 Found 736 hits with Last Name = 'emami' and Initial = 's'
Found 736 hits with Last Name = 'emami' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50052204
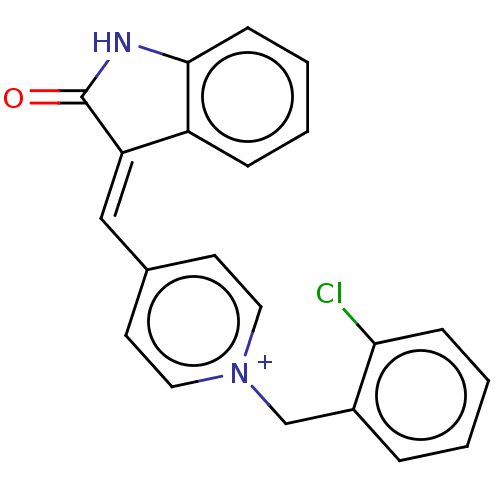
(CHEMBL3318392)Show SMILES [Cl-].Clc1ccccc1C[n+]1ccc(\C=C2\C(=O)Nc3ccccc23)cc1 Show InChI InChI=1S/C21H15ClN2O/c22-19-7-3-1-5-16(19)14-24-11-9-15(10-12-24)13-18-17-6-2-4-8-20(17)23-21(18)25/h1-13H,14H2/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of electric eel AChE using acetylthiocholine iodide substrate by Lineweaver-Burk plot analysis |
Eur J Med Chem 84: 375-81 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.017
BindingDB Entry DOI: 10.7270/Q2H996T1 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medicinal Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE pre-incubated for 3 mins before acetylthiocholine substrate addition by Lineweaver-Burk plot |
Eur J Med Chem 97: 181-9 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.055
BindingDB Entry DOI: 10.7270/Q2P84DM4 |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM82353
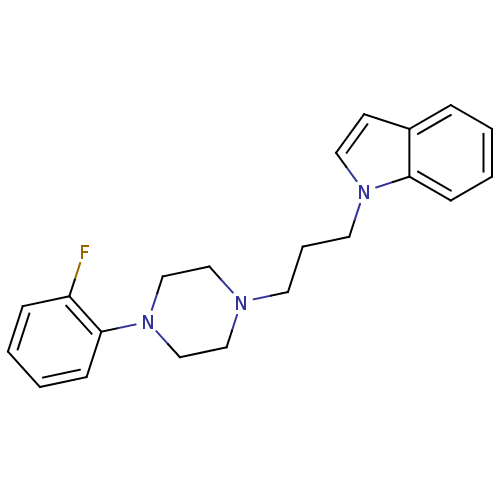
(1-{3-[4-(substitutedphenyl)piperazin1-yl]propyl}-1...)Show InChI InChI=1S/C21H24FN3/c22-19-7-2-4-9-21(19)25-16-14-23(15-17-25)11-5-12-24-13-10-18-6-1-3-8-20(18)24/h1-4,6-10,13H,5,11-12,14-17H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-di-o-tolylguanidine from sigma-2 receptor in rat liver membranes after 180 mins by scintillation counting method |
Eur J Med Chem 150: 9-29 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.065
BindingDB Entry DOI: 10.7270/Q2X92DXX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50457028

(CHEMBL4218303)Show SMILES COc1ccccc1C1c2c(nc3CCCCc3c2N)-n2n1c(=O)c1ccccc1c2=O Show InChI InChI=1S/C25H22N4O3/c1-32-19-13-7-5-11-17(19)22-20-21(26)16-10-4-6-12-18(16)27-23(20)29-25(31)15-9-3-2-8-14(15)24(30)28(22)29/h2-3,5,7-9,11,13,22H,4,6,10,12H2,1H3,(H2,26,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measur... |
Eur J Med Chem 139: 280-289 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.072
BindingDB Entry DOI: 10.7270/Q2SX6GVB |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM82351
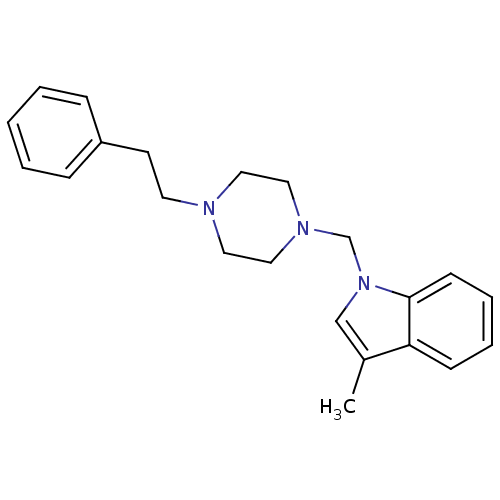
(1-{[4-(substitutedphenyl/phenylethyl)piperazin1-yl...)Show InChI InChI=1S/C22H27N3/c1-19-17-25(22-10-6-5-9-21(19)22)18-24-15-13-23(14-16-24)12-11-20-7-3-2-4-8-20/h2-10,17H,11-16,18H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in guinea pig brain membranes after 180 mins by scintillation counting method |
Eur J Med Chem 150: 9-29 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.065
BindingDB Entry DOI: 10.7270/Q2X92DXX |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50595018
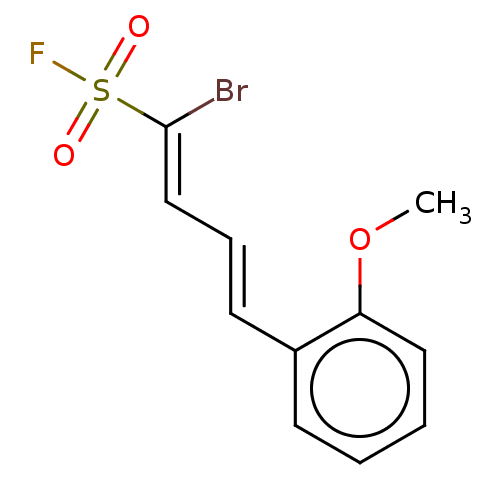
(CHEMBL5208027) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114255
BindingDB Entry DOI: 10.7270/Q20G3Q5H |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50569812
(CHEMBL4869068)Show SMILES Oc1coc(Cn2cc(COc3cc(=O)oc4ccccc34)nn2)cc1=O | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate by Lineweaver-Burk double reciprocal plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112480
BindingDB Entry DOI: 10.7270/Q2WW7NGF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50235419

(CHEMBL4077932)Show SMILES CCOC(=O)C1=C(Oc2nc3CCCCc3c(N)c2C1c1cccc(Br)c1)c1ccccc1 |t:5| Show InChI InChI=1S/C27H25BrN2O3/c1-2-32-27(31)23-21(17-11-8-12-18(28)15-17)22-24(29)19-13-6-7-14-20(19)30-26(22)33-25(23)16-9-4-3-5-10-16/h3-5,8-12,15,21H,2,6-7,13-14H2,1H3,(H2,29,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of electric eel AChE preincubated for 5 mins followed by varying levels acetylthiocholine iodide substrate addition by Lineweav... |
Eur J Med Chem 128: 237-246 (2017)
Article DOI: 10.1016/j.ejmech.2017.01.042
BindingDB Entry DOI: 10.7270/Q2GM89J3 |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM82351

(1-{[4-(substitutedphenyl/phenylethyl)piperazin1-yl...)Show InChI InChI=1S/C22H27N3/c1-19-17-25(22-10-6-5-9-21(19)22)18-24-15-13-23(14-16-24)12-11-20-7-3-2-4-8-20/h2-10,17H,11-16,18H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-di-o-tolylguanidine from sigma-2 receptor in rat liver membranes after 180 mins by scintillation counting method |
Eur J Med Chem 150: 9-29 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.065
BindingDB Entry DOI: 10.7270/Q2X92DXX |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50569806
(CHEMBL4865646)Show SMILES COc1cc(ccc1OCc1cn(Cc2cc(=O)c(O)co2)nn1)C(C)=O | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate by Lineweaver-Burk double reciprocal plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112480
BindingDB Entry DOI: 10.7270/Q2WW7NGF |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50569801

(CHEMBL4867662)Show SMILES CC(C)c1ccc(C)c(OCc2cn(Cc3cc(=O)c(O)co3)nn2)c1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate by Lineweaver-Burk double reciprocal plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112480
BindingDB Entry DOI: 10.7270/Q2WW7NGF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50080392
(CHEMBL3416513)Show SMILES Cl.CCOc1ccc2C(=O)\C(COc2c1)=C\c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C25H29NO4.ClH/c1-2-28-22-10-11-23-24(17-22)30-18-20(25(23)27)16-19-6-8-21(9-7-19)29-15-14-26-12-4-3-5-13-26;/h6-11,16-17H,2-5,12-15,18H2,1H3;1H/b20-16+; | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medicinal Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE pre-incubated for 3 mins before acetylthiocholine substrate addition by Lineweaver-Burk plot |
Eur J Med Chem 97: 181-9 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.055
BindingDB Entry DOI: 10.7270/Q2P84DM4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50204859
(CHEMBL3939483)Show SMILES Nc1c2CCCCc2cc2Oc3[nH]nc(c3C(c3ccc(F)cc3)c12)-c1ccccc1 Show InChI InChI=1S/C26H22FN3O/c27-18-12-10-15(11-13-18)21-22-20(14-17-8-4-5-9-19(17)24(22)28)31-26-23(21)25(29-30-26)16-6-2-1-3-7-16/h1-3,6-7,10-14,21H,4-5,8-9,28H2,(H,29,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Mixed type inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured f... |
Eur J Med Chem 123: 298-308 (2016)
Article DOI: 10.1016/j.ejmech.2016.07.043
BindingDB Entry DOI: 10.7270/Q2V40X55 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50197934
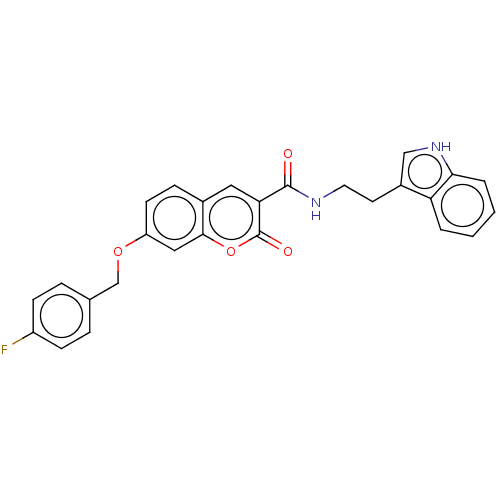
(CHEMBL3889532)Show SMILES Fc1ccc(COc2ccc3cc(C(=O)NCCc4c[nH]c5ccccc45)c(=O)oc3c2)cc1 Show InChI InChI=1S/C27H21FN2O4/c28-20-8-5-17(6-9-20)16-33-21-10-7-18-13-23(27(32)34-25(18)14-21)26(31)29-12-11-19-15-30-24-4-2-1-3-22(19)24/h1-10,13-15,30H,11-12,16H2,(H,29,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured for 2 mins by Lineweaver-Burk plot analysis |
Eur J Med Chem 121: 40-46 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.014
BindingDB Entry DOI: 10.7270/Q2XS5XBG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50038390
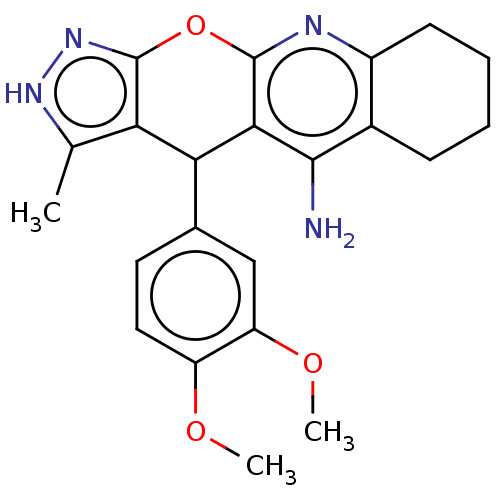
(CHEMBL3352904)Show SMILES COc1ccc(cc1OC)C1c2c(C)[nH]nc2Oc2nc3CCCCc3c(N)c12 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Lineweaver-Burk plot |
Eur J Med Chem 89: 296-303 (2014)
Article DOI: 10.1016/j.ejmech.2014.10.049
BindingDB Entry DOI: 10.7270/Q2T72K1Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50020730
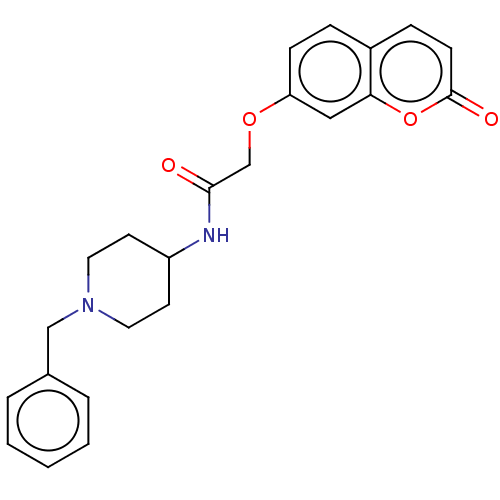
(CHEMBL1469070)Show SMILES O=C(COc1ccc2ccc(=O)oc2c1)NC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C23H24N2O4/c26-22(16-28-20-8-6-18-7-9-23(27)29-21(18)14-20)24-19-10-12-25(13-11-19)15-17-4-2-1-3-5-17/h1-9,14,19H,10-13,15-16H2,(H,24,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine as substrate by Lineweavere-Burk plot |
Eur J Med Chem 82: 536-44 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.056
BindingDB Entry DOI: 10.7270/Q27D2WQV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50595017
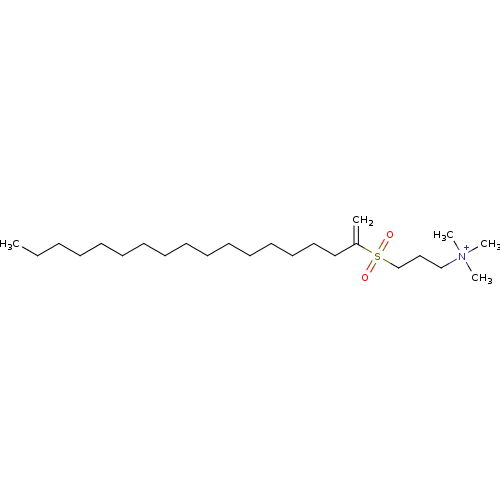
(CHEMBL3606084)Show SMILES CCCCCCCCCCCCCCCCC(=C)S(=O)(=O)CCC[N+](C)(C)C | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114255
BindingDB Entry DOI: 10.7270/Q20G3Q5H |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50406864

(CHEMBL4161244)Show SMILES COc1ccc(cc1OC)-c1cc2ccc(OCCCn3cc(CNC(=O)CCCCC4CCSS4)nn3)cc2oc1=O Show InChI InChI=1S/C31H36N4O6S2/c1-38-27-11-9-21(17-29(27)39-2)26-16-22-8-10-24(18-28(22)41-31(26)37)40-14-5-13-35-20-23(33-34-35)19-32-30(36)7-4-3-6-25-12-15-42-43-25/h8-11,16-18,20,25H,3-7,12-15,19H2,1-2H3,(H,32,36) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of electric eel AChE using varying levels of acetylthiocholine iodide as substrate pretreated for 5 mins followed by subst... |
Eur J Med Chem 152: 600-614 (2018)
Article DOI: 10.1016/j.ejmech.2018.04.058
BindingDB Entry DOI: 10.7270/Q2WW7M8B |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50434353

(CHEMBL2386747 | US9446044, 72)Show SMILES COC(=N)c1nc2ccc3ncnc(Nc4ccc(Cl)cc4Cl)c3c2s1 Show InChI InChI=1S/C17H11Cl2N5OS/c1-25-15(20)17-24-12-5-4-11-13(14(12)26-17)16(22-7-21-11)23-10-3-2-8(18)6-9(10)19/h2-7,20H,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113949
BindingDB Entry DOI: 10.7270/Q2M90DP7 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50434353

(CHEMBL2386747 | US9446044, 72)Show SMILES COC(=N)c1nc2ccc3ncnc(Nc4ccc(Cl)cc4Cl)c3c2s1 Show InChI InChI=1S/C17H11Cl2N5OS/c1-25-15(20)17-24-12-5-4-11-13(14(12)26-17)16(22-7-21-11)23-10-3-2-8(18)6-9(10)19/h2-7,20H,1H3,(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113949
BindingDB Entry DOI: 10.7270/Q2M90DP7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50443517
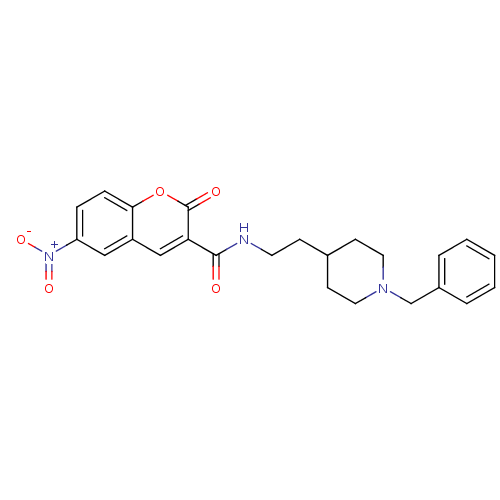
(CHEMBL3087899)Show SMILES [O-][N+](=O)c1ccc2oc(=O)c(cc2c1)C(=O)NCCC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C24H25N3O5/c28-23(21-15-19-14-20(27(30)31)6-7-22(19)32-24(21)29)25-11-8-17-9-12-26(13-10-17)16-18-4-2-1-3-5-18/h1-7,14-15,17H,8-13,16H2,(H,25,28) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kerman University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate preincubated for 15 mins prior to substrate addition by Ellman's method |
Eur J Med Chem 70: 623-30 (2013)
Article DOI: 10.1016/j.ejmech.2013.10.024
BindingDB Entry DOI: 10.7270/Q2GQ7070 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM246383
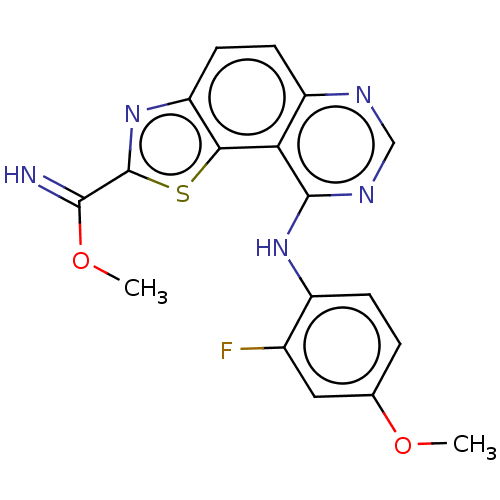
(US9446044, 69)Show InChI InChI=1S/C18H14FN5O2S/c1-25-9-3-4-11(10(19)7-9)23-17-14-12(21-8-22-17)5-6-13-15(14)27-18(24-13)16(20)26-2/h3-8,20H,1-2H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113949
BindingDB Entry DOI: 10.7270/Q2M90DP7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50052204

(CHEMBL3318392)Show SMILES [Cl-].Clc1ccccc1C[n+]1ccc(\C=C2\C(=O)Nc3ccccc23)cc1 Show InChI InChI=1S/C21H15ClN2O/c22-19-7-3-1-5-16(19)14-24-11-9-15(10-12-24)13-18-17-6-2-4-8-20(17)23-21(18)25/h1-13H,14H2/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman method based spectrophotometry |
Eur J Med Chem 84: 375-81 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.017
BindingDB Entry DOI: 10.7270/Q2H996T1 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM246383

(US9446044, 69)Show InChI InChI=1S/C18H14FN5O2S/c1-25-9-3-4-11(10(19)7-9)23-17-14-12(21-8-22-17)5-6-13-15(14)27-18(24-13)16(20)26-2/h3-8,20H,1-2H3,(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113949
BindingDB Entry DOI: 10.7270/Q2M90DP7 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50434352

(CHEMBL2386748 | US9446044, 78)Show InChI InChI=1S/C17H11F2N5OS/c1-25-15(20)17-24-12-5-4-11-13(14(12)26-17)16(22-7-21-11)23-10-3-2-8(18)6-9(10)19/h2-7,20H,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113949
BindingDB Entry DOI: 10.7270/Q2M90DP7 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50434364
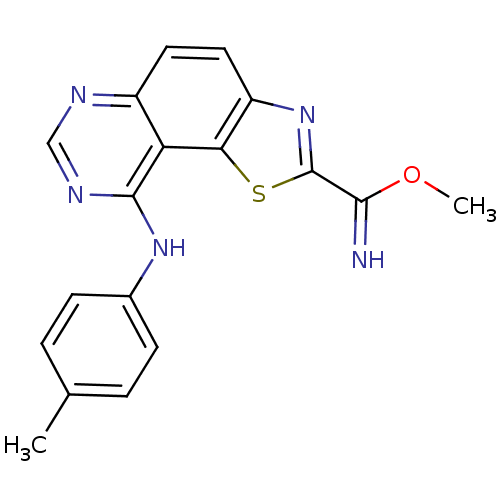
(CHEMBL2386764 | US9446044, 65)Show InChI InChI=1S/C18H15N5OS/c1-10-3-5-11(6-4-10)22-17-14-12(20-9-21-17)7-8-13-15(14)25-18(23-13)16(19)24-2/h3-9,19H,1-2H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113949
BindingDB Entry DOI: 10.7270/Q2M90DP7 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM246384
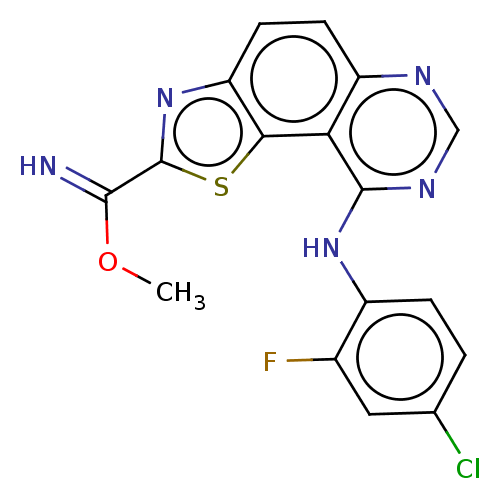
(US9446044, 71)Show InChI InChI=1S/C17H11ClFN5OS/c1-25-15(20)17-24-12-5-4-11-13(14(12)26-17)16(22-7-21-11)23-10-3-2-8(18)6-9(10)19/h2-7,20H,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113949
BindingDB Entry DOI: 10.7270/Q2M90DP7 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50434352

(CHEMBL2386748 | US9446044, 78)Show InChI InChI=1S/C17H11F2N5OS/c1-25-15(20)17-24-12-5-4-11-13(14(12)26-17)16(22-7-21-11)23-10-3-2-8(18)6-9(10)19/h2-7,20H,1H3,(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113949
BindingDB Entry DOI: 10.7270/Q2M90DP7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50052206
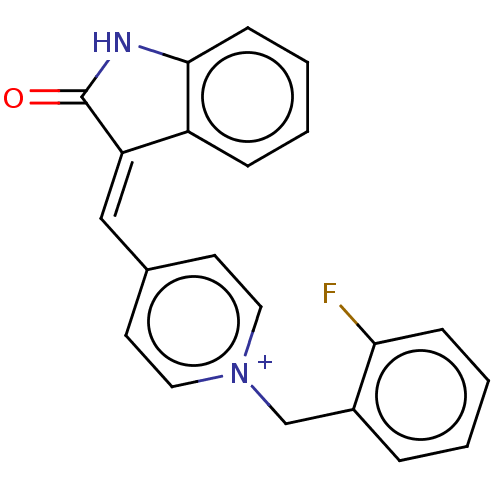
(CHEMBL3318391)Show SMILES [Cl-].Fc1ccccc1C[n+]1ccc(\C=C2\C(=O)Nc3ccccc23)cc1 Show InChI InChI=1S/C21H15FN2O/c22-19-7-3-1-5-16(19)14-24-11-9-15(10-12-24)13-18-17-6-2-4-8-20(17)23-21(18)25/h1-13H,14H2/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman method based spectrophotometry |
Eur J Med Chem 84: 375-81 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.017
BindingDB Entry DOI: 10.7270/Q2H996T1 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50052208
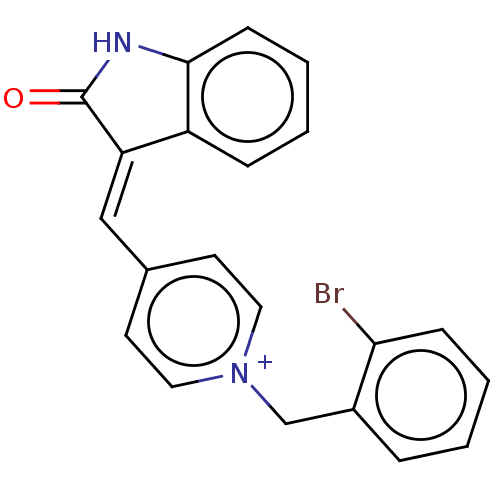
(CHEMBL3318394)Show SMILES [Cl-].Brc1ccccc1C[n+]1ccc(\C=C2\C(=O)Nc3ccccc23)cc1 Show InChI InChI=1S/C21H15BrN2O/c22-19-7-3-1-5-16(19)14-24-11-9-15(10-12-24)13-18-17-6-2-4-8-20(17)23-21(18)25/h1-13H,14H2/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman method based spectrophotometry |
Eur J Med Chem 84: 375-81 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.017
BindingDB Entry DOI: 10.7270/Q2H996T1 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM246384

(US9446044, 71)Show InChI InChI=1S/C17H11ClFN5OS/c1-25-15(20)17-24-12-5-4-11-13(14(12)26-17)16(22-7-21-11)23-10-3-2-8(18)6-9(10)19/h2-7,20H,1H3,(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113949
BindingDB Entry DOI: 10.7270/Q2M90DP7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50443513
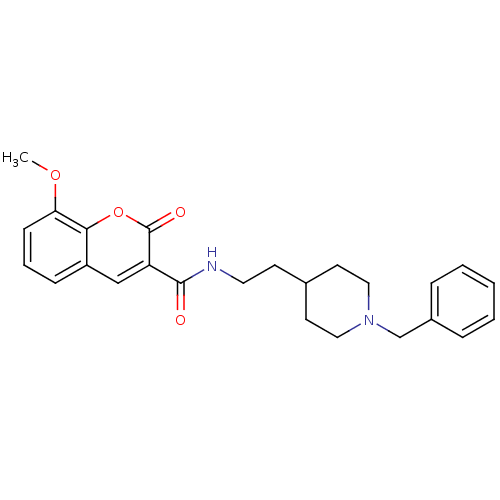
(CHEMBL3087903)Show SMILES COc1cccc2cc(C(=O)NCCC3CCN(Cc4ccccc4)CC3)c(=O)oc12 Show InChI InChI=1S/C25H28N2O4/c1-30-22-9-5-8-20-16-21(25(29)31-23(20)22)24(28)26-13-10-18-11-14-27(15-12-18)17-19-6-3-2-4-7-19/h2-9,16,18H,10-15,17H2,1H3,(H,26,28) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kerman University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate preincubated for 15 mins prior to substrate addition by Ellman's method |
Eur J Med Chem 70: 623-30 (2013)
Article DOI: 10.1016/j.ejmech.2013.10.024
BindingDB Entry DOI: 10.7270/Q2GQ7070 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50443518

(CHEMBL3087898)Show SMILES Brc1ccc2oc(=O)c(cc2c1)C(=O)NCCC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C24H25BrN2O3/c25-20-6-7-22-19(14-20)15-21(24(29)30-22)23(28)26-11-8-17-9-12-27(13-10-17)16-18-4-2-1-3-5-18/h1-7,14-15,17H,8-13,16H2,(H,26,28) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kerman University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate preincubated for 15 mins prior to substrate addition by Ellman's method |
Eur J Med Chem 70: 623-30 (2013)
Article DOI: 10.1016/j.ejmech.2013.10.024
BindingDB Entry DOI: 10.7270/Q2GQ7070 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50097456

(CHEMBL3585822)Show InChI InChI=1S/C17H16N4O4/c18-20-15(22)8-21-16(10-5-6-13-14(7-10)25-9-24-13)19-12-4-2-1-3-11(12)17(21)23/h1-7,16,19H,8-9,18H2,(H,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113949
BindingDB Entry DOI: 10.7270/Q2M90DP7 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50434364

(CHEMBL2386764 | US9446044, 65)Show InChI InChI=1S/C18H15N5OS/c1-10-3-5-11(6-4-10)22-17-14-12(20-9-21-17)7-8-13-15(14)25-18(23-13)16(19)24-2/h3-9,19H,1-2H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113949
BindingDB Entry DOI: 10.7270/Q2M90DP7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50052210

(CHEMBL3318398)Show SMILES [Cl-].Fc1cccc(Cl)c1C[n+]1ccc(\C=C2\C(=O)Nc3ccccc23)cc1 Show InChI InChI=1S/C21H14ClFN2O/c22-18-5-3-6-19(23)17(18)13-25-10-8-14(9-11-25)12-16-15-4-1-2-7-20(15)24-21(16)26/h1-12H,13H2/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman method based spectrophotometry |
Eur J Med Chem 84: 375-81 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.017
BindingDB Entry DOI: 10.7270/Q2H996T1 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50073207
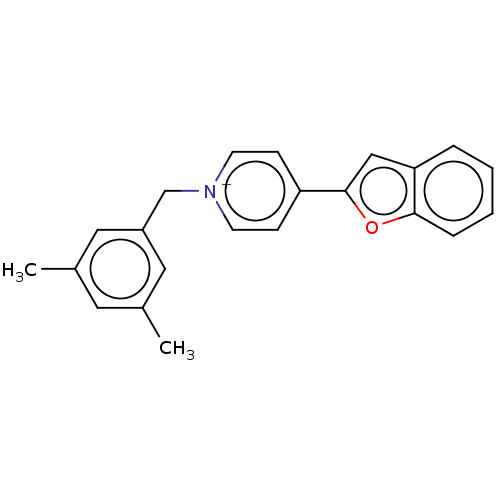
(CHEMBL3408398)Show SMILES [Br-].Cc1cc(C)cc(C[n+]2ccc(cc2)-c2cc3ccccc3o2)c1 Show InChI InChI=1S/C22H20NO.BrH/c1-16-11-17(2)13-18(12-16)15-23-9-7-19(8-10-23)22-14-20-5-3-4-6-21(20)24-22;/h3-14H,15H2,1-2H3;1H/q+1;/p-1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine substrate by Ellman's reagent based method |
Eur J Med Chem 93: 196-201 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.009
BindingDB Entry DOI: 10.7270/Q2CF9RTS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50052212

(CHEMBL3318400)Show SMILES [Br-].Clc1cccc(C[n+]2ccc(\C=C3\C(=O)Nc4ccccc34)cc2)c1 Show InChI InChI=1S/C21H15ClN2O/c22-17-5-3-4-16(12-17)14-24-10-8-15(9-11-24)13-19-18-6-1-2-7-20(18)23-21(19)25/h1-13H,14H2/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman method based spectrophotometry |
Eur J Med Chem 84: 375-81 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.017
BindingDB Entry DOI: 10.7270/Q2H996T1 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50073217
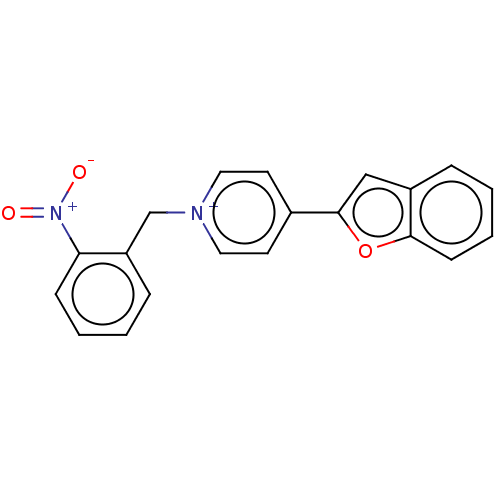
(CHEMBL3408242)Show SMILES [Br-].[O-][N+](=O)c1ccccc1C[n+]1ccc(cc1)-c1cc2ccccc2o1 Show InChI InChI=1S/C20H15N2O3/c23-22(24)18-7-3-1-6-17(18)14-21-11-9-15(10-12-21)20-13-16-5-2-4-8-19(16)25-20/h1-13H,14H2/q+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine substrate by Ellman's reagent based method |
Eur J Med Chem 93: 196-201 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.009
BindingDB Entry DOI: 10.7270/Q2CF9RTS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50204847

(CHEMBL3895802)Show SMILES Nc1c2CCCCc2nc2Oc3c(C(c4ccccc4)c12)c(nn3-c1ccccc1)-c1ccco1 Show InChI InChI=1S/C29H24N4O2/c30-26-20-14-7-8-15-21(20)31-28-24(26)23(18-10-3-1-4-11-18)25-27(22-16-9-17-34-22)32-33(29(25)35-28)19-12-5-2-6-13-19/h1-6,9-13,16-17,23H,7-8,14-15H2,(H2,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured for 1 ... |
Eur J Med Chem 123: 298-308 (2016)
Article DOI: 10.1016/j.ejmech.2016.07.043
BindingDB Entry DOI: 10.7270/Q2V40X55 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50443515

(CHEMBL3087901)Show SMILES COc1ccc2cc(C(=O)NCCC3CCN(Cc4ccccc4)CC3)c(=O)oc2c1 Show InChI InChI=1S/C25H28N2O4/c1-30-21-8-7-20-15-22(25(29)31-23(20)16-21)24(28)26-12-9-18-10-13-27(14-11-18)17-19-5-3-2-4-6-19/h2-8,15-16,18H,9-14,17H2,1H3,(H,26,28) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kerman University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate preincubated for 15 mins prior to substrate addition by Ellman's method |
Eur J Med Chem 70: 623-30 (2013)
Article DOI: 10.1016/j.ejmech.2013.10.024
BindingDB Entry DOI: 10.7270/Q2GQ7070 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50052214

(CHEMBL3318402)Show SMILES [Cl-].Cc1cccc(C[n+]2ccc(\C=C3\C(=O)Nc4ccccc34)cc2)c1 Show InChI InChI=1S/C22H18N2O.ClH/c1-16-5-4-6-18(13-16)15-24-11-9-17(10-12-24)14-20-19-7-2-3-8-21(19)23-22(20)25;/h2-14H,15H2,1H3;1H | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman method based spectrophotometry |
Eur J Med Chem 84: 375-81 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.017
BindingDB Entry DOI: 10.7270/Q2H996T1 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50594712
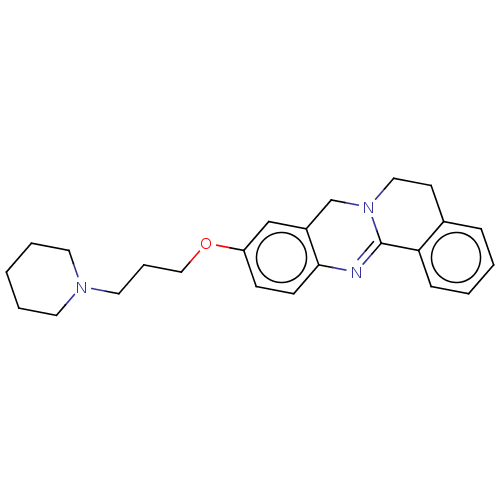
(CHEMBL5181664)Show SMILES C(COc1ccc2N=C3N(CCc4ccccc34)Cc2c1)CN1CCCCC1 |c:7| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113949
BindingDB Entry DOI: 10.7270/Q2M90DP7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50073208

(CHEMBL3408397)Show SMILES [Br-].[O-][N+](=O)c1ccc(C[n+]2ccc(cc2)-c2cc3ccccc3o2)cc1 Show InChI InChI=1S/C20H15N2O3.BrH/c23-22(24)18-7-5-15(6-8-18)14-21-11-9-16(10-12-21)20-13-17-3-1-2-4-19(17)25-20;/h1-13H,14H2;1H/q+1;/p-1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine substrate by Ellman's reagent based method |
Eur J Med Chem 93: 196-201 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.009
BindingDB Entry DOI: 10.7270/Q2CF9RTS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50204842
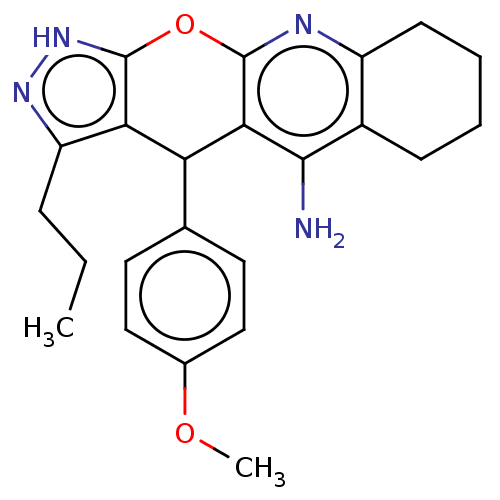
(CHEMBL3927698)Show SMILES CCCc1n[nH]c2Oc3nc4CCCCc4c(N)c3C(c12)c1ccc(OC)cc1 Show InChI InChI=1S/C23H26N4O2/c1-3-6-17-19-18(13-9-11-14(28-2)12-10-13)20-21(24)15-7-4-5-8-16(15)25-22(20)29-23(19)27-26-17/h9-12,18H,3-8H2,1-2H3,(H2,24,25)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured for 1 ... |
Eur J Med Chem 123: 298-308 (2016)
Article DOI: 10.1016/j.ejmech.2016.07.043
BindingDB Entry DOI: 10.7270/Q2V40X55 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50052215
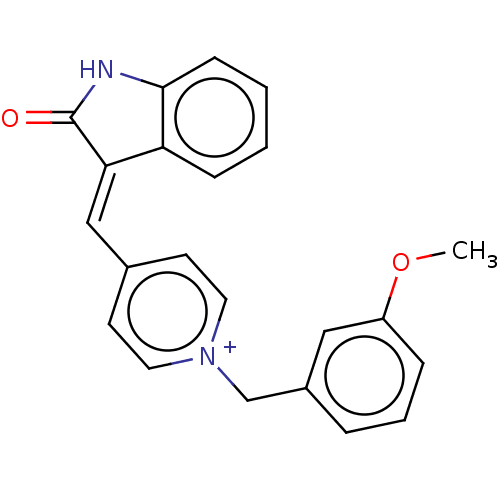
(CHEMBL3318403)Show SMILES [Cl-].COc1cccc(C[n+]2ccc(\C=C3\C(=O)Nc4ccccc34)cc2)c1 Show InChI InChI=1S/C22H18N2O2.ClH/c1-26-18-6-4-5-17(13-18)15-24-11-9-16(10-12-24)14-20-19-7-2-3-8-21(19)23-22(20)25;/h2-14H,15H2,1H3;1H | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman method based spectrophotometry |
Eur J Med Chem 84: 375-81 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.017
BindingDB Entry DOI: 10.7270/Q2H996T1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50594714
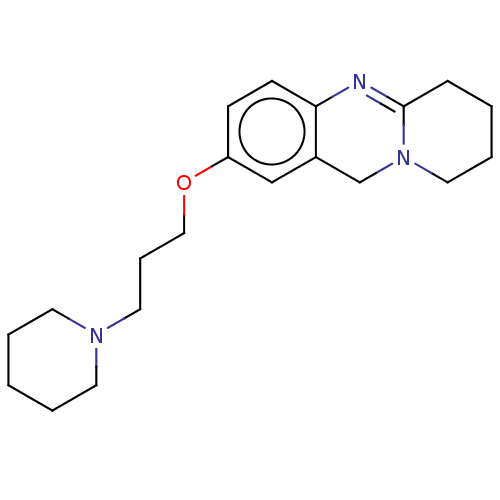
(CHEMBL5208725) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113949
BindingDB Entry DOI: 10.7270/Q2M90DP7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50073220
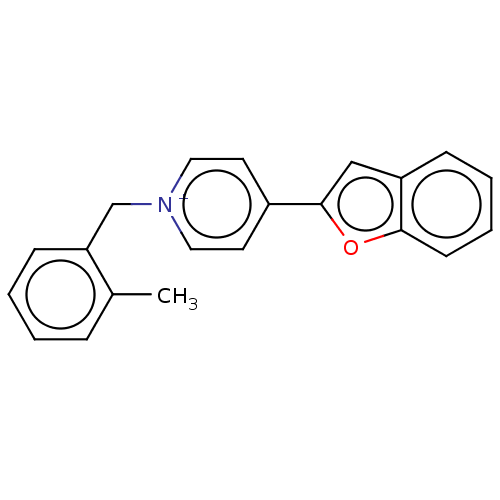
(CHEMBL3408241)Show SMILES [Br-].Cc1ccccc1C[n+]1ccc(cc1)-c1cc2ccccc2o1 Show InChI InChI=1S/C21H18NO/c1-16-6-2-3-8-19(16)15-22-12-10-17(11-13-22)21-14-18-7-4-5-9-20(18)23-21/h2-14H,15H2,1H3/q+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine substrate by Ellman's reagent based method |
Eur J Med Chem 93: 196-201 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.009
BindingDB Entry DOI: 10.7270/Q2CF9RTS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50594711

(CHEMBL5208529)Show SMILES C(COc1ccc2N=C3N(Cc4ccccc34)Cc2c1)CN1CCCCC1 |c:7| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113949
BindingDB Entry DOI: 10.7270/Q2M90DP7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50204851
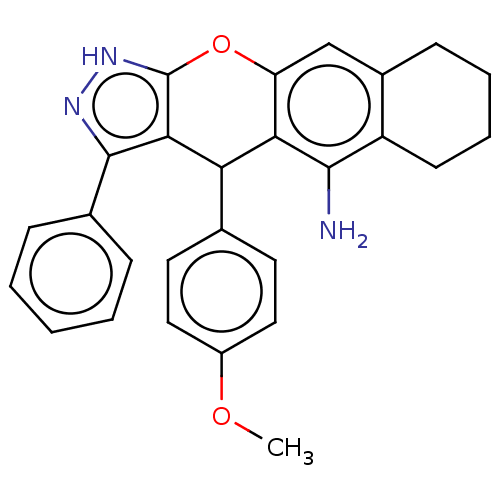
(CHEMBL3947445)Show SMILES COc1ccc(cc1)C1c2c(Oc3cc4CCCCc4c(N)c13)[nH]nc2-c1ccccc1 Show InChI InChI=1S/C27H25N3O2/c1-31-19-13-11-16(12-14-19)22-23-21(15-18-9-5-6-10-20(18)25(23)28)32-27-24(22)26(29-30-27)17-7-3-2-4-8-17/h2-4,7-8,11-15,22H,5-6,9-10,28H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured for 1 ... |
Eur J Med Chem 123: 298-308 (2016)
Article DOI: 10.1016/j.ejmech.2016.07.043
BindingDB Entry DOI: 10.7270/Q2V40X55 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data