 Found 44 hits with Last Name = 'dos santos' and Initial = 'jl'
Found 44 hits with Last Name = 'dos santos' and Initial = 'jl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50587724
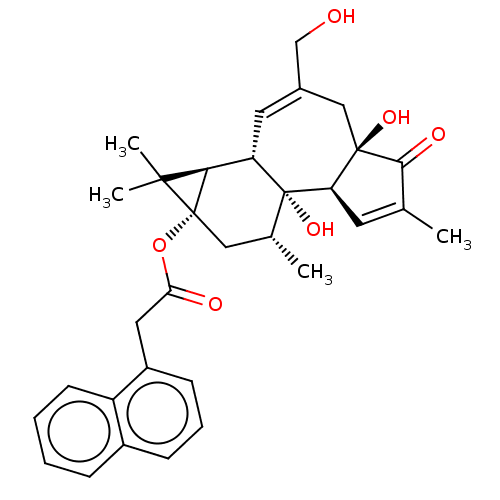
(CHEMBL5175742)Show SMILES [H][C@]12[C@]3([H])C=C(CO)C[C@]4(O)C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)C[C@@]1(OC(=O)Cc1cccc3ccccc13)C2(C)C |r,c:14,t:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113213
BindingDB Entry DOI: 10.7270/Q26Q2260 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50516759
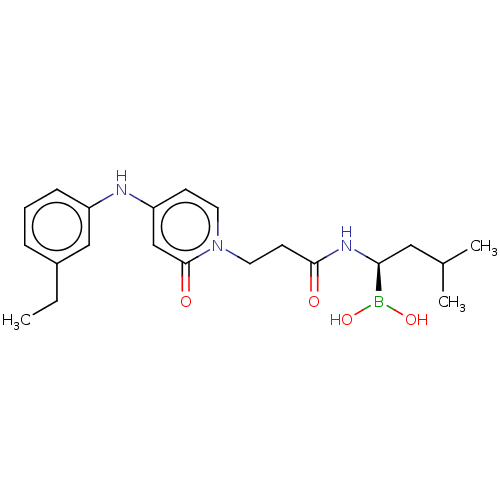
(CHEMBL4458610)Show SMILES CCc1cccc(Nc2ccn(CCC(=O)N[C@@H](CC(C)C)B(O)O)c(=O)c2)c1 |r| Show InChI InChI=1S/C21H30BN3O4/c1-4-16-6-5-7-17(13-16)23-18-8-10-25(21(27)14-18)11-9-20(26)24-19(22(28)29)12-15(2)3/h5-8,10,13-15,19,23,28-29H,4,9,11-12H2,1-3H3,(H,24,26)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S£o Paulo State University
Curated by ChEMBL
| Assay Description
Inhibition of human chymotrypsin-like activity of 20S proteasome (unknown origin) |
Eur J Med Chem 179: 791-804 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.092
BindingDB Entry DOI: 10.7270/Q28055ZM |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50368315

(PROSTRATIN)Show SMILES C[C@@H]1C[C@]2(OC(C)=O)[C@H]([C@@H]3C=C(CO)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]13O)C2(C)C |r,t:10,18| Show InChI InChI=1S/C22H30O6/c1-11-6-16-20(26,18(11)25)9-14(10-23)7-15-17-19(4,5)21(17,28-13(3)24)8-12(2)22(15,16)27/h6-7,12,15-17,23,26-27H,8-10H2,1-5H3/t12-,15+,16-,17?,20-,21+,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113213
BindingDB Entry DOI: 10.7270/Q26Q2260 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50516761
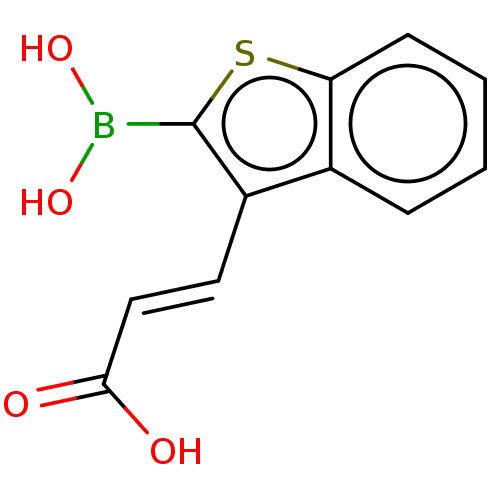
(CHEMBL3286879)Show InChI InChI=1S/C11H9BO4S/c13-10(14)6-5-8-7-3-1-2-4-9(7)17-11(8)12(15)16/h1-6,15-16H,(H,13,14)/b6-5+ | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S£o Paulo State University
Curated by ChEMBL
| Assay Description
Inhibition of bacterial beta lactamase TEM-1 |
Eur J Med Chem 179: 791-804 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.092
BindingDB Entry DOI: 10.7270/Q28055ZM |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Mus musculus) | BDBM50531479
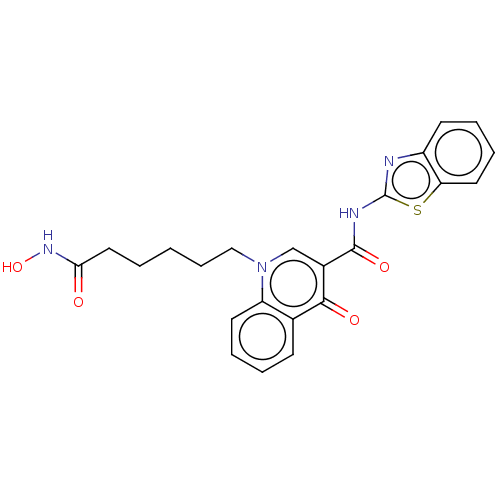
(CHEMBL4439518)Show SMILES ONC(=O)CCCCCn1cc(C(=O)Nc2nc3ccccc3s2)c(=O)c2ccccc12 Show InChI InChI=1S/C23H22N4O4S/c28-20(26-31)12-2-1-7-13-27-14-16(21(29)15-8-3-5-10-18(15)27)22(30)25-23-24-17-9-4-6-11-19(17)32-23/h3-6,8-11,14,31H,1-2,7,12-13H2,(H,26,28)(H,24,25,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
S£o Paulo State University (UNESP)
Curated by ChEMBL
| Assay Description
Inhibition of mouse liver HDAC using Boc-Lys (Ac)-AMC as substrate preincubated for 5 mins followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 164: 8-26 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.033
BindingDB Entry DOI: 10.7270/Q20R9SVB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM255614
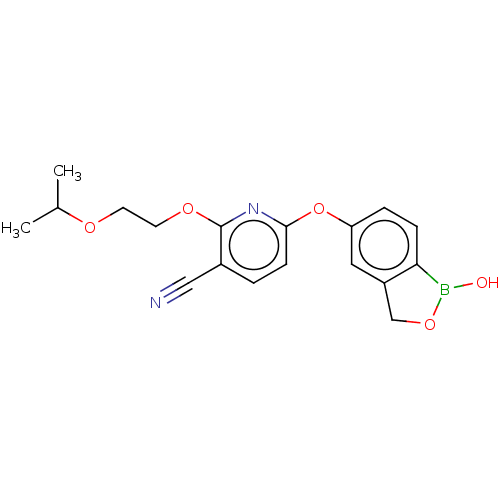
(US9499570, D140)Show InChI InChI=1S/C18H19BN2O5/c1-12(2)23-7-8-24-18-13(10-20)3-6-17(21-18)26-15-4-5-16-14(9-15)11-25-19(16)22/h3-6,9,12,22H,7-8,11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
S£o Paulo State University
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant PDE4B catalytic domain (152 to 484 residues) |
Eur J Med Chem 179: 791-804 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.092
BindingDB Entry DOI: 10.7270/Q28055ZM |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50516760
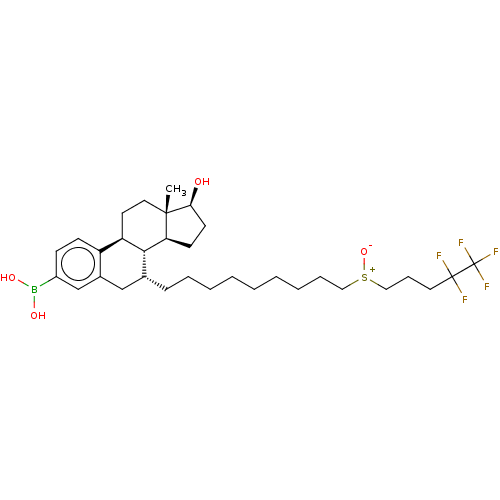
(CHEMBL4526356)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(cc3C[C@@H](CCCCCCCCC[S+]([O-])CCCC(F)(F)C(F)(F)F)[C@@]21[H])B(O)O |r| Show InChI InChI=1S/C32H48BF5O4S/c1-30-17-15-26-25-12-11-24(33(40)41)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-43(42)19-9-16-31(34,35)32(36,37)38/h11-12,21-22,26-29,39-41H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,43?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
S£o Paulo State University
Curated by ChEMBL
| Assay Description
Selective estrogen receptor down regulator activity at ERalpha (unknown origin) |
Eur J Med Chem 179: 791-804 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.092
BindingDB Entry DOI: 10.7270/Q28055ZM |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50516758
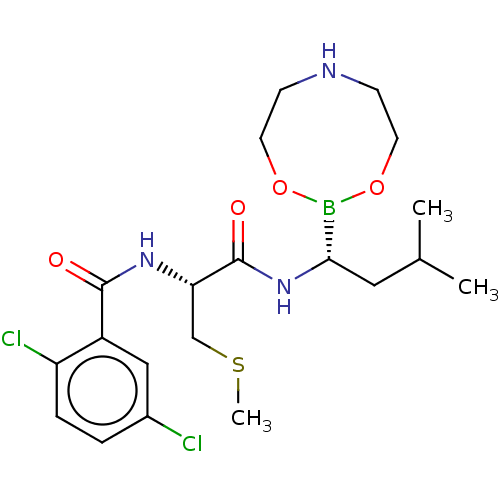
(CHEMBL4444471 | US11542283, Compound V-9A)Show SMILES CSC[C@H](NC(=O)c1cc(Cl)ccc1Cl)C(=O)N[C@@H](CC(C)C)B1OCCNCCO1 |r| Show InChI InChI=1S/C20H30BCl2N3O4S/c1-13(2)10-18(21-29-8-6-24-7-9-30-21)26-20(28)17(12-31-3)25-19(27)15-11-14(22)4-5-16(15)23/h4-5,11,13,17-18,24H,6-10,12H2,1-3H3,(H,25,27)(H,26,28)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
S£o Paulo State University
Curated by ChEMBL
| Assay Description
Inhibition of human chymotrypsin-like activity of 20S proteasome (unknown origin) |
Eur J Med Chem 179: 791-804 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.092
BindingDB Entry DOI: 10.7270/Q28055ZM |
More data for this
Ligand-Target Pair | |
Decaprenylphosphoryl-beta-D-ribose oxidase
(Mycobacterium smegmatis (strain ATCC 700084 / mc(2...) | BDBM50517949
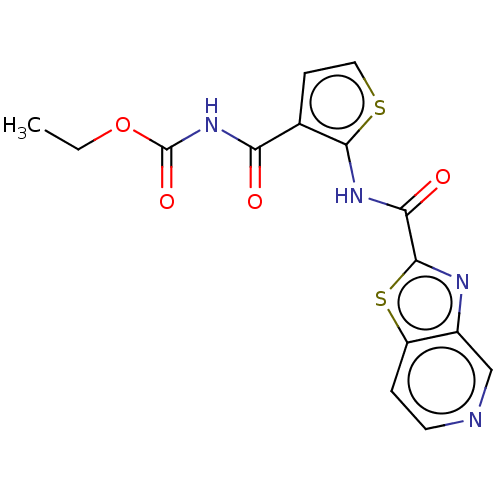
(CHEMBL4447540)Show InChI InChI=1S/C15H12N4O4S2/c1-2-23-15(22)19-11(20)8-4-6-24-13(8)18-12(21)14-17-9-7-16-5-3-10(9)25-14/h3-7H,2H2,1H3,(H,18,21)(H,19,20,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50550527
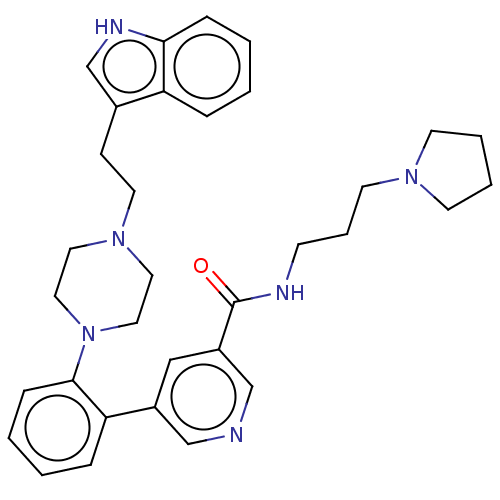
(CHEMBL4754902)Show SMILES O=C(NCCCN1CCCC1)c1cncc(c1)-c1ccccc1N1CCN(CCc2c[nH]c3ccccc23)CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113213
BindingDB Entry DOI: 10.7270/Q26Q2260 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50550519

(CHEMBL4755178)Show SMILES O=C(NCCCN1CCCC1)c1cc(cc(c1)-c1ccccc1N1CCN(CCc2c[nH]c3ccccc23)CC1)C#N | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113213
BindingDB Entry DOI: 10.7270/Q26Q2260 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Mus musculus) | BDBM50531480
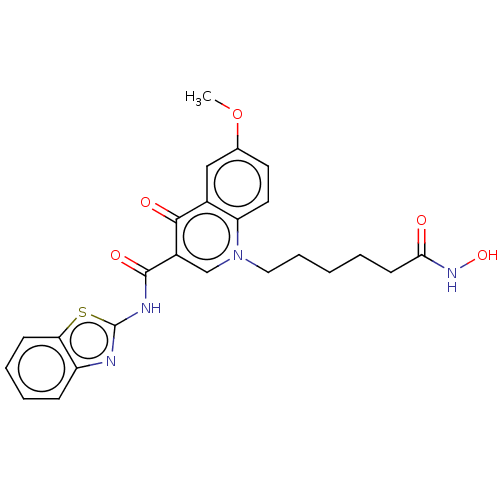
(CHEMBL4447524)Show SMILES COc1ccc2n(CCCCCC(=O)NO)cc(C(=O)Nc3nc4ccccc4s3)c(=O)c2c1 Show InChI InChI=1S/C24H24N4O5S/c1-33-15-10-11-19-16(13-15)22(30)17(14-28(19)12-6-2-3-9-21(29)27-32)23(31)26-24-25-18-7-4-5-8-20(18)34-24/h4-5,7-8,10-11,13-14,32H,2-3,6,9,12H2,1H3,(H,27,29)(H,25,26,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
S£o Paulo State University (UNESP)
Curated by ChEMBL
| Assay Description
Inhibition of mouse liver HDAC using Boc-Lys (Ac)-AMC as substrate preincubated for 5 mins followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 164: 8-26 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.033
BindingDB Entry DOI: 10.7270/Q20R9SVB |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50589215
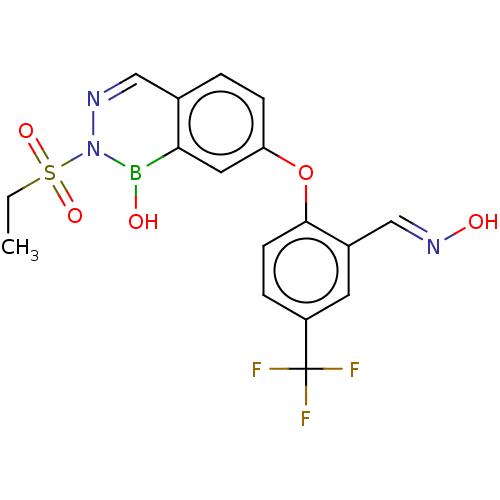
(CHEMBL5190515)Show SMILES CCS(=O)(=O)N1N=Cc2ccc(Oc3ccc(cc3\C=N\O)C(F)(F)F)cc2B1O |c:6| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50517949

(CHEMBL4447540)Show InChI InChI=1S/C15H12N4O4S2/c1-2-23-15(22)19-11(20)8-4-6-24-13(8)18-12(21)14-17-9-7-16-5-3-10(9)25-14/h3-7H,2H2,1H3,(H,18,21)(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyketide synthase Pks13
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50520948
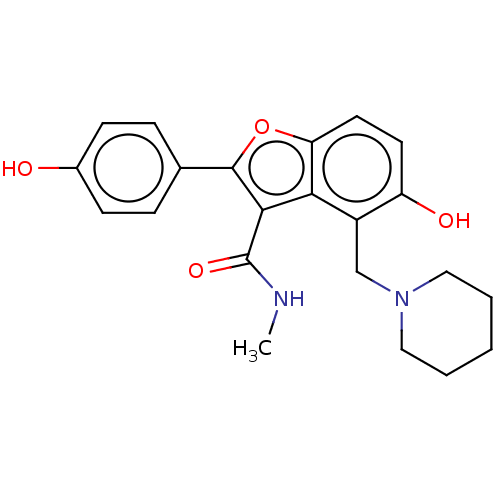
(CHEMBL4443524)Show SMILES CNC(=O)c1c(oc2ccc(O)c(CN3CCCCC3)c12)-c1ccc(O)cc1 Show InChI InChI=1S/C22H24N2O4/c1-23-22(27)20-19-16(13-24-11-3-2-4-12-24)17(26)9-10-18(19)28-21(20)14-5-7-15(25)8-6-14/h5-10,25-26H,2-4,11-13H2,1H3,(H,23,27) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50598090
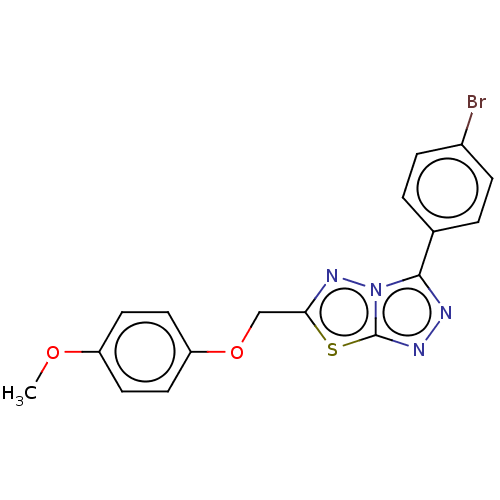
(CHEMBL5207531) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50598090

(CHEMBL5207531) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50598090

(CHEMBL5207531) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50598093
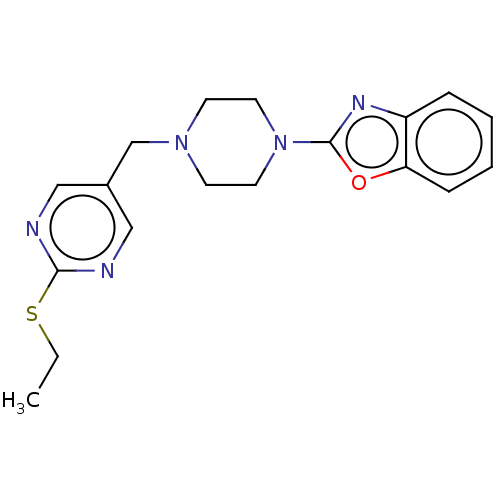
(CHEMBL5199365) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50598086
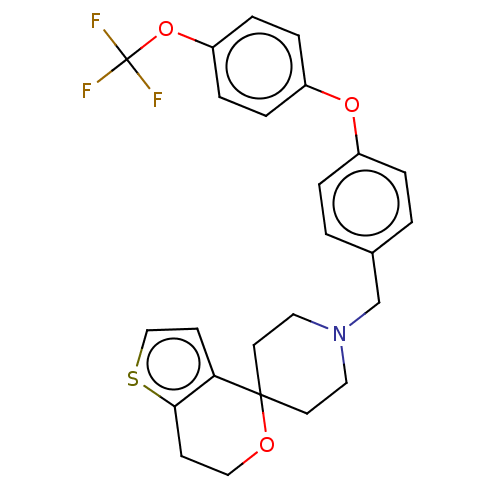
(CHEMBL5181337)Show SMILES FC(F)(F)Oc1ccc(Oc2ccc(CN3CCC4(CC3)OCCc3sccc43)cc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50516762
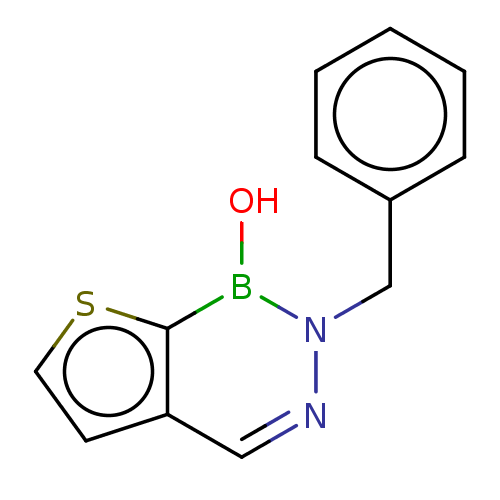
(CHEMBL4474485)Show InChI InChI=1S/C12H11BN2OS/c16-13-12-11(6-7-17-12)8-14-15(13)9-10-4-2-1-3-5-10/h1-8,16H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
S£o Paulo State University
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil Elastase |
Eur J Med Chem 179: 791-804 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.092
BindingDB Entry DOI: 10.7270/Q28055ZM |
More data for this
Ligand-Target Pair | |
O43924/P16499/P18545/P35913/P51160/Q13956
(Homo sapiens (Human)) | BDBM50019654

(CHEMBL3109802)Show InChI InChI=1S/C18H21N5O3/c1-11-6-15-16(20-7-11)13(17(25)19-4-5-24)8-23(15)9-14-12(2)18(26-3)22-10-21-14/h6-8,10,24H,4-5,9H2,1-3H3,(H,19,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50250181
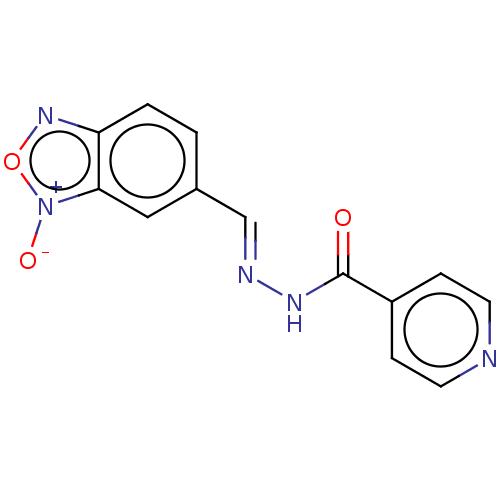
(CHEMBL2338421 | N''-(5-Benzofuroxanylmethylidene)I...)Show InChI InChI=1S/C13H9N5O3/c19-13(10-3-5-14-6-4-10)16-15-8-9-1-2-11-12(7-9)18(20)21-17-11/h1-8H,(H,16,19)/b15-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
São Paulo State University (UNESP) , Institute of Chemistry, Araraquara 14800060, Brazil.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes in presence of NADPH by LC-MS/MS analysis |
J Med Chem 60: 8647-8660 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01332
BindingDB Entry DOI: 10.7270/Q20G3NK2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50250181

(CHEMBL2338421 | N''-(5-Benzofuroxanylmethylidene)I...)Show InChI InChI=1S/C13H9N5O3/c19-13(10-3-5-14-6-4-10)16-15-8-9-1-2-11-12(7-9)18(20)21-17-11/h1-8H,(H,16,19)/b15-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
São Paulo State University (UNESP) , Institute of Chemistry, Araraquara 14800060, Brazil.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes in presence of NADPH by LC-MS/MS analysis |
J Med Chem 60: 8647-8660 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01332
BindingDB Entry DOI: 10.7270/Q20G3NK2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50250181

(CHEMBL2338421 | N''-(5-Benzofuroxanylmethylidene)I...)Show InChI InChI=1S/C13H9N5O3/c19-13(10-3-5-14-6-4-10)16-15-8-9-1-2-11-12(7-9)18(20)21-17-11/h1-8H,(H,16,19)/b15-8+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
São Paulo State University (UNESP) , Institute of Chemistry, Araraquara 14800060, Brazil.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes in presence of NADPH by LC-MS/MS analysis |
J Med Chem 60: 8647-8660 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01332
BindingDB Entry DOI: 10.7270/Q20G3NK2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50250181

(CHEMBL2338421 | N''-(5-Benzofuroxanylmethylidene)I...)Show InChI InChI=1S/C13H9N5O3/c19-13(10-3-5-14-6-4-10)16-15-8-9-1-2-11-12(7-9)18(20)21-17-11/h1-8H,(H,16,19)/b15-8+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
São Paulo State University (UNESP) , Institute of Chemistry, Araraquara 14800060, Brazil.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2B6 in human liver microsomes in presence of NADPH by LC-MS/MS analysis |
J Med Chem 60: 8647-8660 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01332
BindingDB Entry DOI: 10.7270/Q20G3NK2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50520948

(CHEMBL4443524)Show SMILES CNC(=O)c1c(oc2ccc(O)c(CN3CCCCC3)c12)-c1ccc(O)cc1 Show InChI InChI=1S/C22H24N2O4/c1-23-22(27)20-19-16(13-24-11-3-2-4-12-24)17(26)9-10-18(19)28-21(20)14-5-7-15(25)8-6-14/h5-10,25-26H,2-4,11-13H2,1H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50598092
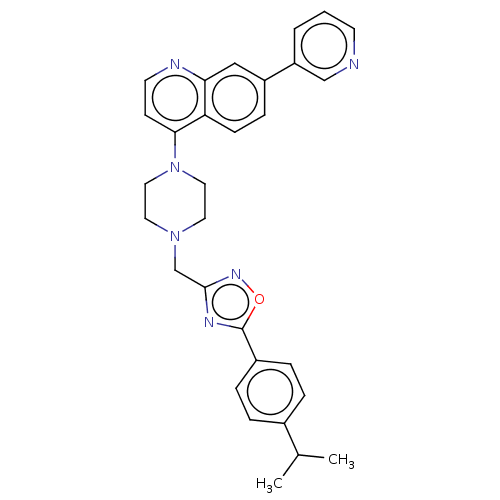
(CHEMBL4473719)Show SMILES CC(C)c1ccc(cc1)-c1nc(CN2CCN(CC2)c2ccnc3cc(ccc23)-c2cccnc2)no1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50598091
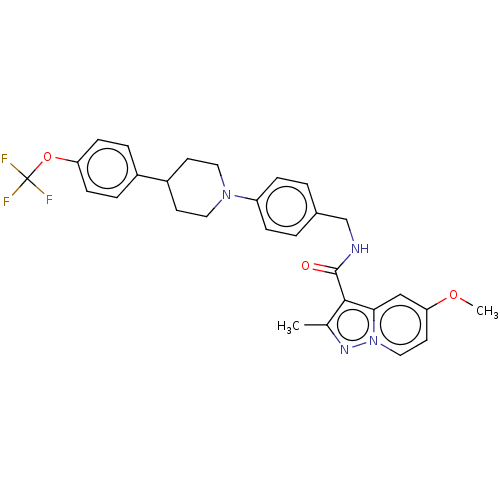
(CHEMBL3612958)Show SMILES COc1ccn2nc(C)c(C(=O)NCc3ccc(cc3)N3CCC(CC3)c3ccc(OC(F)(F)F)cc3)c2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50583310
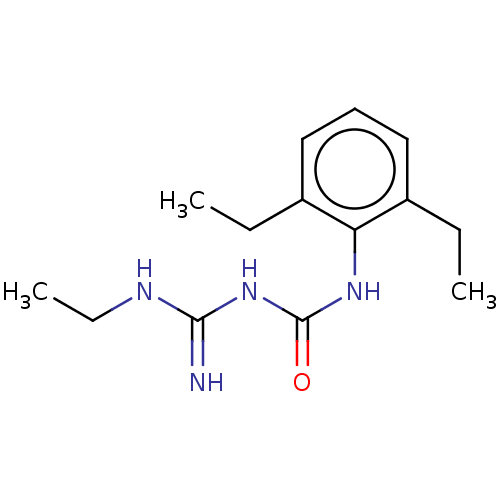
(CHEMBL5082711) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50583310

(CHEMBL5082711) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50517949

(CHEMBL4447540)Show InChI InChI=1S/C15H12N4O4S2/c1-2-23-15(22)19-11(20)8-4-6-24-13(8)18-12(21)14-17-9-7-16-5-3-10(9)25-14/h3-7H,2H2,1H3,(H,18,21)(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50598088
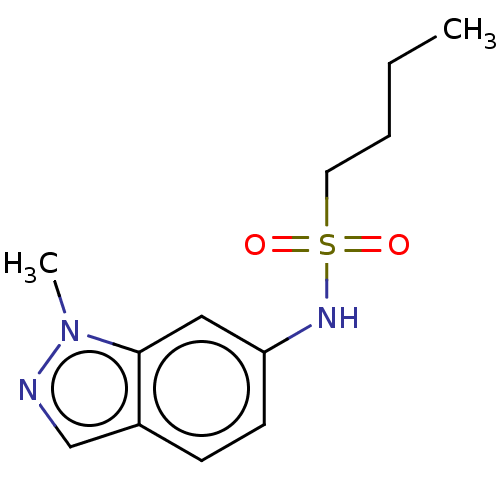
(CHEMBL2098221 | GSK3011724A | TCMDC-142399) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50598089
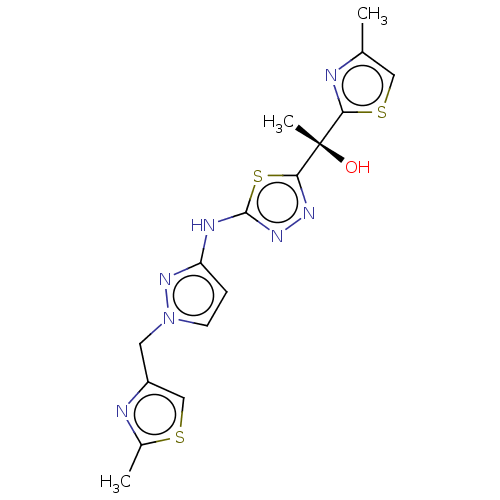
(CHEMBL5186224)Show SMILES Cc1csc(n1)[C@](C)(O)c1nnc(Nc2ccn(Cc3csc(C)n3)n2)s1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50598087
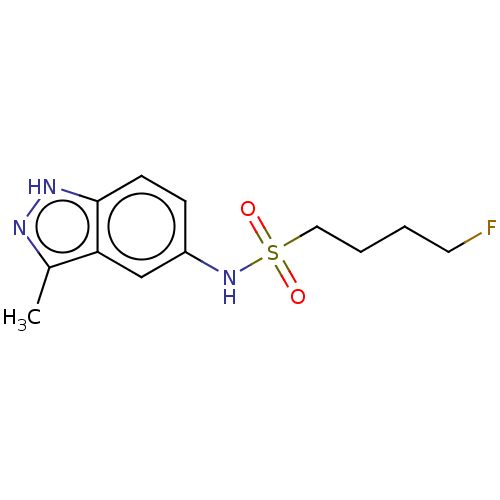
(CHEMBL5181555) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM25150

((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 82 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113213
BindingDB Entry DOI: 10.7270/Q26Q2260 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM25150

((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 67 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113213
BindingDB Entry DOI: 10.7270/Q26Q2260 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM25150

((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 216 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113213
BindingDB Entry DOI: 10.7270/Q26Q2260 |
More data for this
Ligand-Target Pair | |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50396022
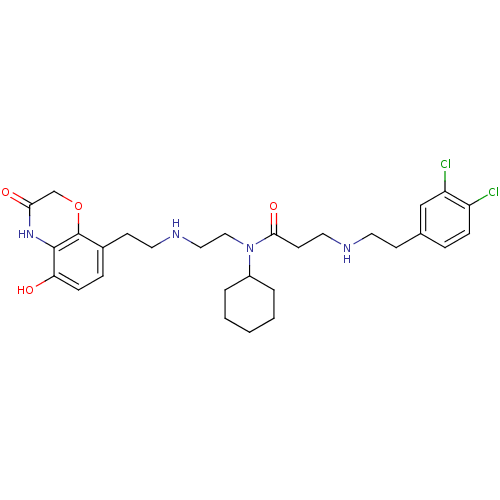
(CHEMBL2169920)Show SMILES Oc1ccc(CCNCCN(C2CCCCC2)C(=O)CCNCCc2ccc(Cl)c(Cl)c2)c2OCC(=O)Nc12 Show InChI InChI=1S/C29H38Cl2N4O4/c30-23-8-6-20(18-24(23)31)10-13-32-15-12-27(38)35(22-4-2-1-3-5-22)17-16-33-14-11-21-7-9-25(36)28-29(21)39-19-26(37)34-28/h6-9,18,22,32-33,36H,1-5,10-17,19H2,(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113213
BindingDB Entry DOI: 10.7270/Q26Q2260 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM25150

((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113213
BindingDB Entry DOI: 10.7270/Q26Q2260 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM25150

((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 115 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113213
BindingDB Entry DOI: 10.7270/Q26Q2260 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM25150

((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113213
BindingDB Entry DOI: 10.7270/Q26Q2260 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM25150

((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 125 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113213
BindingDB Entry DOI: 10.7270/Q26Q2260 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM25150

((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 128 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113213
BindingDB Entry DOI: 10.7270/Q26Q2260 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data