 Found 93 hits with Last Name = 'einspahr' and Initial = 'h'
Found 93 hits with Last Name = 'einspahr' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18867
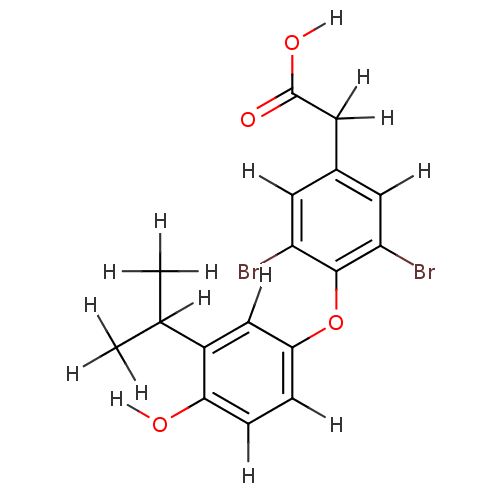
(2-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...)Show InChI InChI=1S/C17H16Br2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18913
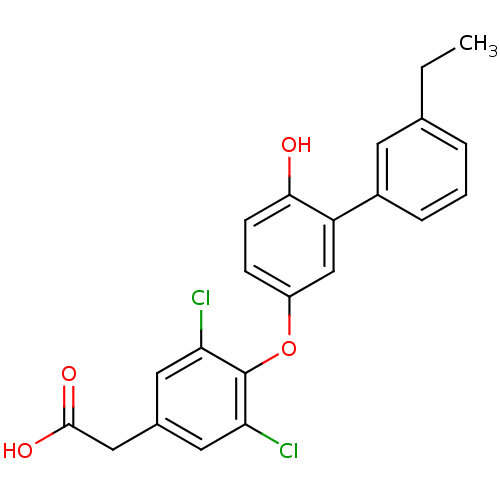
(2-{3,5-dichloro-4-[3-(3-ethylphenyl)-4-hydroxyphen...)Show SMILES CCc1cccc(c1)-c1cc(Oc2c(Cl)cc(CC(O)=O)cc2Cl)ccc1O Show InChI InChI=1S/C22H18Cl2O4/c1-2-13-4-3-5-15(8-13)17-12-16(6-7-20(17)25)28-22-18(23)9-14(10-19(22)24)11-21(26)27/h3-10,12,25H,2,11H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18954
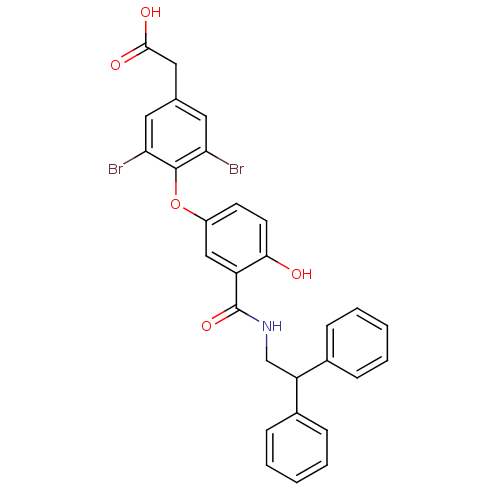
(2-(3,5-dibromo-4-{3-[(2,2-diphenylethyl)carbamoyl]...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(=O)NCC(c2ccccc2)c2ccccc2)c(Br)c1 Show InChI InChI=1S/C29H23Br2NO5/c30-24-13-18(15-27(34)35)14-25(31)28(24)37-21-11-12-26(33)22(16-21)29(36)32-17-23(19-7-3-1-4-8-19)20-9-5-2-6-10-20/h1-14,16,23,33H,15,17H2,(H,32,36)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18948
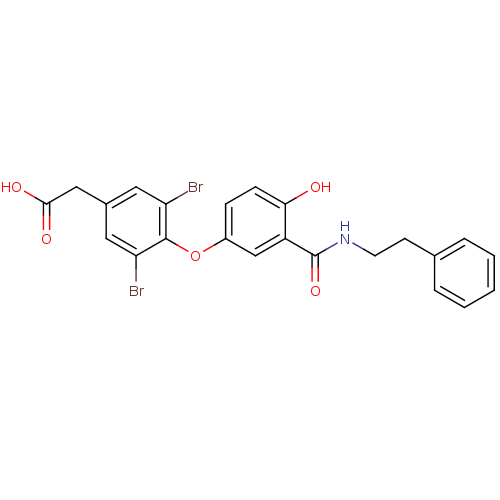
(2-(3,5-dibromo-4-{4-hydroxy-3-[(2-phenylethyl)carb...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(=O)NCCc2ccccc2)c(Br)c1 Show InChI InChI=1S/C23H19Br2NO5/c24-18-10-15(12-21(28)29)11-19(25)22(18)31-16-6-7-20(27)17(13-16)23(30)26-9-8-14-4-2-1-3-5-14/h1-7,10-11,13,27H,8-9,12H2,(H,26,30)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18927

(2-(3,5-dichloro-4-{3-[3-(difluoromethoxy)phenyl]-4...)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)-c2cccc(OC(F)F)c2)c(Cl)c1 Show InChI InChI=1S/C21H14Cl2F2O5/c22-16-6-11(8-19(27)28)7-17(23)20(16)29-14-4-5-18(26)15(10-14)12-2-1-3-13(9-12)30-21(24)25/h1-7,9-10,21,26H,8H2,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18922
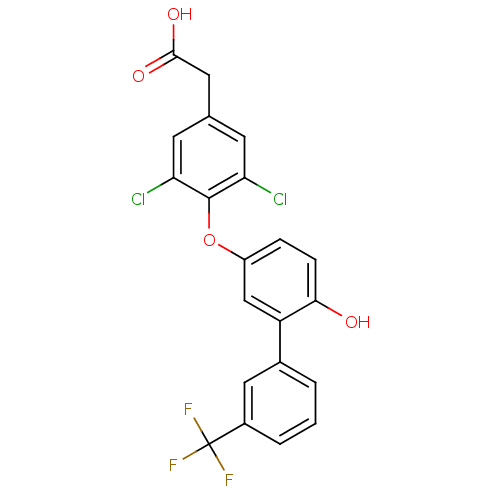
(2-(3,5-dichloro-4-{4-hydroxy-3-[3-(trifluoromethyl...)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)-c2cccc(c2)C(F)(F)F)c(Cl)c1 Show InChI InChI=1S/C21H13Cl2F3O4/c22-16-6-11(8-19(28)29)7-17(23)20(16)30-14-4-5-18(27)15(10-14)12-2-1-3-13(9-12)21(24,25)26/h1-7,9-10,27H,8H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18869
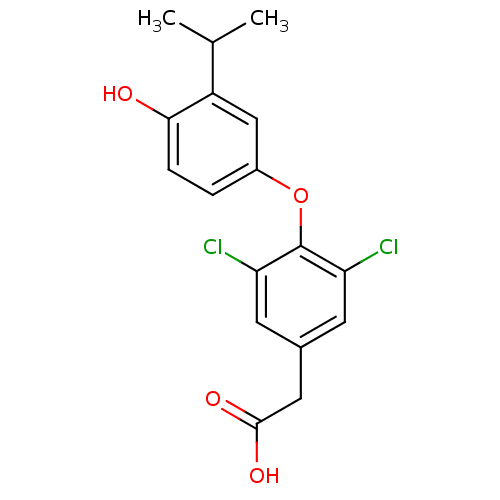
(2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C17H16Cl2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18940
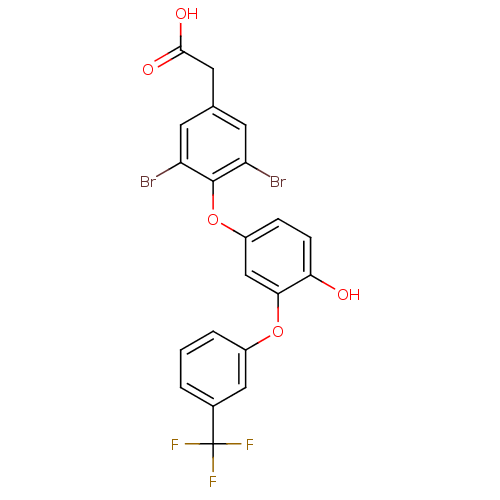
(2-(3,5-dibromo-4-{4-hydroxy-3-[3-(trifluoromethyl)...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(Oc3cccc(c3)C(F)(F)F)c2)c(Br)c1 Show InChI InChI=1S/C21H13Br2F3O5/c22-15-6-11(8-19(28)29)7-16(23)20(15)31-14-4-5-17(27)18(10-14)30-13-3-1-2-12(9-13)21(24,25)26/h1-7,9-10,27H,8H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18867

(2-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...)Show InChI InChI=1S/C17H16Br2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18920
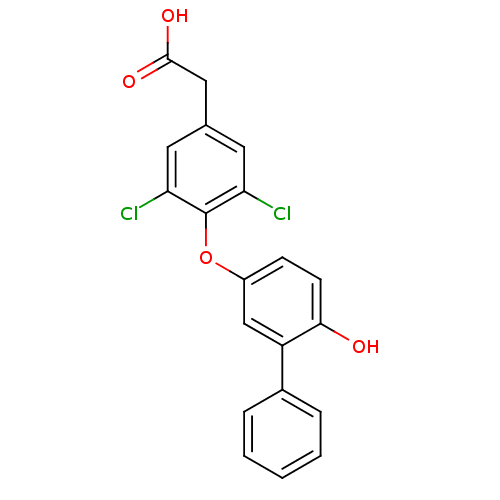
(2-[3,5-dichloro-4-(4-hydroxy-3-phenylphenoxy)pheny...)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)-c2ccccc2)c(Cl)c1 Show InChI InChI=1S/C20H14Cl2O4/c21-16-8-12(10-19(24)25)9-17(22)20(16)26-14-6-7-18(23)15(11-14)13-4-2-1-3-5-13/h1-9,11,23H,10H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50199036
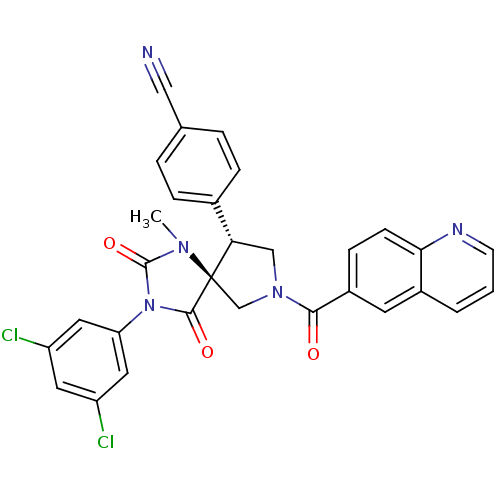
(4-[(5S,9R)-3-(3,5-dichloro-phenyl)-1-methyl-2,4-di...)Show SMILES CN1C(=O)N(C(=O)[C@]11CN(C[C@H]1c1ccc(cc1)C#N)C(=O)c1ccc2ncccc2c1)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C30H21Cl2N5O3/c1-35-29(40)37(24-13-22(31)12-23(32)14-24)28(39)30(35)17-36(16-25(30)19-6-4-18(15-33)5-7-19)27(38)21-8-9-26-20(11-21)3-2-10-34-26/h2-14,25H,16-17H2,1H3/t25-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cerep
Curated by ChEMBL
| Assay Description
Inhibition of LFA1-mediated adhesion of T cell to HUVEC |
J Med Chem 49: 6946-9 (2006)
Article DOI: 10.1021/jm0610806
BindingDB Entry DOI: 10.7270/Q2SF2XC3 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18921
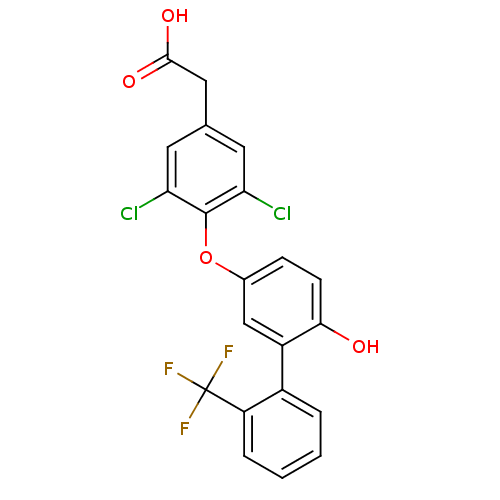
(2-(3,5-dichloro-4-{4-hydroxy-3-[2-(trifluoromethyl...)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)-c2ccccc2C(F)(F)F)c(Cl)c1 Show InChI InChI=1S/C21H13Cl2F3O4/c22-16-7-11(9-19(28)29)8-17(23)20(16)30-12-5-6-18(27)14(10-12)13-3-1-2-4-15(13)21(24,25)26/h1-8,10,27H,9H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18928
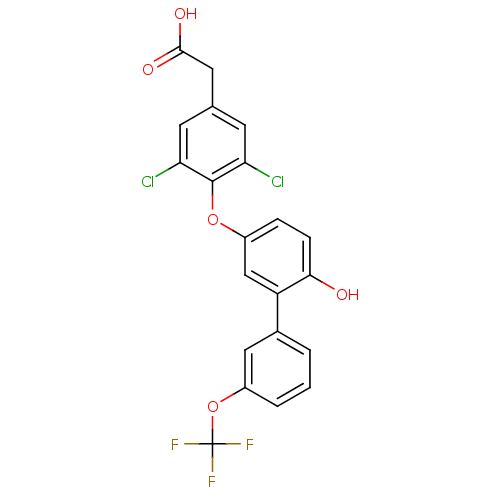
(2-(3,5-dichloro-4-{4-hydroxy-3-[3-(trifluoromethox...)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)-c2cccc(OC(F)(F)F)c2)c(Cl)c1 Show InChI InChI=1S/C21H13Cl2F3O5/c22-16-6-11(8-19(28)29)7-17(23)20(16)30-13-4-5-18(27)15(10-13)12-2-1-3-14(9-12)31-21(24,25)26/h1-7,9-10,27H,8H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18956

(2-(3,5-dichloro-4-{4-hydroxy-3-[(2-phenylethyl)car...)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)C(=O)NCCc2ccccc2)c(Cl)c1 Show InChI InChI=1S/C23H19Cl2NO5/c24-18-10-15(12-21(28)29)11-19(25)22(18)31-16-6-7-20(27)17(13-16)23(30)26-9-8-14-4-2-1-3-5-14/h1-7,10-11,13,27H,8-9,12H2,(H,26,30)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18924
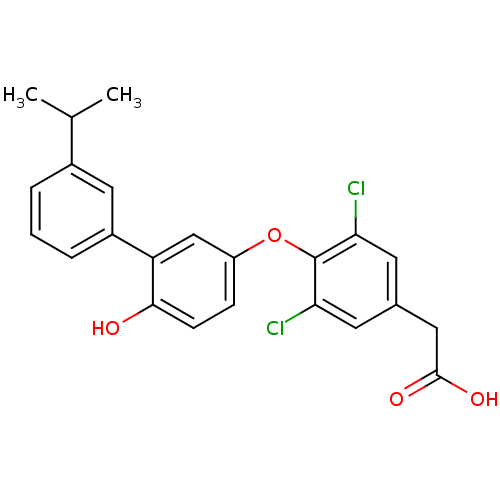
(2-(3,5-dichloro-4-{4-hydroxy-3-[3-(propan-2-yl)phe...)Show SMILES CC(C)c1cccc(c1)-c1cc(Oc2c(Cl)cc(CC(O)=O)cc2Cl)ccc1O Show InChI InChI=1S/C23H20Cl2O4/c1-13(2)15-4-3-5-16(11-15)18-12-17(6-7-21(18)26)29-23-19(24)8-14(9-20(23)25)10-22(27)28/h3-9,11-13,26H,10H2,1-2H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18947

(2-{4-[3-(benzylcarbamoyl)-4-hydroxyphenoxy]-3,5-di...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(=O)NCc2ccccc2)c(Br)c1 Show InChI InChI=1S/C22H17Br2NO5/c23-17-8-14(10-20(27)28)9-18(24)21(17)30-15-6-7-19(26)16(11-15)22(29)25-12-13-4-2-1-3-5-13/h1-9,11,26H,10,12H2,(H,25,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18952
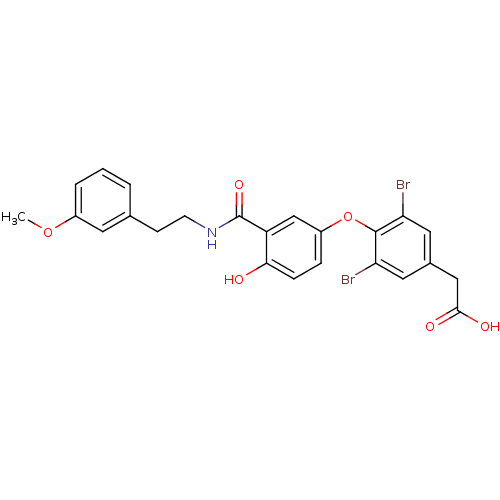
(2-[3,5-dibromo-4-(4-hydroxy-3-{[2-(3-methoxyphenyl...)Show SMILES COc1cccc(CCNC(=O)c2cc(Oc3c(Br)cc(CC(O)=O)cc3Br)ccc2O)c1 Show InChI InChI=1S/C24H21Br2NO6/c1-32-16-4-2-3-14(9-16)7-8-27-24(31)18-13-17(5-6-21(18)28)33-23-19(25)10-15(11-20(23)26)12-22(29)30/h2-6,9-11,13,28H,7-8,12H2,1H3,(H,27,31)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18938
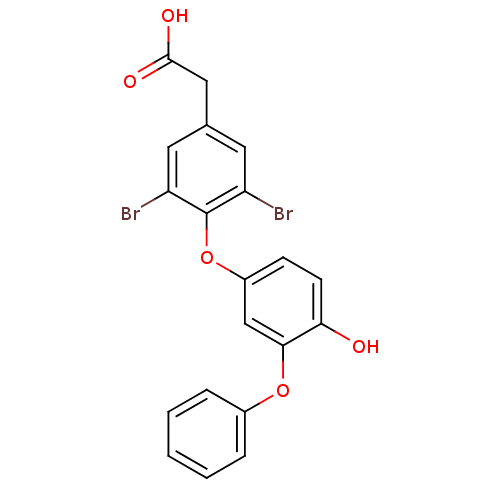
(2-[3,5-dibromo-4-(4-hydroxy-3-phenoxyphenoxy)pheny...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(Oc3ccccc3)c2)c(Br)c1 Show InChI InChI=1S/C20H14Br2O5/c21-15-8-12(10-19(24)25)9-16(22)20(15)27-14-6-7-17(23)18(11-14)26-13-4-2-1-3-5-13/h1-9,11,23H,10H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.97 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18945
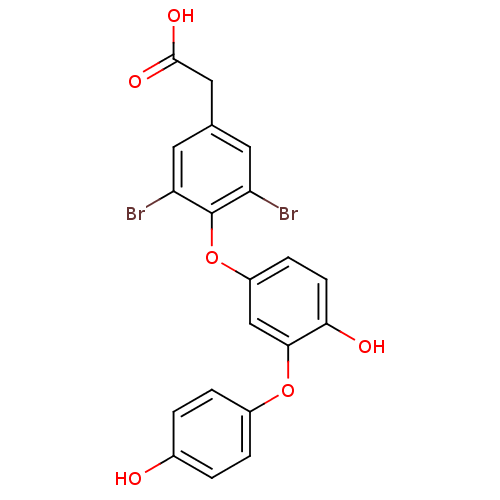
(2-{3,5-dibromo-4-[4-hydroxy-3-(4-hydroxyphenoxy)ph...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(Oc3ccc(O)cc3)c2)c(Br)c1 Show InChI InChI=1S/C20H14Br2O6/c21-15-7-11(9-19(25)26)8-16(22)20(15)28-14-5-6-17(24)18(10-14)27-13-3-1-12(23)2-4-13/h1-8,10,23-24H,9H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50199031
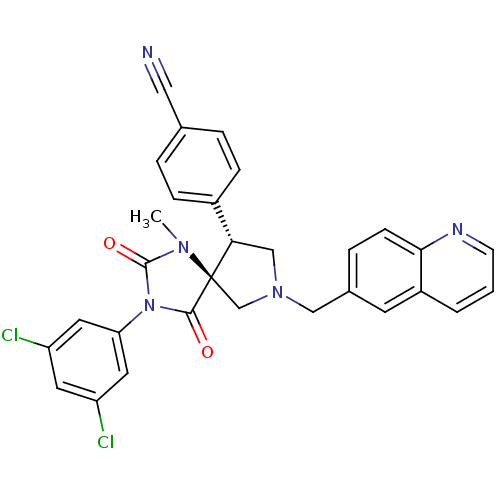
(4-[(5S,9R)-3-(3,5-dichloro-phenyl)-1-methyl-2,4-di...)Show SMILES CN1C(=O)N(C(=O)[C@]11CN(Cc2ccc3ncccc3c2)C[C@H]1c1ccc(cc1)C#N)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C30H23Cl2N5O2/c1-35-29(39)37(25-13-23(31)12-24(32)14-25)28(38)30(35)18-36(17-26(30)21-7-4-19(15-33)5-8-21)16-20-6-9-27-22(11-20)3-2-10-34-27/h2-14,26H,16-18H2,1H3/t26-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cerep
Curated by ChEMBL
| Assay Description
Inhibition of LFA1-mediated adhesion of T cell to HUVEC |
J Med Chem 49: 6946-9 (2006)
Article DOI: 10.1021/jm0610806
BindingDB Entry DOI: 10.7270/Q2SF2XC3 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18949
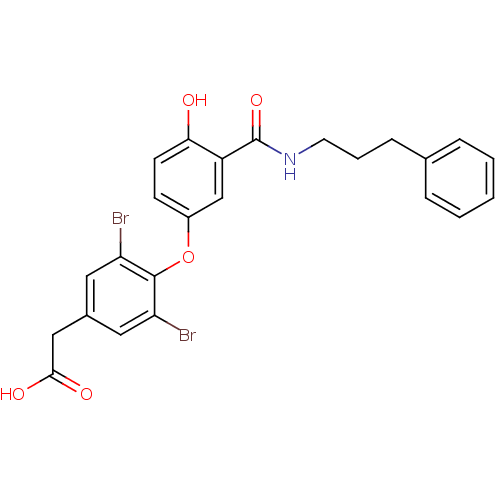
(2-(3,5-dibromo-4-{4-hydroxy-3-[(3-phenylpropyl)car...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(=O)NCCCc2ccccc2)c(Br)c1 Show InChI InChI=1S/C24H21Br2NO5/c25-19-11-16(13-22(29)30)12-20(26)23(19)32-17-8-9-21(28)18(14-17)24(31)27-10-4-7-15-5-2-1-3-6-15/h1-3,5-6,8-9,11-12,14,28H,4,7,10,13H2,(H,27,31)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18939

(2-(3,5-dibromo-4-{4-hydroxy-3-[2-(trifluoromethyl)...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(Oc3ccccc3C(F)(F)F)c2)c(Br)c1 Show InChI InChI=1S/C21H13Br2F3O5/c22-14-7-11(9-19(28)29)8-15(23)20(14)30-12-5-6-16(27)18(10-12)31-17-4-2-1-3-13(17)21(24,25)26/h1-8,10,27H,9H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18937

(2-[3,5-dichloro-4-(3-cyclohexyl-4-hydroxyphenoxy)p...)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)C2CCCCC2)c(Cl)c1 Show InChI InChI=1S/C20H20Cl2O4/c21-16-8-12(10-19(24)25)9-17(22)20(16)26-14-6-7-18(23)15(11-14)13-4-2-1-3-5-13/h6-9,11,13,23H,1-5,10H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50199037
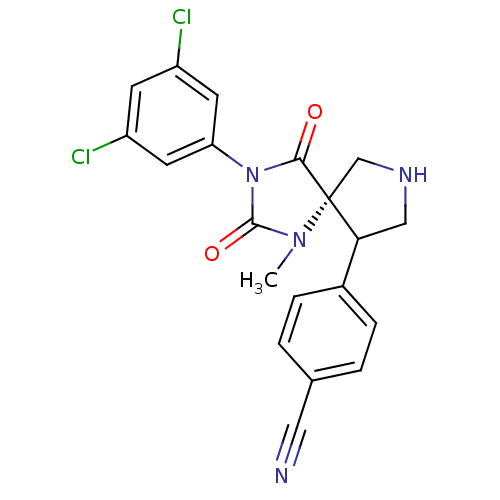
(4-[(5S,9R)-3-(3,5-dichloro-phenyl)-1-methyl-2,4-di...)Show SMILES CN1C(=O)N(C(=O)[C@]11CNCC1c1ccc(cc1)C#N)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C20H16Cl2N4O2/c1-25-19(28)26(16-7-14(21)6-15(22)8-16)18(27)20(25)11-24-10-17(20)13-4-2-12(9-23)3-5-13/h2-8,17,24H,10-11H2,1H3/t17?,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cerep
Curated by ChEMBL
| Assay Description
Inhibition of LFA1-mediated adhesion of T cell to HUVEC |
J Med Chem 49: 6946-9 (2006)
Article DOI: 10.1021/jm0610806
BindingDB Entry DOI: 10.7270/Q2SF2XC3 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18953

(2-[3,5-dibromo-4-(4-hydroxy-3-{[2-(2-methoxyphenyl...)Show SMILES COc1ccccc1CCNC(=O)c1cc(Oc2c(Br)cc(CC(O)=O)cc2Br)ccc1O Show InChI InChI=1S/C24H21Br2NO6/c1-32-21-5-3-2-4-15(21)8-9-27-24(31)17-13-16(6-7-20(17)28)33-23-18(25)10-14(11-19(23)26)12-22(29)30/h2-7,10-11,13,28H,8-9,12H2,1H3,(H,27,31)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18941
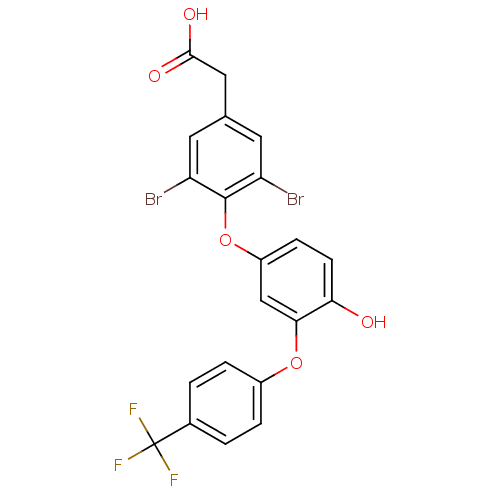
(2-(3,5-dibromo-4-{4-hydroxy-3-[4-(trifluoromethyl)...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(Oc3ccc(cc3)C(F)(F)F)c2)c(Br)c1 Show InChI InChI=1S/C21H13Br2F3O5/c22-15-7-11(9-19(28)29)8-16(23)20(15)31-14-5-6-17(27)18(10-14)30-13-3-1-12(2-4-13)21(24,25)26/h1-8,10,27H,9H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18926
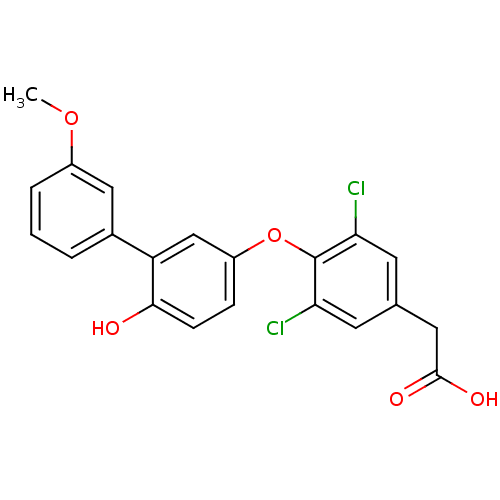
(2-{3,5-dichloro-4-[4-hydroxy-3-(3-methoxyphenyl)ph...)Show SMILES COc1cccc(c1)-c1cc(Oc2c(Cl)cc(CC(O)=O)cc2Cl)ccc1O Show InChI InChI=1S/C21H16Cl2O5/c1-27-14-4-2-3-13(10-14)16-11-15(5-6-19(16)24)28-21-17(22)7-12(8-18(21)23)9-20(25)26/h2-8,10-11,24H,9H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50199030
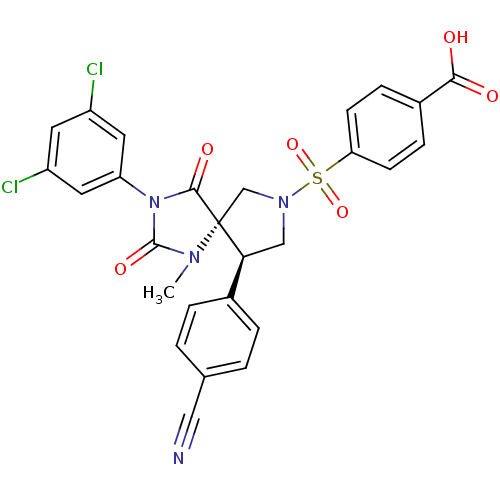
(4-[(5S,9R)-9-(4-cyano-phenyl)-3-(3,5-dichloro-phen...)Show SMILES CN1C(=O)N(C(=O)[C@]11CN(C[C@H]1c1ccc(cc1)C#N)S(=O)(=O)c1ccc(cc1)C(O)=O)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C27H20Cl2N4O6S/c1-31-26(37)33(21-11-19(28)10-20(29)12-21)25(36)27(31)15-32(14-23(27)17-4-2-16(13-30)3-5-17)40(38,39)22-8-6-18(7-9-22)24(34)35/h2-12,23H,14-15H2,1H3,(H,34,35)/t23-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cerep
Curated by ChEMBL
| Assay Description
Inhibition of LFA1-mediated adhesion of T cell to HUVEC |
J Med Chem 49: 6946-9 (2006)
Article DOI: 10.1021/jm0610806
BindingDB Entry DOI: 10.7270/Q2SF2XC3 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18913

(2-{3,5-dichloro-4-[3-(3-ethylphenyl)-4-hydroxyphen...)Show SMILES CCc1cccc(c1)-c1cc(Oc2c(Cl)cc(CC(O)=O)cc2Cl)ccc1O Show InChI InChI=1S/C22H18Cl2O4/c1-2-13-4-3-5-15(8-13)17-12-16(6-7-20(17)25)28-22-18(23)9-14(10-19(22)24)11-21(26)27/h3-10,12,25H,2,11H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50199035
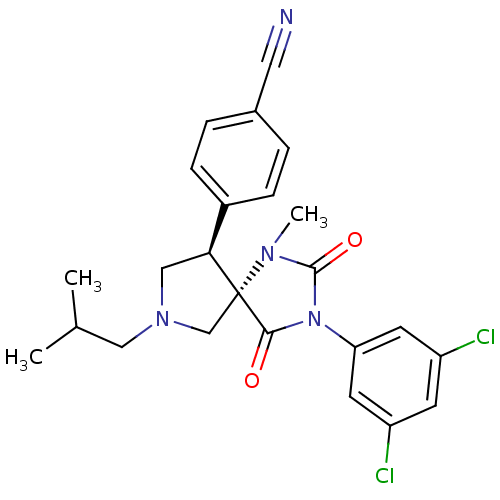
(4-[(5S,9R)-3-(3,5-dichloro-phenyl)-7-isobutyl-1-me...)Show SMILES CC(C)CN1C[C@@H](c2ccc(cc2)C#N)[C@@]2(C1)N(C)C(=O)N(C2=O)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C24H24Cl2N4O2/c1-15(2)12-29-13-21(17-6-4-16(11-27)5-7-17)24(14-29)22(31)30(23(32)28(24)3)20-9-18(25)8-19(26)10-20/h4-10,15,21H,12-14H2,1-3H3/t21-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Cerep
Curated by ChEMBL
| Assay Description
Inhibition of LFA1-mediated adhesion of T cell to HUVEC |
J Med Chem 49: 6946-9 (2006)
Article DOI: 10.1021/jm0610806
BindingDB Entry DOI: 10.7270/Q2SF2XC3 |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50199039
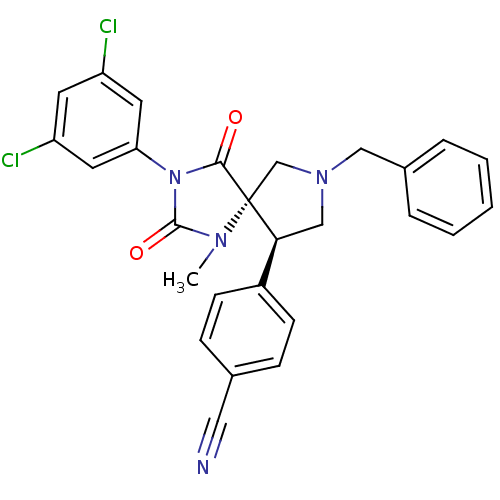
(4-[(5S,9R)-7-benzyl-3-(3,5-dichloro-phenyl)-1-meth...)Show SMILES CN1C(=O)N(C(=O)[C@]11CN(Cc2ccccc2)C[C@H]1c1ccc(cc1)C#N)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C27H22Cl2N4O2/c1-31-26(35)33(23-12-21(28)11-22(29)13-23)25(34)27(31)17-32(15-19-5-3-2-4-6-19)16-24(27)20-9-7-18(14-30)8-10-20/h2-13,24H,15-17H2,1H3/t24-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cerep
Curated by ChEMBL
| Assay Description
Inhibition of LFA1-mediated adhesion of T cell to HUVEC |
J Med Chem 49: 6946-9 (2006)
Article DOI: 10.1021/jm0610806
BindingDB Entry DOI: 10.7270/Q2SF2XC3 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18948

(2-(3,5-dibromo-4-{4-hydroxy-3-[(2-phenylethyl)carb...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(=O)NCCc2ccccc2)c(Br)c1 Show InChI InChI=1S/C23H19Br2NO5/c24-18-10-15(12-21(28)29)11-19(25)22(18)31-16-6-7-20(27)17(13-16)23(30)26-9-8-14-4-2-1-3-5-14/h1-7,10-11,13,27H,8-9,12H2,(H,26,30)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18921

(2-(3,5-dichloro-4-{4-hydroxy-3-[2-(trifluoromethyl...)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)-c2ccccc2C(F)(F)F)c(Cl)c1 Show InChI InChI=1S/C21H13Cl2F3O4/c22-16-7-11(9-19(28)29)8-17(23)20(16)30-12-5-6-18(27)14(10-12)13-3-1-2-4-15(13)21(24,25)26/h1-8,10,27H,9H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18950

(2-(3,5-dibromo-4-{4-hydroxy-3-[(4-phenylbutyl)carb...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(=O)NCCCCc2ccccc2)c(Br)c1 Show InChI InChI=1S/C25H23Br2NO5/c26-20-12-17(14-23(30)31)13-21(27)24(20)33-18-9-10-22(29)19(15-18)25(32)28-11-5-4-8-16-6-2-1-3-7-16/h1-3,6-7,9-10,12-13,15,29H,4-5,8,11,14H2,(H,28,32)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18954

(2-(3,5-dibromo-4-{3-[(2,2-diphenylethyl)carbamoyl]...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(=O)NCC(c2ccccc2)c2ccccc2)c(Br)c1 Show InChI InChI=1S/C29H23Br2NO5/c30-24-13-18(15-27(34)35)14-25(31)28(24)37-21-11-12-26(33)22(16-21)29(36)32-17-23(19-7-3-1-4-8-19)20-9-5-2-6-10-20/h1-14,16,23,33H,15,17H2,(H,32,36)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50199033
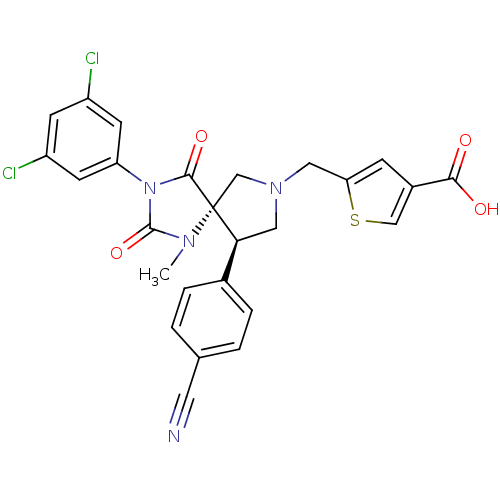
(5-[(5S,9R)-9-(4-CYANOPHENYL)-3-(3,5-DICHLOROPHENYL...)Show SMILES CN1C(=O)N(C(=O)[C@]11CN(Cc2cc(cs2)C(O)=O)C[C@H]1c1ccc(cc1)C#N)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C26H20Cl2N4O4S/c1-30-25(36)32(20-8-18(27)7-19(28)9-20)24(35)26(30)14-31(11-21-6-17(13-37-21)23(33)34)12-22(26)16-4-2-15(10-29)3-5-16/h2-9,13,22H,11-12,14H2,1H3,(H,33,34)/t22-,26+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cerep
Curated by ChEMBL
| Assay Description
Inhibition of LFA1-mediated adhesion of T cell to HUVEC |
J Med Chem 49: 6946-9 (2006)
Article DOI: 10.1021/jm0610806
BindingDB Entry DOI: 10.7270/Q2SF2XC3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50199033

(5-[(5S,9R)-9-(4-CYANOPHENYL)-3-(3,5-DICHLOROPHENYL...)Show SMILES CN1C(=O)N(C(=O)[C@]11CN(Cc2cc(cs2)C(O)=O)C[C@H]1c1ccc(cc1)C#N)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C26H20Cl2N4O4S/c1-30-25(36)32(20-8-18(27)7-19(28)9-20)24(35)26(30)14-31(11-21-6-17(13-37-21)23(33)34)12-22(26)16-4-2-15(10-29)3-5-16/h2-9,13,22H,11-12,14H2,1H3,(H,33,34)/t22-,26+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Cerep
Curated by ChEMBL
| Assay Description
Inhibition of LFA1-mediated adhesion of T cell to HUVEC |
J Med Chem 49: 6946-9 (2006)
Article DOI: 10.1021/jm0610806
BindingDB Entry DOI: 10.7270/Q2SF2XC3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18925

(2-{3,5-dichloro-4-[4-hydroxy-3-(3-phenylphenyl)phe...)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)-c2cccc(c2)-c2ccccc2)c(Cl)c1 Show InChI InChI=1S/C26H18Cl2O4/c27-22-11-16(13-25(30)31)12-23(28)26(22)32-20-9-10-24(29)21(15-20)19-8-4-7-18(14-19)17-5-2-1-3-6-17/h1-12,14-15,29H,13H2,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18869

(2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C17H16Cl2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18936
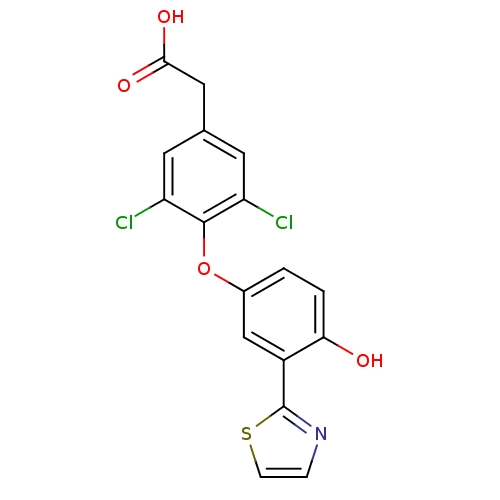
(2-{3,5-dichloro-4-[4-hydroxy-3-(1,3-thiazol-2-yl)p...)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)-c2nccs2)c(Cl)c1 Show InChI InChI=1S/C17H11Cl2NO4S/c18-12-5-9(7-15(22)23)6-13(19)16(12)24-10-1-2-14(21)11(8-10)17-20-3-4-25-17/h1-6,8,21H,7H2,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50199038
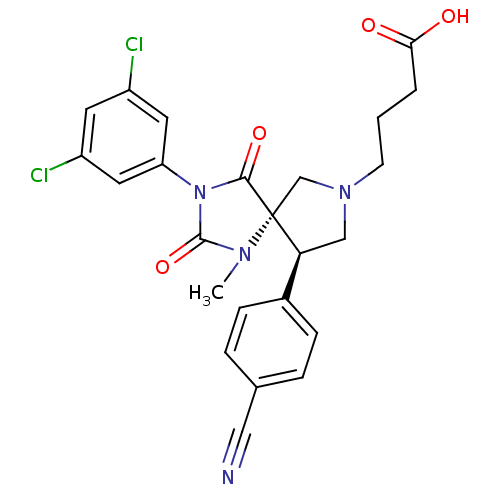
(4-[(5S,9R)-9-(4-cyano-phenyl)-3-(3,5-dichloro-phen...)Show SMILES CN1C(=O)N(C(=O)[C@]11CN(CCCC(O)=O)C[C@H]1c1ccc(cc1)C#N)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C24H22Cl2N4O4/c1-28-23(34)30(19-10-17(25)9-18(26)11-19)22(33)24(28)14-29(8-2-3-21(31)32)13-20(24)16-6-4-15(12-27)5-7-16/h4-7,9-11,20H,2-3,8,13-14H2,1H3,(H,31,32)/t20-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Cerep
Curated by ChEMBL
| Assay Description
Inhibition of LFA1-mediated adhesion of T cell to HUVEC |
J Med Chem 49: 6946-9 (2006)
Article DOI: 10.1021/jm0610806
BindingDB Entry DOI: 10.7270/Q2SF2XC3 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18927

(2-(3,5-dichloro-4-{3-[3-(difluoromethoxy)phenyl]-4...)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)-c2cccc(OC(F)F)c2)c(Cl)c1 Show InChI InChI=1S/C21H14Cl2F2O5/c22-16-6-11(8-19(27)28)7-17(23)20(16)29-14-4-5-18(26)15(10-14)12-2-1-3-13(9-12)30-21(24)25/h1-7,9-10,21,26H,8H2,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18946

(2-{3,5-dibromo-4-[4-hydroxy-3-(phenylcarbamoyl)phe...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(=O)Nc2ccccc2)c(Br)c1 Show InChI InChI=1S/C21H15Br2NO5/c22-16-8-12(10-19(26)27)9-17(23)20(16)29-14-6-7-18(25)15(11-14)21(28)24-13-4-2-1-3-5-13/h1-9,11,25H,10H2,(H,24,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18951

(2-[3,5-dibromo-4-(4-hydroxy-3-{[2-(4-methoxyphenyl...)Show SMILES COc1ccc(CCNC(=O)c2cc(Oc3c(Br)cc(CC(O)=O)cc3Br)ccc2O)cc1 Show InChI InChI=1S/C24H21Br2NO6/c1-32-16-4-2-14(3-5-16)8-9-27-24(31)18-13-17(6-7-21(18)28)33-23-19(25)10-15(11-20(23)26)12-22(29)30/h2-7,10-11,13,28H,8-9,12H2,1H3,(H,27,31)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18922

(2-(3,5-dichloro-4-{4-hydroxy-3-[3-(trifluoromethyl...)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)-c2cccc(c2)C(F)(F)F)c(Cl)c1 Show InChI InChI=1S/C21H13Cl2F3O4/c22-16-6-11(8-19(28)29)7-17(23)20(16)30-14-4-5-18(27)15(10-14)12-2-1-3-13(9-12)21(24,25)26/h1-7,9-10,27H,8H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18933

(2-{3,5-dichloro-4-[4-hydroxy-3-(pyridin-3-yl)pheno...)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)-c2cccnc2)c(Cl)c1 Show InChI InChI=1S/C19H13Cl2NO4/c20-15-6-11(8-18(24)25)7-16(21)19(15)26-13-3-4-17(23)14(9-13)12-2-1-5-22-10-12/h1-7,9-10,23H,8H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18944

(2-{3,5-dibromo-4-[4-hydroxy-3-(3-hydroxyphenoxy)ph...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(Oc3cccc(O)c3)c2)c(Br)c1 Show InChI InChI=1S/C20H14Br2O6/c21-15-6-11(8-19(25)26)7-16(22)20(15)28-14-4-5-17(24)18(10-14)27-13-3-1-2-12(23)9-13/h1-7,9-10,23-24H,8H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18929
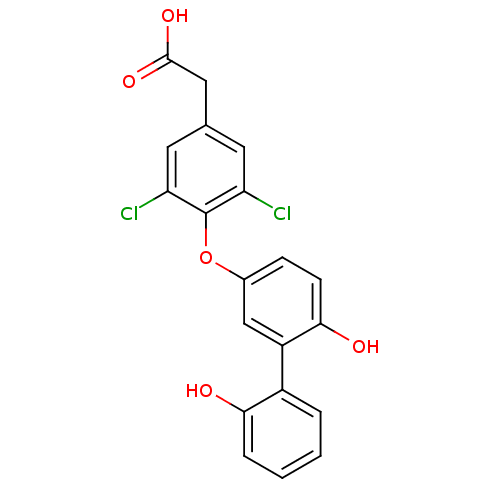
(2-{3,5-dichloro-4-[4-hydroxy-3-(2-hydroxyphenyl)ph...)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)-c2ccccc2O)c(Cl)c1 Show InChI InChI=1S/C20H14Cl2O5/c21-15-7-11(9-19(25)26)8-16(22)20(15)27-12-5-6-18(24)14(10-12)13-3-1-2-4-17(13)23/h1-8,10,23-24H,9H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data