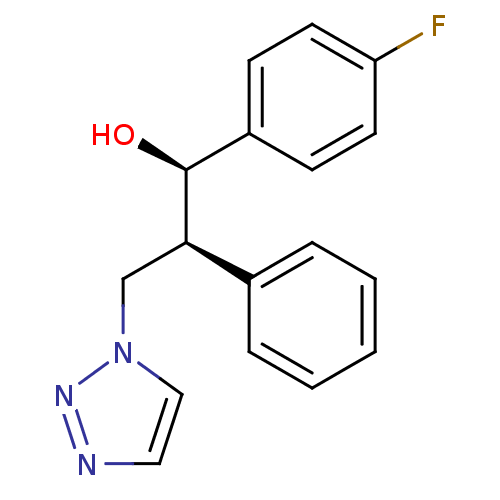 (CHEMBL2443359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay | Bioorg Med Chem Lett 23: 6060-3 (2013) Article DOI: 10.1016/j.bmcl.2013.09.030 BindingDB Entry DOI: 10.7270/Q2QJ7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442769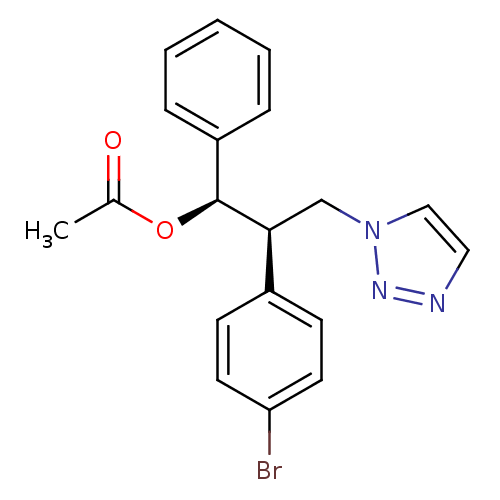 (CHEMBL2443355) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 237 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay | Bioorg Med Chem Lett 23: 6060-3 (2013) Article DOI: 10.1016/j.bmcl.2013.09.030 BindingDB Entry DOI: 10.7270/Q2QJ7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50360384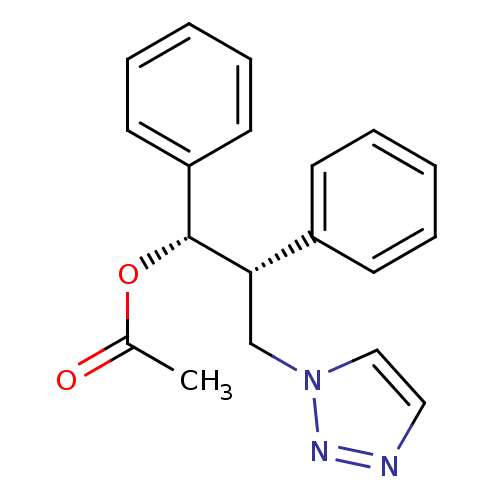 (CHEMBL1933701) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442774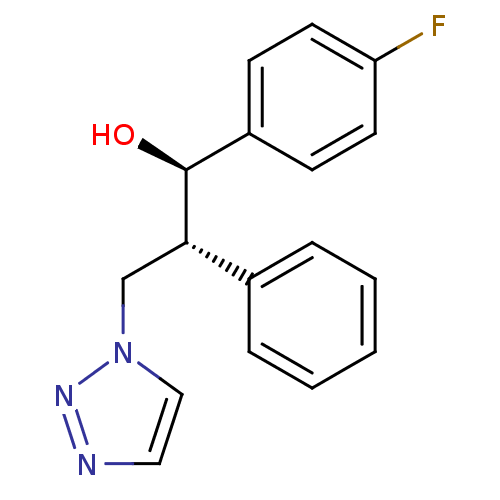 (CHEMBL2443350) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay | Bioorg Med Chem Lett 23: 6060-3 (2013) Article DOI: 10.1016/j.bmcl.2013.09.030 BindingDB Entry DOI: 10.7270/Q2QJ7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442762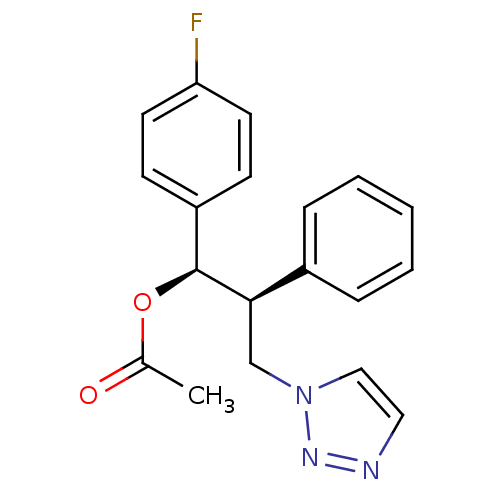 (CHEMBL2443362) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay | Bioorg Med Chem Lett 23: 6060-3 (2013) Article DOI: 10.1016/j.bmcl.2013.09.030 BindingDB Entry DOI: 10.7270/Q2QJ7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM31768 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM31768 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442761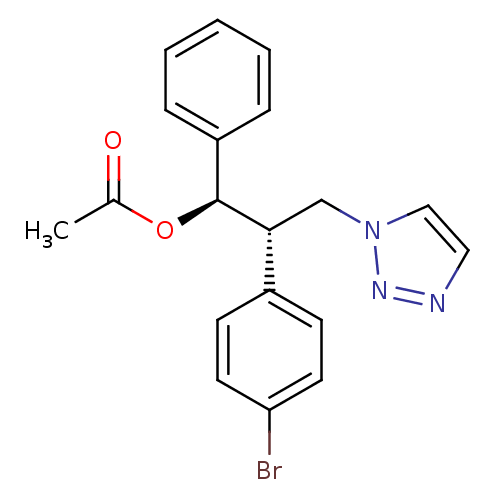 (CHEMBL2443364) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 647 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay | Bioorg Med Chem Lett 23: 6060-3 (2013) Article DOI: 10.1016/j.bmcl.2013.09.030 BindingDB Entry DOI: 10.7270/Q2QJ7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50360378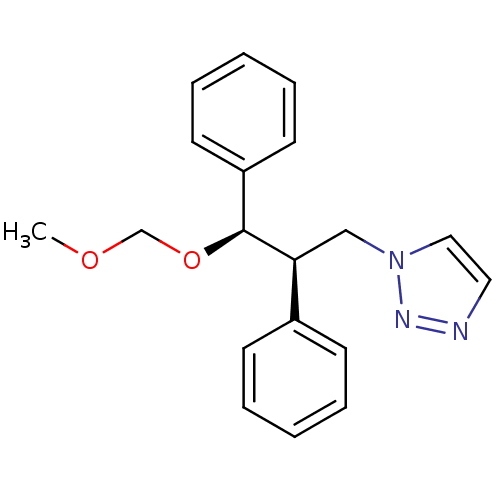 (CHEMBL1933690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442772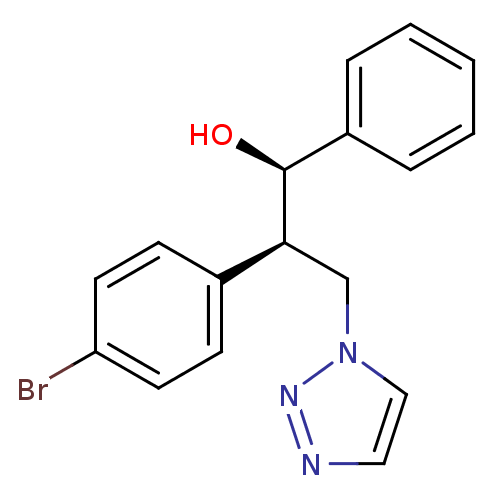 (CHEMBL2443352) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay | Bioorg Med Chem Lett 23: 6060-3 (2013) Article DOI: 10.1016/j.bmcl.2013.09.030 BindingDB Entry DOI: 10.7270/Q2QJ7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442763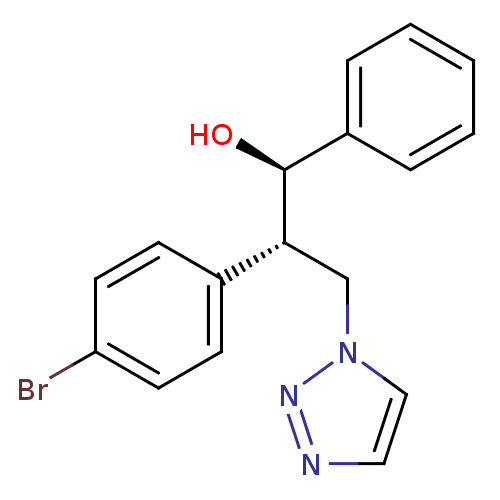 (CHEMBL2443361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 841 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay | Bioorg Med Chem Lett 23: 6060-3 (2013) Article DOI: 10.1016/j.bmcl.2013.09.030 BindingDB Entry DOI: 10.7270/Q2QJ7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50360379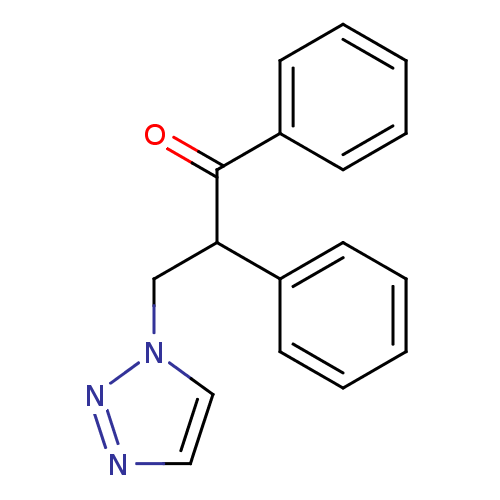 (CHEMBL1933692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidoglycan-N-acetylglucosamine deacetylase (Streptococcus pneumoniae (Firmicutes)) | BDBM50466399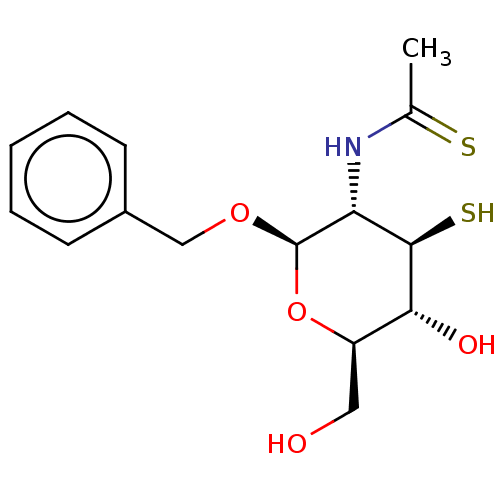 (CHEMBL4293388) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Partial mixed inhibition of Streptococcus pneumoniae apo-Pgda using N,N',N\"-triacetyl chitotriose as substrate in presence of ZnCl2 by fluorescamine... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50360379 (CHEMBL1933692) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of CYP1A1 | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50360380 (CHEMBL1933693) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of CYP1A1 | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidoglycan-N-acetylglucosamine deacetylase (Streptococcus pneumoniae (Firmicutes)) | BDBM50466409 (CHEMBL4286586) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of His6-tagged Streptococcus pneumoniae Pgda C-terminal de-N-acetylase domain (232 to 431 residues) expressed in Escherichia coli BL21 (DE... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidoglycan-N-acetylglucosamine deacetylase (Streptococcus pneumoniae (Firmicutes)) | BDBM50466403 (CHEMBL4292978) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of His6-tagged Streptococcus pneumoniae Pgda C-terminal de-N-acetylase domain (232 to 431 residues) expressed in Escherichia coli BL21 (DE... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50360383 (CHEMBL1933700) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50360378 (CHEMBL1933690) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidoglycan-N-acetylglucosamine deacetylase (Streptococcus pneumoniae (Firmicutes)) | BDBM50466399 (CHEMBL4293388) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae Pgda expressed in Escherichia coli BL21 (DE3) cells transformed with pET28bSpPgdA232-431 plasmid using compoun... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly-beta-1,6-N-acetyl-D-glucosamine N-deacetylase (Escherichia coli (Enterobacteria)) | BDBM50466399 (CHEMBL4293388) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Escherichia coli PgaB expressed in Escherichia coli BL21 (DE3) cells transformed with pET28 plasmid coding for PgaB42-655 using compoun... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidoglycan-N-acetylglucosamine deacetylase (Streptococcus pneumoniae (Firmicutes)) | BDBM50466402 (CHEMBL4291068) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae Pgda expressed in Escherichia coli BL21 (DE3) cells transformed with pET28bSpPgdA232-431 plasmid using compoun... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly-beta-1,6-N-acetyl-D-glucosamine N-deacetylase (Escherichia coli (Enterobacteria)) | BDBM50466399 (CHEMBL4293388) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Escherichia coli PgaB expressed in Escherichia coli BL21 (DE3) cells transformed with pET28 plasmid coding for PgaB42-655 using compoun... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly-beta-1,6-N-acetyl-D-glucosamine N-deacetylase (Escherichia coli (Enterobacteria)) | BDBM50466402 (CHEMBL4291068) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Escherichia coli PgaB expressed in Escherichia coli BL21 (DE3) cells transformed with pET28 plasmid coding for PgaB42-655 using compoun... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidoglycan-N-acetylglucosamine deacetylase (Streptococcus pneumoniae (Firmicutes)) | BDBM50466402 (CHEMBL4291068) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae Pgda expressed in Escherichia coli BL21 (DE3) cells transformed with pET28bSpPgdA232-431 plasmid using compoun... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidoglycan-N-acetylglucosamine deacetylase (Streptococcus pneumoniae (Firmicutes)) | BDBM50466399 (CHEMBL4293388) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae Pgda expressed in Escherichia coli BL21 (DE3) cells transformed with pET28bSpPgdA232-431 plasmid using compoun... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly-beta-1,6-N-acetyl-D-glucosamine N-deacetylase (Escherichia coli (Enterobacteria)) | BDBM50466402 (CHEMBL4291068) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Escherichia coli PgaB expressed in Escherichia coli BL21 (DE3) cells transformed with pET28 plasmid coding for PgaB42-655 using compoun... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly-beta-1,6-N-acetyl-D-glucosamine N-deacetylase (Escherichia coli (Enterobacteria)) | BDBM50466410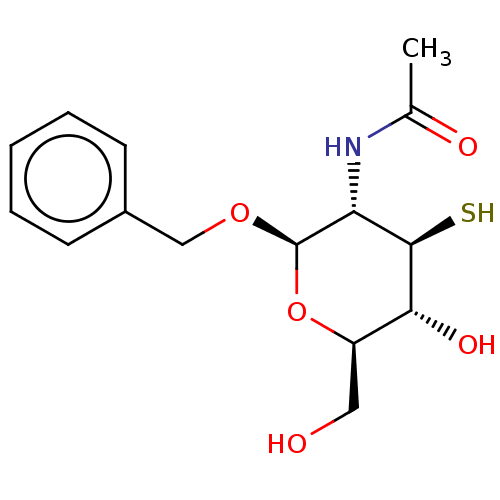 (CHEMBL4294745) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Escherichia coli PgaB expressed in Escherichia coli BL21 (DE3) cells transformed with pET28 plasmid coding for PgaB42-655 using compoun... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidoglycan-N-acetylglucosamine deacetylase (Streptococcus pneumoniae (Firmicutes)) | BDBM50466404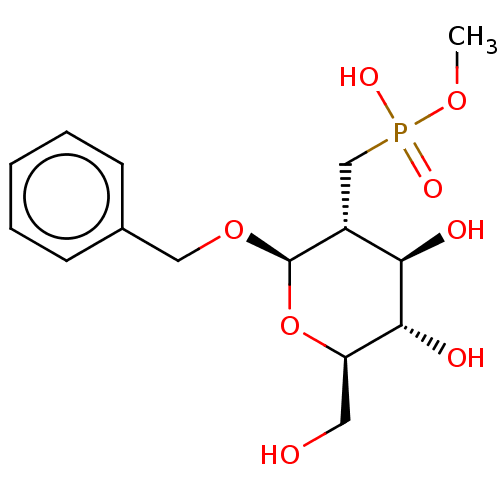 (CHEMBL4282480) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae Pgda expressed in Escherichia coli BL21 (DE3) cells transformed with pET28bSpPgdA232-431 plasmid using acetoxy... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly-beta-1,6-N-acetyl-D-glucosamine N-deacetylase (Escherichia coli (Enterobacteria)) | BDBM50466405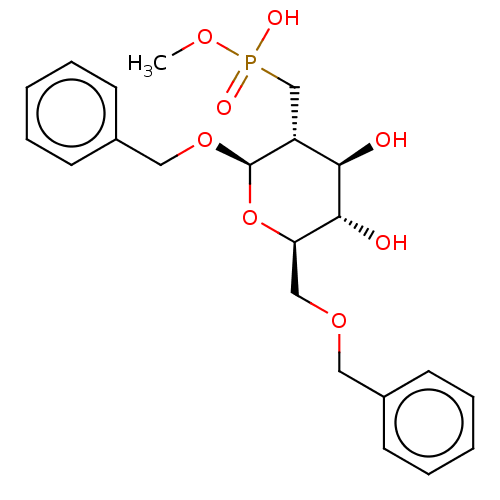 (CHEMBL4289694) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Escherichia coli PgaB expressed in Escherichia coli BL21 (DE3) cells transformed with pET28 plasmid coding for PgaB42-655 using acetoxy... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidoglycan-N-acetylglucosamine deacetylase (Streptococcus pneumoniae (Firmicutes)) | BDBM50466398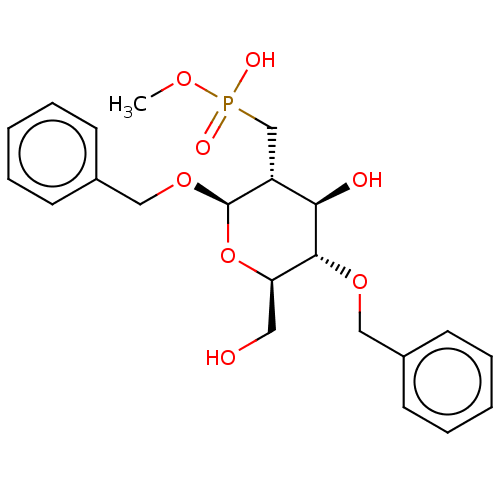 (CHEMBL4279006) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae Pgda expressed in Escherichia coli BL21 (DE3) cells transformed with pET28bSpPgdA232-431 plasmid using acetoxy... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly-beta-1,6-N-acetyl-D-glucosamine N-deacetylase (Escherichia coli (Enterobacteria)) | BDBM50466406 (CHEMBL4283218) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Escherichia coli PgaB expressed in Escherichia coli BL21 (DE3) cells transformed with pET28 plasmid coding for PgaB42-655 using acetoxy... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidoglycan-N-acetylglucosamine deacetylase (Streptococcus pneumoniae (Firmicutes)) | BDBM50466410 (CHEMBL4294745) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae Pgda expressed in Escherichia coli BL21 (DE3) cells transformed with pET28bSpPgdA232-431 plasmid using compoun... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidoglycan-N-acetylglucosamine deacetylase (Streptococcus pneumoniae (Firmicutes)) | BDBM50466405 (CHEMBL4289694) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae Pgda expressed in Escherichia coli BL21 (DE3) cells transformed with pET28bSpPgdA232-431 plasmid using acetoxy... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidoglycan-N-acetylglucosamine deacetylase (Streptococcus pneumoniae (Firmicutes)) | BDBM50466406 (CHEMBL4283218) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae Pgda expressed in Escherichia coli BL21 (DE3) cells transformed with pET28bSpPgdA232-431 plasmid using acetoxy... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly-beta-1,6-N-acetyl-D-glucosamine N-deacetylase (Escherichia coli (Enterobacteria)) | BDBM50466398 (CHEMBL4279006) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Escherichia coli PgaB expressed in Escherichia coli BL21 (DE3) cells transformed with pET28 plasmid coding for PgaB42-655 using acetoxy... | Bioorg Med Chem 26: 5631-5643 (2018) Article DOI: 10.1016/j.bmc.2018.10.008 BindingDB Entry DOI: 10.7270/Q208681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 63 total ) | Next | Last >> |