

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50066770 ((3R,6S,8aS)-5-Oxo-6-phenylmethanesulfonylamino-hex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human Thrombin. | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066766 ((3R,6R,8aS)-5-Oxo-6-phenylmethanesulfonylamino-hex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human Thrombin. | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066769 ((3R,6R,8aS)-6-(Naphthalene-1-sulfonylamino)-5-oxo-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 751 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human Thrombin. | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066768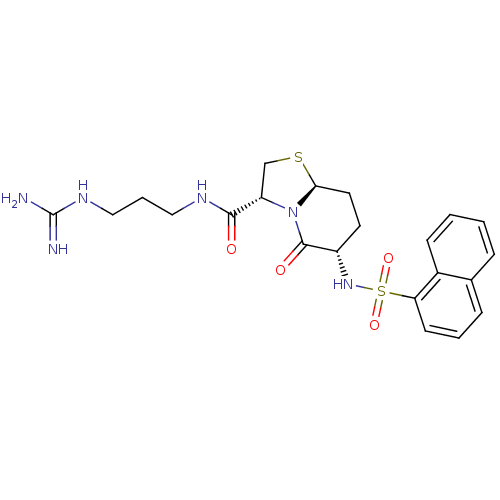 ((3R,6S,8aS)-6-(Naphthalene-1-sulfonylamino)-5-oxo-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human Thrombin. | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175982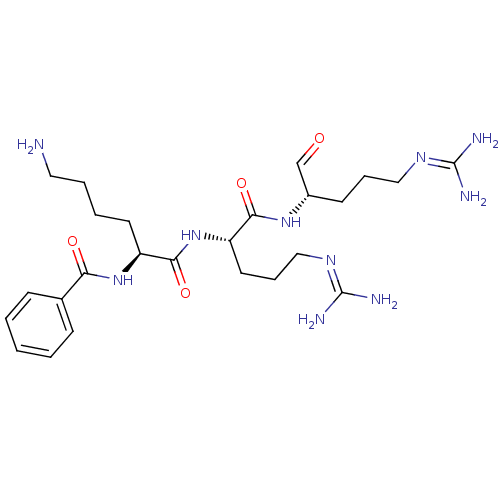 (Bz-Lys-Arg-Arg-H | CHEMBL199510) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175984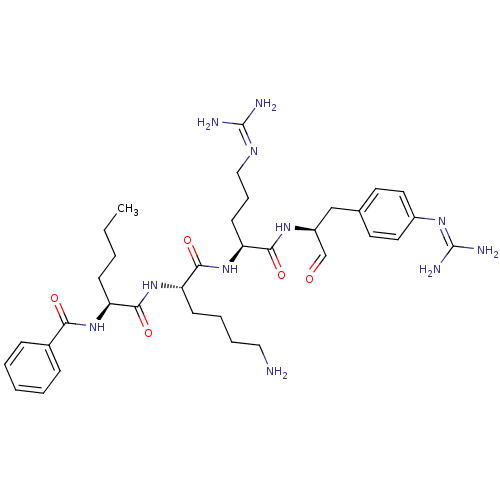 (BZ-Nle-Lys-Arg-(4-guanidinyl)-Phe-H | Bz-Nle-Lys-A...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50066769 ((3R,6R,8aS)-6-(Naphthalene-1-sulfonylamino)-5-oxo-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human bovine pancreatic trypsin. | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50066773 ((3R,6S,8aS)-6-Amino-5-oxo-hexahydro-thiazolo[3,2-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human bovine pancreatic trypsin. | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50066766 ((3R,6R,8aS)-5-Oxo-6-phenylmethanesulfonylamino-hex...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human bovine pancreatic trypsin. | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066771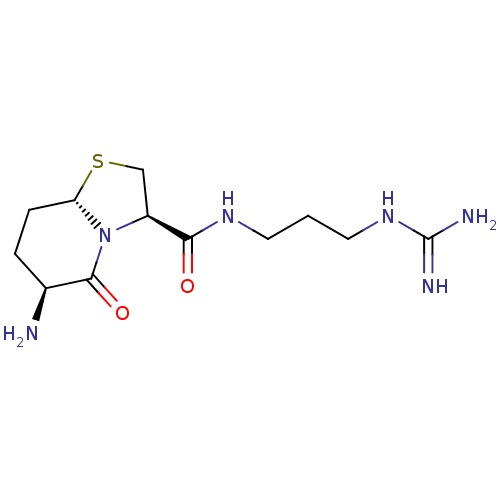 ((3R,6S,8aS)-6-Amino-5-oxo-hexahydro-thiazolo[3,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human Thrombin. | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175976 (Bz-Ala-Lys-Arg-Arg-H | CHEMBL197765) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50066767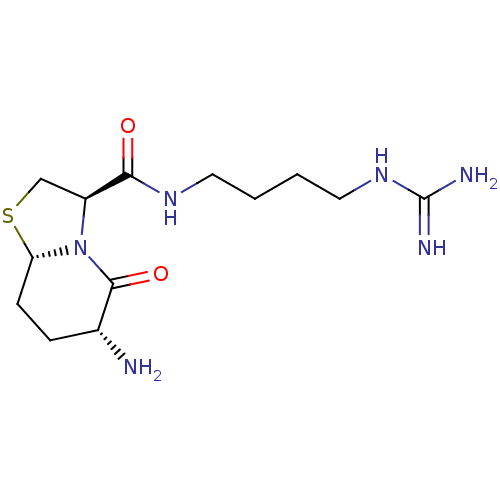 ((3R,6R,8aS)-6-Amino-5-oxo-hexahydro-thiazolo[3,2-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human bovine pancreatic trypsin. | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM33259 (Bz-NKRR-H | Bz-Nle-Lys-Arg-Arg-H | CHEMBL256877) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175988 (Bz-Nle-Lys-Arg-(p-Me)Phe-H | CHEMBL199396) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066769 ((3R,6R,8aS)-6-(Naphthalene-1-sulfonylamino)-5-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human Coagulation factor X | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175987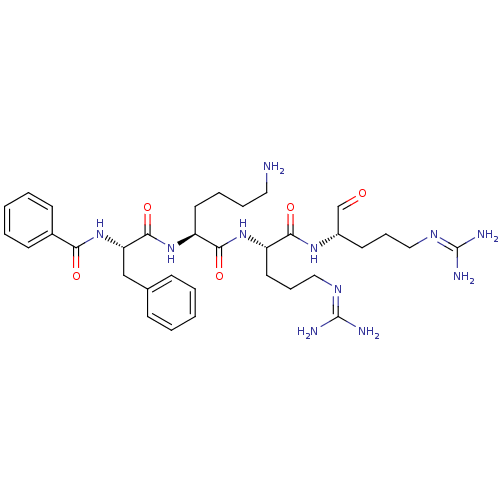 (Bz-Phe-Lys-Arg-Arg-H | CHEMBL199736) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175974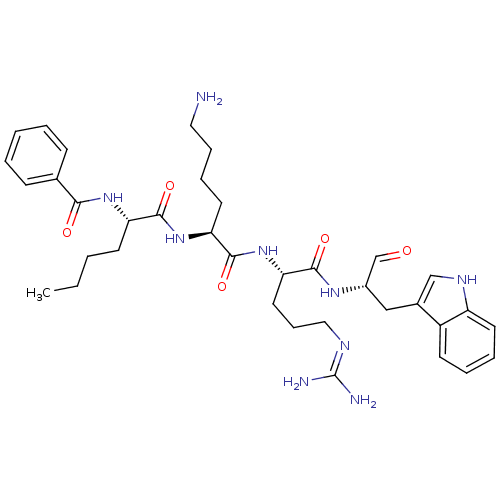 (Bz-Nle-Lys-Arg-Trp-H | CHEMBL433993) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50066770 ((3R,6S,8aS)-5-Oxo-6-phenylmethanesulfonylamino-hex...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human bovine pancreatic trypsin. | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175993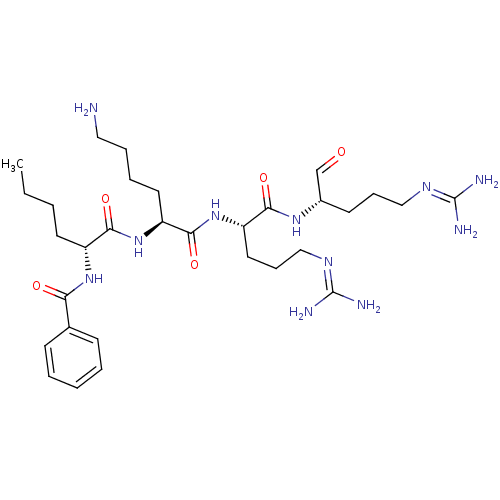 (Bz-D-Nle-Lys-Arg-Arg-H | CHEMBL201775) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Homo sapiens (Human)) | BDBM50033369 (AMANTADINE | CHEMBL660 | SYMADINE | SYMMETREL) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-MK-801 from phencyclidine binding site of NMDA receptor in human frontal cortex after 22 hrs by scintillation counting analy... | Bioorg Med Chem 23: 4277-85 (2015) Article DOI: 10.1016/j.bmc.2015.06.030 BindingDB Entry DOI: 10.7270/Q29Z96NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066772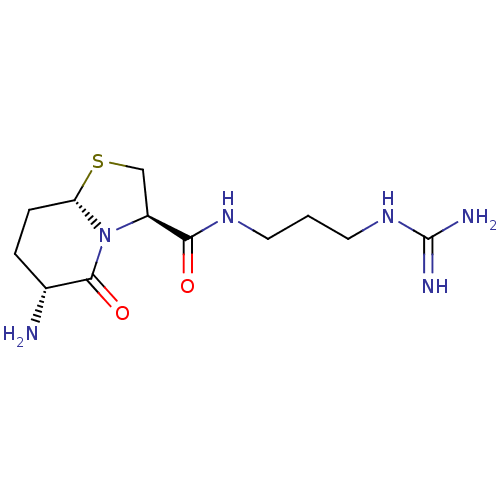 ((3R,6R,8aS)-6-Amino-5-oxo-hexahydro-thiazolo[3,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human Thrombin. | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175978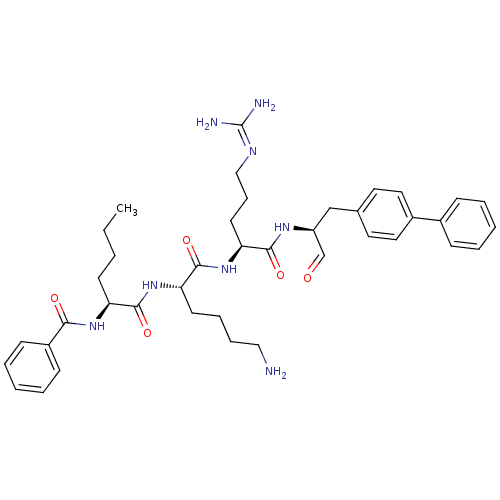 (Bz-Nle-Lys-Arg-(p-Ph)Phe-H | CHEMBL381854) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175981 (Bz-Arg-Arg-H | CHEMBL197766) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50066771 ((3R,6S,8aS)-6-Amino-5-oxo-hexahydro-thiazolo[3,2-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human bovine pancreatic trypsin. | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175985 (Bz-Nle-Phe-Arg-Arg-H | CHEMBL199582) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175986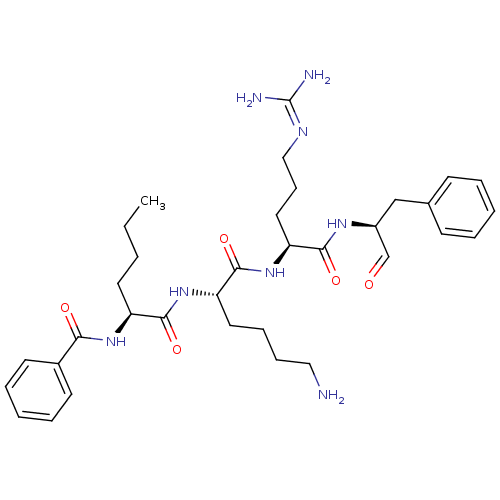 (Bz-Nle-Lys-Arg-Phe-H | CHEMBL199727) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066768 ((3R,6S,8aS)-6-(Naphthalene-1-sulfonylamino)-5-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human Coagulation factor X | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175968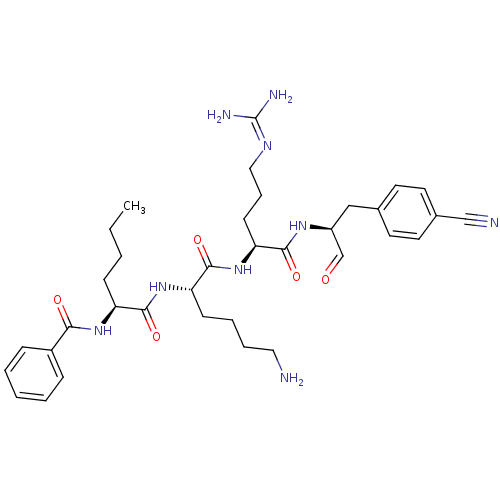 (Bz-Nle-Lys-Arg-(p-CN)Phe-H | CHEMBL200095) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066773 ((3R,6S,8aS)-6-Amino-5-oxo-hexahydro-thiazolo[3,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human Thrombin. | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50066768 ((3R,6S,8aS)-6-(Naphthalene-1-sulfonylamino)-5-oxo-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Bovine pancreatic trypsin. | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175971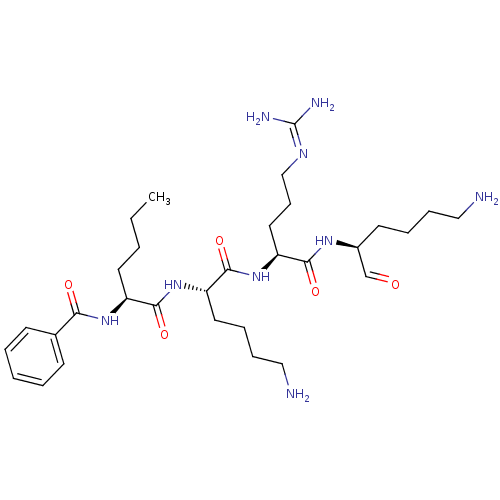 (Bz-Nle-Lys-Arg-Lys-H | CHEMBL377076) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175970 (Bz-Nle-Ala-Arg-Arg-H | CHEMBL369916) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50066772 ((3R,6R,8aS)-6-Amino-5-oxo-hexahydro-thiazolo[3,2-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human bovine pancreatic trypsin. | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066766 ((3R,6R,8aS)-5-Oxo-6-phenylmethanesulfonylamino-hex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human Coagulation factor X | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175975 (Bz-Nle-D-Lys-Arg-Arg-H | CHEMBL370138) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175991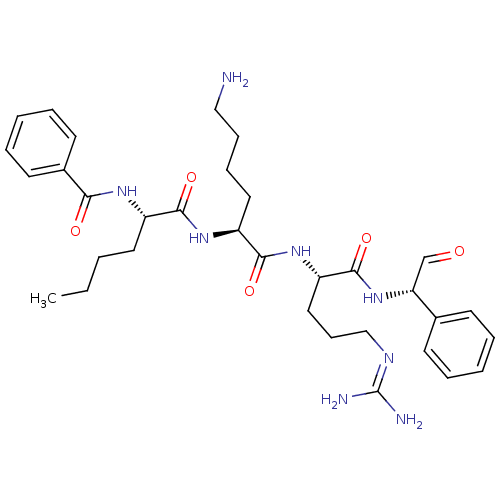 (Bz-Nle-Lys-Arg-Phg-H | CHEMBL200972) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066770 ((3R,6S,8aS)-5-Oxo-6-phenylmethanesulfonylamino-hex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human Coagulation factor X | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066771 ((3R,6S,8aS)-6-Amino-5-oxo-hexahydro-thiazolo[3,2-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human Coagulation factor X | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175990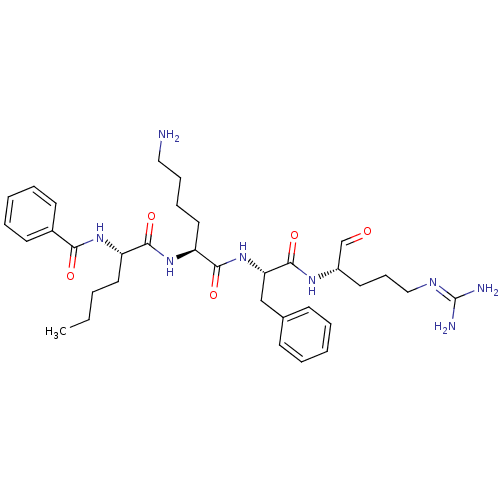 (Bz-Nle-Lys-Phe-Arg-H | CHEMBL435122) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175977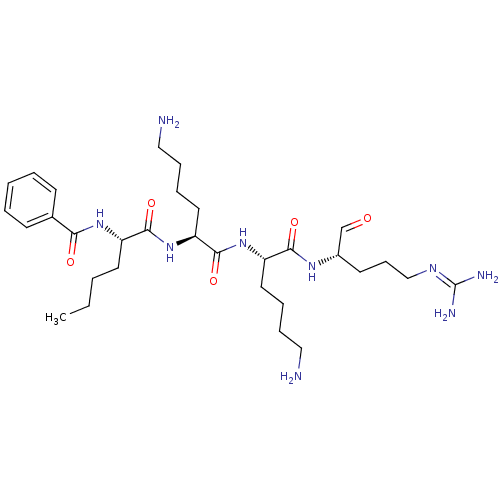 (Bz-Nle-Lys-Lys-Arg-H | CHEMBL201609) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175969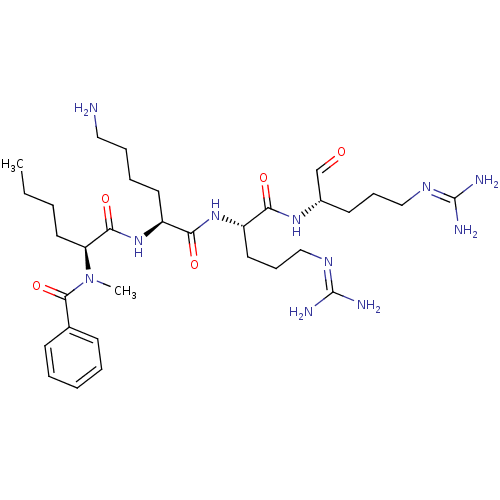 (Bz-N-Me-Nle-Lys-Arg-Arg-H | CHEMBL382143) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175967 (Bz-Nle-Lys-N-Me-Arg-Arg-H | CHEMBL199726) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175980 (Bz-Nle-Lys-Arg-D-Arg-H | CHEMBL199352) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175979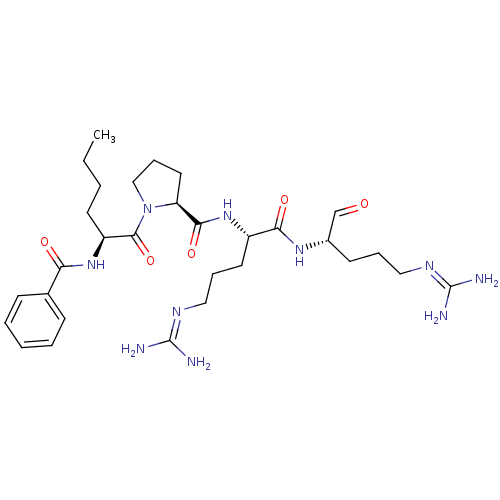 (Bz-Nle-Pro-Arg-Arg-H | CHEMBL382983) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066767 ((3R,6R,8aS)-6-Amino-5-oxo-hexahydro-thiazolo[3,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Invitro inhibition was measured against Human Thrombin. | J Med Chem 41: 3664-74 (1998) Article DOI: 10.1021/jm981013e BindingDB Entry DOI: 10.7270/Q22R3QSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175965 (Bz-Nle-Lys-Pro-Arg-H | CHEMBL199987) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175973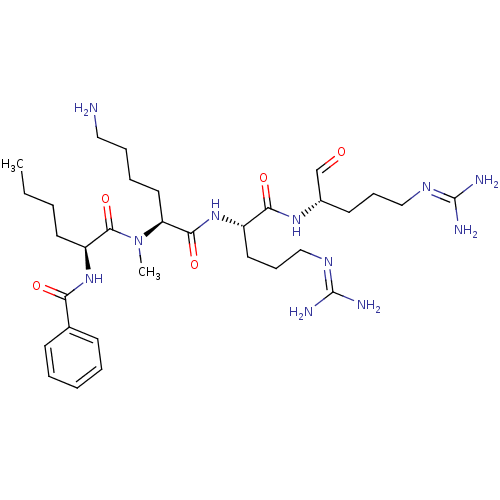 (Bz-Nle-N-Me-Lys-Arg-Arg-H | CHEMBL381639) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175989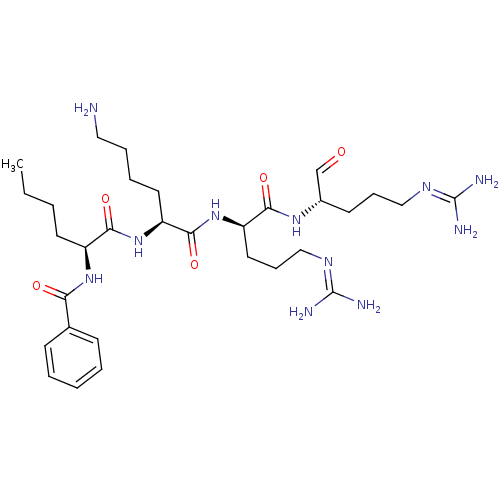 (Bz-Nle-Lys-D-Arg-Arg-H | CHEMBL371447) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175972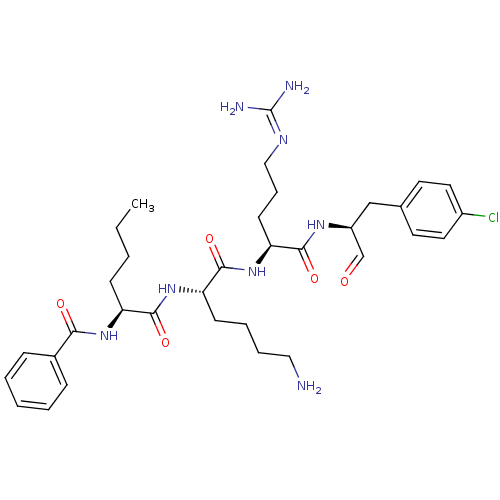 (Bz-Nle-Lys-Arg-(p-Cl)Phe-H | CHEMBL199531) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175966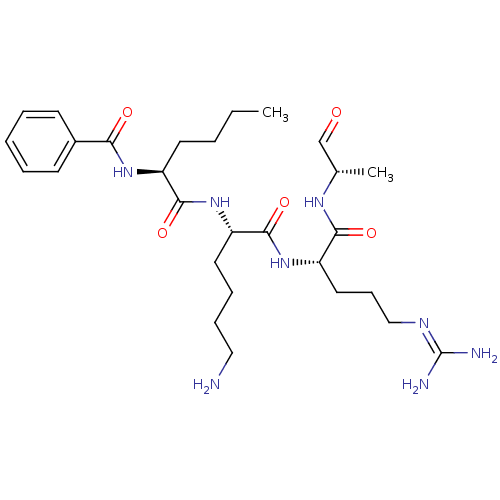 (Bz-Nle-Lys-Arg-Ala-H | CHEMBL199376) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 420 total ) | Next | Last >> |