

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239078 (US9416103, TV-5-157) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 min... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239078 (US9416103, TV-5-157) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in mouse Neuro2a cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 mins by liq... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50374634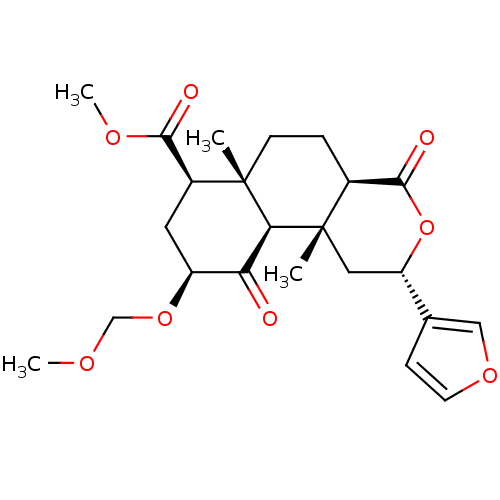 (CHEMBL258098) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50374645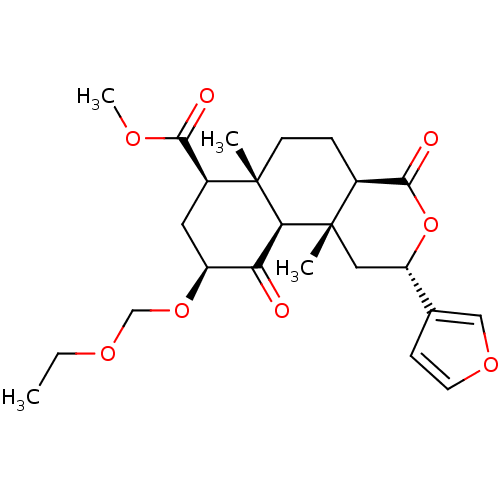 (CHEMBL272939) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50496725 (CHEMBL3219933) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50496728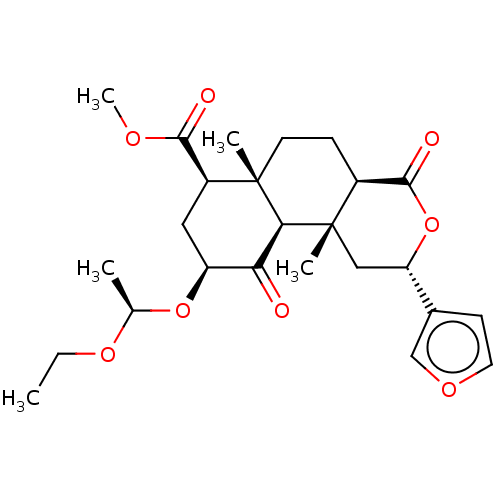 (CHEMBL3219937) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50159165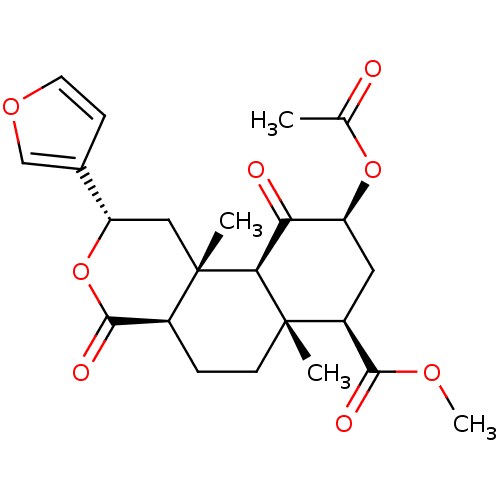 ((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(fura...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor | J Nat Prod 74: 718-26 (2011) Article DOI: 10.1021/np1007872 BindingDB Entry DOI: 10.7270/Q2PR7W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM85804 (1-Naphthyl(1-butyl-1H-indole-3-yl)methanone | JWH-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 min... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239077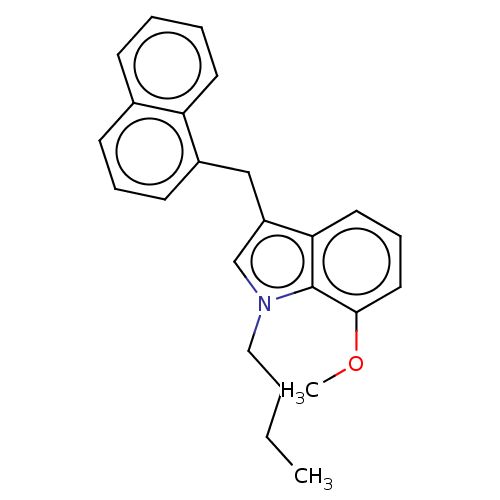 (US9416103, TV-5-249) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 min... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM85804 (1-Naphthyl(1-butyl-1H-indole-3-yl)methanone | JWH-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in mouse Neuro2a cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 mins by liq... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239077 (US9416103, TV-5-249) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in mouse Neuro2a cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 mins by liq... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239075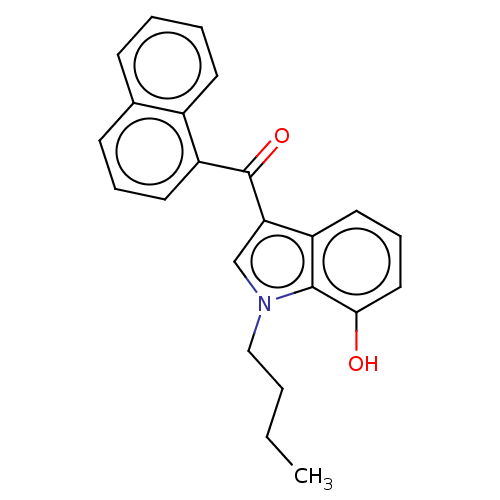 (US9416103, JWH-073-M4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in mouse Neuro2a cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 mins by liq... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50491525 (CHEMBL2380408) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 min... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50491525 (CHEMBL2380408) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in mouse Neuro2a cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 mins by liq... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50159172 ((3S,4aR,4bS,6S,8R,8aR,10aR)-6-Formyloxy-3-furan-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50374645 (CHEMBL272939) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membrane after 2 hrs by liquid scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50496723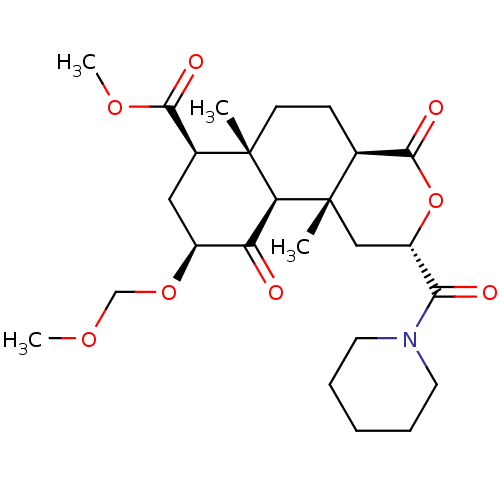 (CHEMBL3219943) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50343253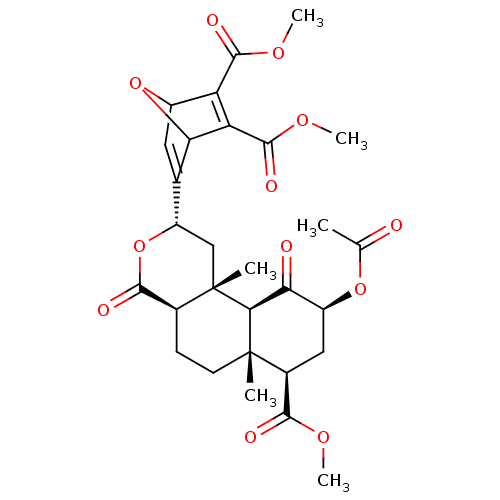 (CHEMBL1773748 | Dimethyl 5-((2S,4aR,6aR,7R,9S,10aS...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor | J Nat Prod 74: 718-26 (2011) Article DOI: 10.1021/np1007872 BindingDB Entry DOI: 10.7270/Q2PR7W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50496722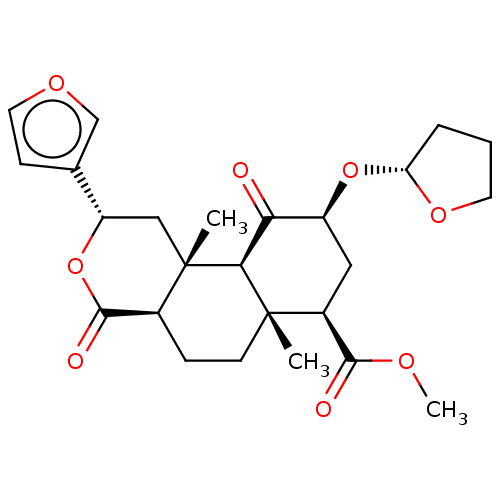 (CHEMBL3219935) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239075 (US9416103, JWH-073-M4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 min... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50496720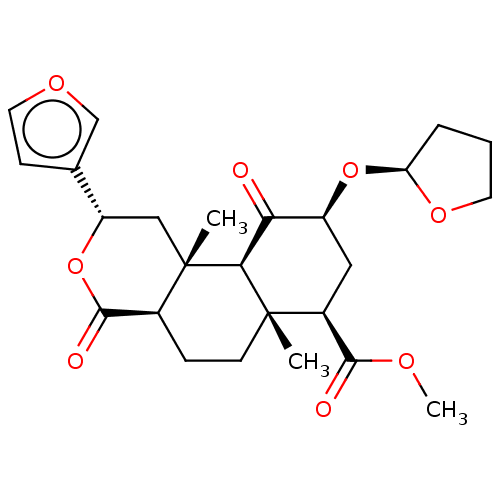 (CHEMBL3219936) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50343252 (CHEMBL1773747 | Diethyl 5-((2S,4aR,6aR,7R,9S,10aS,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor | J Nat Prod 74: 718-26 (2011) Article DOI: 10.1021/np1007872 BindingDB Entry DOI: 10.7270/Q2PR7W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50496731 (CHEMBL3219944) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50496721 (CHEMBL3219941) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50343257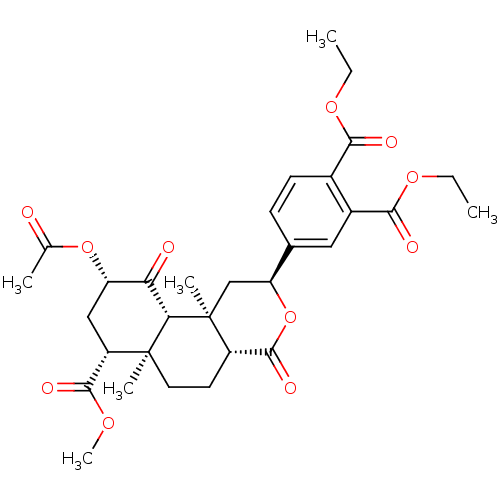 (CHEMBL1773752 | Diethyl 4-((2S,4aR,6aR,7R,9S,10aS,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 228 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor | J Nat Prod 74: 718-26 (2011) Article DOI: 10.1021/np1007872 BindingDB Entry DOI: 10.7270/Q2PR7W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239076 (US9416103, TV-5-129) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 281 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 min... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50343250 (CHEMBL1773753 | Dimethyl 4-((2S,4aR,6aR,7R,9S,10aS...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 286 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor | J Nat Prod 74: 718-26 (2011) Article DOI: 10.1021/np1007872 BindingDB Entry DOI: 10.7270/Q2PR7W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50343256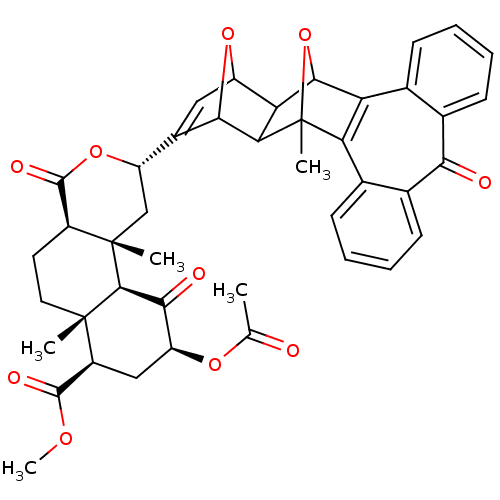 ((2S,4aR,6aR,7R,9S,10aS,10bR)-Methyl 9-acetoxy-2-(8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor | J Nat Prod 74: 718-26 (2011) Article DOI: 10.1021/np1007872 BindingDB Entry DOI: 10.7270/Q2PR7W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50496729 (CHEMBL3219934) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239076 (US9416103, TV-5-129) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 387 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in mouse Neuro2a cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 mins by liq... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50374634 (CHEMBL258098) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membrane after 2 hrs by liquid scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50496730 (CHEMBL3219942) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 462 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membrane | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50496728 (CHEMBL3219937) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 777 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membrane after 2 hrs by liquid scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50343254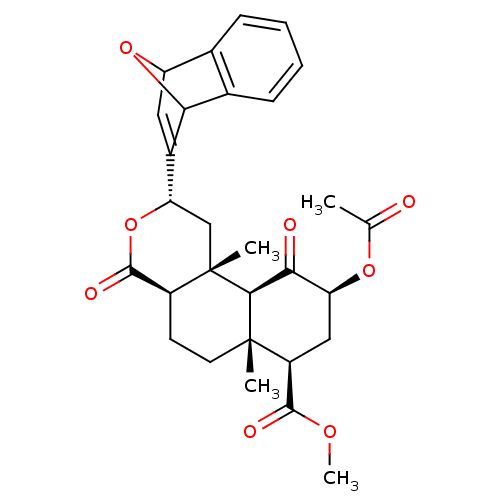 ((2S,4aR,6aR,7R,9S,10aS,10bR)-Methyl 9-acetoxy-2-(7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor | J Nat Prod 74: 718-26 (2011) Article DOI: 10.1021/np1007872 BindingDB Entry DOI: 10.7270/Q2PR7W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50374645 (CHEMBL272939) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cell membrane after 2 hrs by liquid scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50496724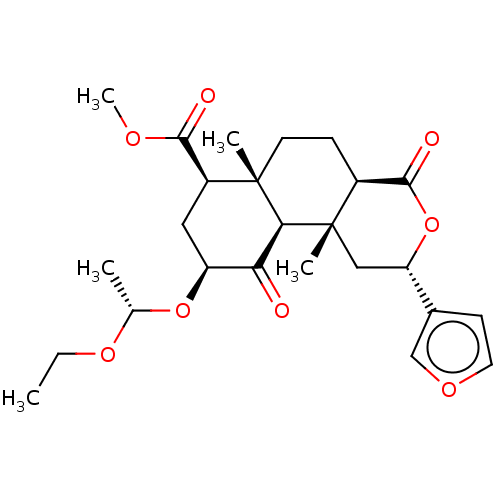 (CHEMBL3219938) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50496730 (CHEMBL3219942) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membrane after 2 hrs by liquid scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50343256 ((2S,4aR,6aR,7R,9S,10aS,10bR)-Methyl 9-acetoxy-2-(8...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor assessed as stimulation of [35S]GTPgammaS binding | J Nat Prod 74: 718-26 (2011) Article DOI: 10.1021/np1007872 BindingDB Entry DOI: 10.7270/Q2PR7W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50343252 (CHEMBL1773747 | Diethyl 5-((2S,4aR,6aR,7R,9S,10aS,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from delta opioid receptor | J Nat Prod 74: 718-26 (2011) Article DOI: 10.1021/np1007872 BindingDB Entry DOI: 10.7270/Q2PR7W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50343254 ((2S,4aR,6aR,7R,9S,10aS,10bR)-Methyl 9-acetoxy-2-(7...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor assessed as stimulation of [35S]GTPgammaS binding | J Nat Prod 74: 718-26 (2011) Article DOI: 10.1021/np1007872 BindingDB Entry DOI: 10.7270/Q2PR7W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50343255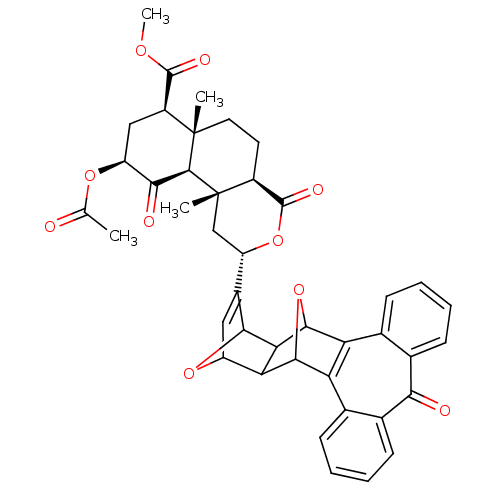 ((2S,4aR,6aR,7R,9S,10aS,10bR)-Methyl 9-acetoxy-2-(8...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor assessed as stimulation of [35S]GTPgammaS binding | J Nat Prod 74: 718-26 (2011) Article DOI: 10.1021/np1007872 BindingDB Entry DOI: 10.7270/Q2PR7W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50374634 (CHEMBL258098) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cell membrane after 2 hrs by liquid scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50496726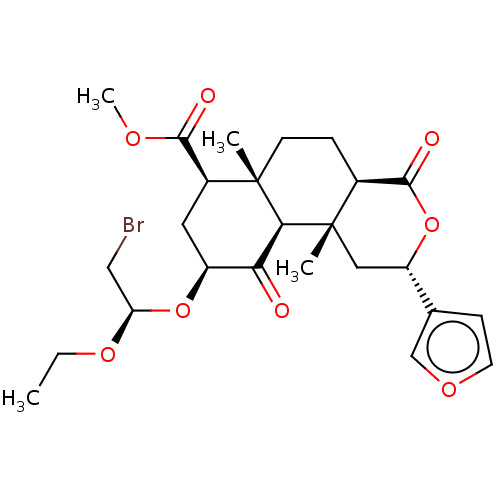 (CHEMBL3219939) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membrane after 2 hrs by liquid scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50343251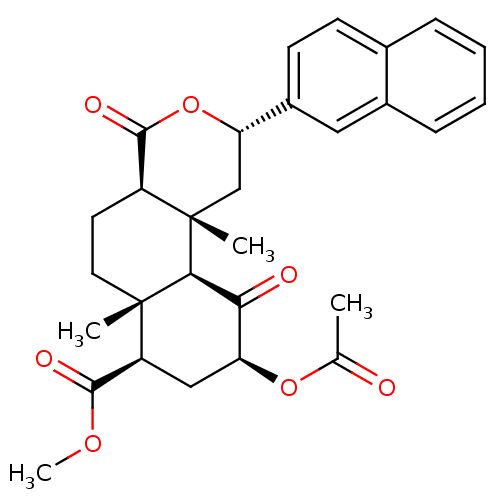 ((2S,4aR,6aR,7R,9S,10aS,10bR)-Methyl 9-acetoxy-6a,1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor assessed as stimulation of [35S]GTPgammaS binding | J Nat Prod 74: 718-26 (2011) Article DOI: 10.1021/np1007872 BindingDB Entry DOI: 10.7270/Q2PR7W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50343250 (CHEMBL1773753 | Dimethyl 4-((2S,4aR,6aR,7R,9S,10aS...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor assessed as stimulation of [35S]GTPgammaS binding | J Nat Prod 74: 718-26 (2011) Article DOI: 10.1021/np1007872 BindingDB Entry DOI: 10.7270/Q2PR7W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50496723 (CHEMBL3219943) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cell membrane after 2 hrs by liquid scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50343257 (CHEMBL1773752 | Diethyl 4-((2S,4aR,6aR,7R,9S,10aS,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor assessed as stimulation of [35S]GTPgammaS binding | J Nat Prod 74: 718-26 (2011) Article DOI: 10.1021/np1007872 BindingDB Entry DOI: 10.7270/Q2PR7W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50496723 (CHEMBL3219943) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membrane after 2 hrs by liquid scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50343252 (CHEMBL1773747 | Diethyl 5-((2S,4aR,6aR,7R,9S,10aS,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor assessed as stimulation of [35S]GTPgammaS binding | J Nat Prod 74: 718-26 (2011) Article DOI: 10.1021/np1007872 BindingDB Entry DOI: 10.7270/Q2PR7W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50496727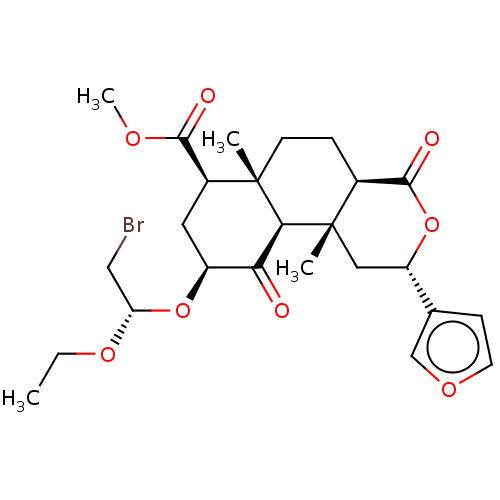 (CHEMBL3219940) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membrane after 2 hrs by liquid scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 86 total ) | Next | Last >> |