 Found 198 hits with Last Name = 'erat' and Initial = 'm'
Found 198 hits with Last Name = 'erat' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
6-phosphogluconate dehydrogenase, decarboxylating
(Homo sapiens (Human)) | BDBM87064
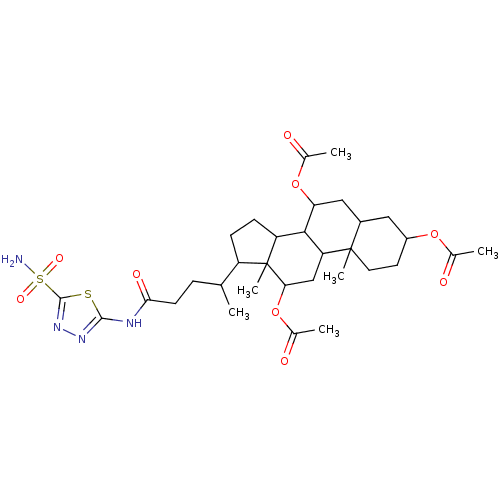
(Sulfonamide derivative, 5)Show SMILES CC(CCC(=O)Nc1nnc(s1)S(N)(=O)=O)C1CCC2C3C(CC4CC(CCC4(C)C3CC(OC(C)=O)C12C)OC(C)=O)OC(C)=O Show InChI InChI=1S/C32H48N4O9S2/c1-16(7-10-27(40)34-29-35-36-30(46-29)47(33,41)42)22-8-9-23-28-24(15-26(32(22,23)6)45-19(4)39)31(5)12-11-21(43-17(2)37)13-20(31)14-25(28)44-18(3)38/h16,20-26,28H,7-15H2,1-6H3,(H2,33,41,42)(H,34,35,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20E+3 | n/a | 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
| Assay Description
The enzyme activities in the absence of drugs or chemicals were taken as 100%. For each drug or chemical, an activity%-(drug) graph was drawn and dr... |
J Enzyme Inhib Med Chem 23: 418-23 (2008)
Article DOI: 10.1080/14756360701546413
BindingDB Entry DOI: 10.7270/Q2F18XBP |
More data for this
Ligand-Target Pair | |
Glutathione-disulfide reductase
(Escherichia coli) | BDBM87062
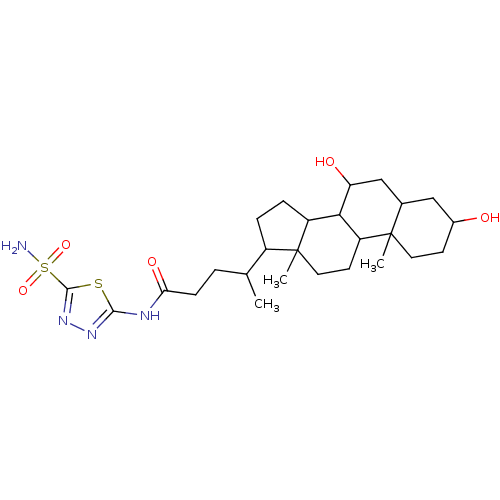
(Sulfonamide derivative, 2)Show SMILES CC(CCC(=O)Nc1nnc(s1)S(N)(=O)=O)C1CCC2C3C(O)CC4CC(O)CCC4(C)C3CCC12C Show InChI InChI=1S/C26H42N4O5S2/c1-14(4-7-21(33)28-23-29-30-24(36-23)37(27,34)35)17-5-6-18-22-19(9-11-26(17,18)3)25(2)10-8-16(31)12-15(25)13-20(22)32/h14-20,22,31-32H,4-13H2,1-3H3,(H2,27,34,35)(H,28,29,33) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10E+3 | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
| Assay Description
The enzyme activities in the absence of drugs or chemicals were taken as 100%. For each drug or chemical, an activity%-(drug) graph was drawn and dr... |
J Enzyme Inhib Med Chem 23: 418-23 (2008)
Article DOI: 10.1080/14756360701546413
BindingDB Entry DOI: 10.7270/Q2F18XBP |
More data for this
Ligand-Target Pair | |
Glutathione-disulfide reductase
(Escherichia coli) | BDBM87061
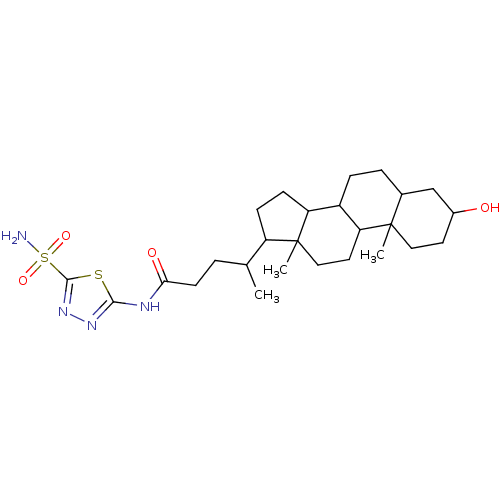
(Sulfonamide derivative, 1)Show SMILES CC(CCC(=O)Nc1nnc(s1)S(N)(=O)=O)C1CCC2C3CCC4CC(O)CCC4(C)C3CCC12C Show InChI InChI=1S/C26H42N4O4S2/c1-15(4-9-22(32)28-23-29-30-24(35-23)36(27,33)34)19-7-8-20-18-6-5-16-14-17(31)10-12-25(16,2)21(18)11-13-26(19,20)3/h15-21,31H,4-14H2,1-3H3,(H2,27,33,34)(H,28,29,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.23E+4 | n/a | 4.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
| Assay Description
The enzyme activities in the absence of drugs or chemicals were taken as 100%. For each drug or chemical, an activity%-(drug) graph was drawn and dr... |
J Enzyme Inhib Med Chem 23: 418-23 (2008)
Article DOI: 10.1080/14756360701546413
BindingDB Entry DOI: 10.7270/Q2F18XBP |
More data for this
Ligand-Target Pair | |
6-phosphogluconate dehydrogenase, decarboxylating
(Homo sapiens (Human)) | BDBM87063
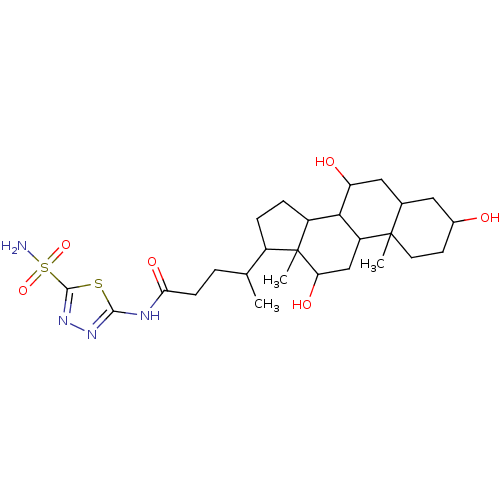
(Sulfonamide derivative, 3)Show SMILES CC(CCC(=O)Nc1nnc(s1)S(N)(=O)=O)C1CCC2C3C(O)CC4CC(O)CCC4(C)C3CC(O)C12C Show InChI InChI=1S/C26H42N4O6S2/c1-13(4-7-21(34)28-23-29-30-24(37-23)38(27,35)36)16-5-6-17-22-18(12-20(33)26(16,17)3)25(2)9-8-15(31)10-14(25)11-19(22)32/h13-20,22,31-33H,4-12H2,1-3H3,(H2,27,35,36)(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.78E+4 | n/a | 6.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
| Assay Description
The enzyme activities in the absence of drugs or chemicals were taken as 100%. For each drug or chemical, an activity%-(drug) graph was drawn and dr... |
J Enzyme Inhib Med Chem 23: 418-23 (2008)
Article DOI: 10.1080/14756360701546413
BindingDB Entry DOI: 10.7270/Q2F18XBP |
More data for this
Ligand-Target Pair | |
6-phosphogluconate dehydrogenase, decarboxylating
(Homo sapiens (Human)) | BDBM50043906
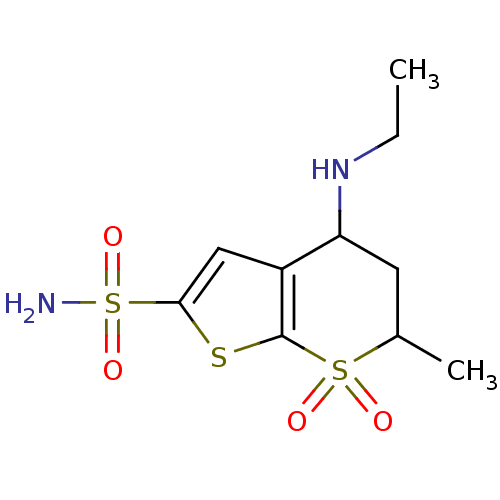
(CHEMBL269001 | Dorzolamide | MK-507 | US10172837, ...)Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.14E+6 | n/a | 1.41E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
| Assay Description
The enzyme activities in the absence of drugs or chemicals were taken as 100%. For each drug or chemical, an activity%-(drug) graph was drawn and dr... |
J Enzyme Inhib Med Chem 23: 418-23 (2008)
Article DOI: 10.1080/14756360701546413
BindingDB Entry DOI: 10.7270/Q2F18XBP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588566
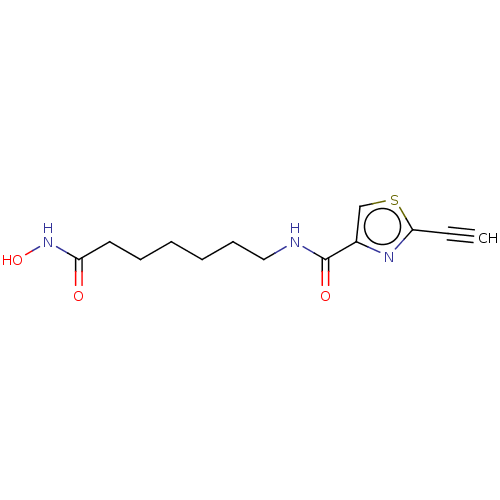
(CHEMBL5209294) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588561
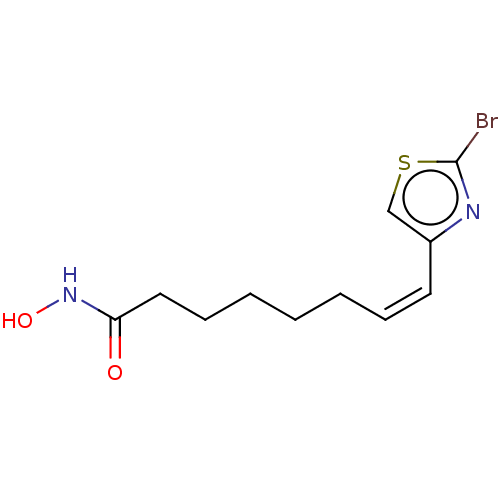
(CHEMBL5193020) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588560
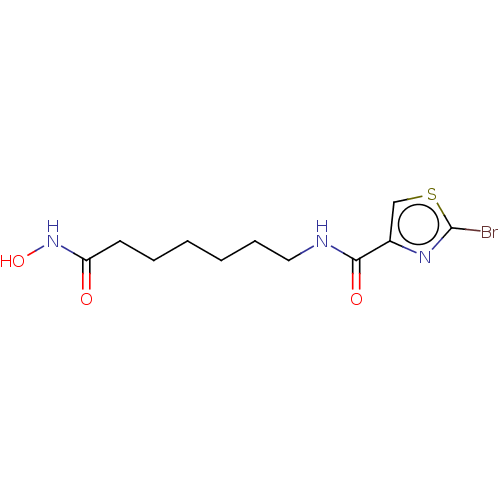
(CHEMBL5172507) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223720
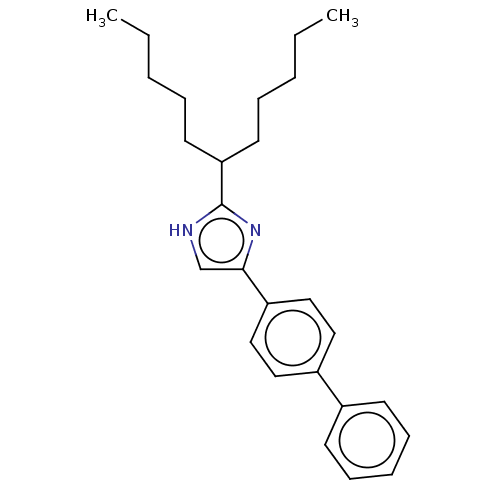
(CHEMBL332257)Show SMILES CCCCCC(CCCCC)c1nc(c[nH]1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C26H34N2/c1-3-5-8-14-24(15-9-6-4-2)26-27-20-25(28-26)23-18-16-22(17-19-23)21-12-10-7-11-13-21/h7,10-13,16-20,24H,3-6,8-9,14-15H2,1-2H3,(H,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588568
(CHEMBL5186798) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Competitive inhibition of androgen binding to androgen receptor (unknown origin) by invitrogen polar screen assay |
J Med Chem 58: 6984-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00867
BindingDB Entry DOI: 10.7270/Q2GF0W97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223731
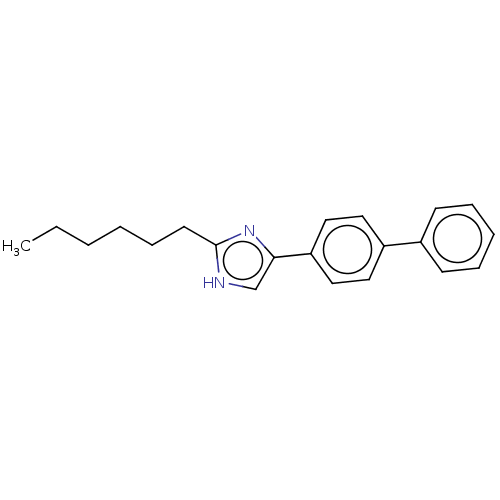
(CHEMBL118603)Show InChI InChI=1S/C21H24N2/c1-2-3-4-8-11-21-22-16-20(23-21)19-14-12-18(13-15-19)17-9-6-5-7-10-17/h5-7,9-10,12-16H,2-4,8,11H2,1H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223721
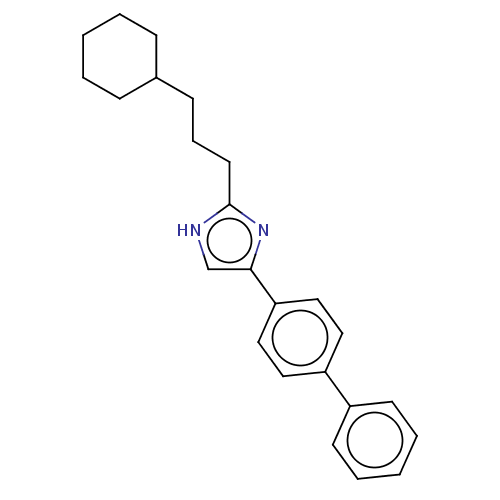
(CHEMBL331513)Show SMILES C(CC1CCCCC1)Cc1nc(c[nH]1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C24H28N2/c1-3-8-19(9-4-1)10-7-13-24-25-18-23(26-24)22-16-14-21(15-17-22)20-11-5-2-6-12-20/h2,5-6,11-12,14-19H,1,3-4,7-10,13H2,(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50550344

(CHEMBL4798363)Show SMILES CCCCCCCC(=O)SCC\C=C\[C@@H]1CC(=O)NCc2cccc(n2)-c2cccc(n2)C(=O)N[C@@H](C(C)C)C(=O)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant HDAC1 (unknown origin) incubated for 15 mins before substrate addition and measured after 30 to 45 mins by HDAC-Glo substra... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VH5SG7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588563
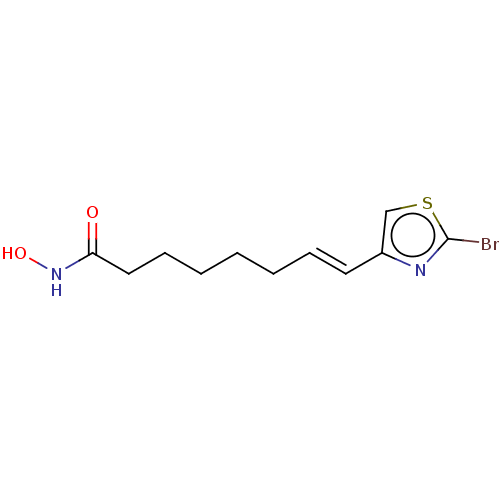
(CHEMBL5174162) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588567

(CHEMBL5181798) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223726
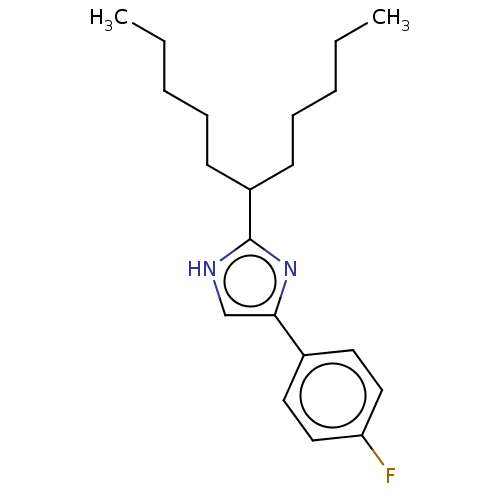
(CHEMBL117331)Show InChI InChI=1S/C20H29FN2/c1-3-5-7-9-17(10-8-6-4-2)20-22-15-19(23-20)16-11-13-18(21)14-12-16/h11-15,17H,3-10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223735
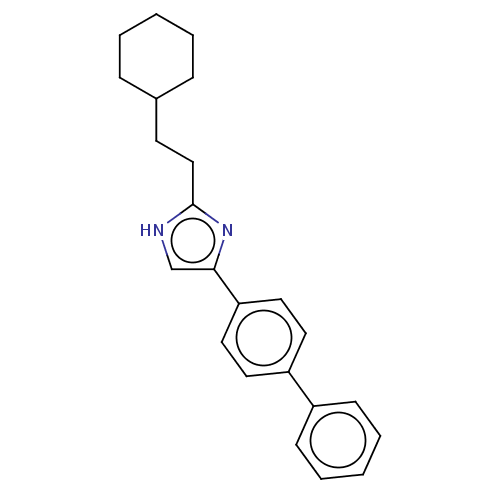
(CHEMBL119139)Show SMILES C(Cc1nc(c[nH]1)-c1ccc(cc1)-c1ccccc1)C1CCCCC1 Show InChI InChI=1S/C23H26N2/c1-3-7-18(8-4-1)11-16-23-24-17-22(25-23)21-14-12-20(13-15-21)19-9-5-2-6-10-19/h2,5-6,9-10,12-15,17-18H,1,3-4,7-8,11,16H2,(H,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50550344

(CHEMBL4798363)Show SMILES CCCCCCCC(=O)SCC\C=C\[C@@H]1CC(=O)NCc2cccc(n2)-c2cccc(n2)C(=O)N[C@@H](C(C)C)C(=O)O1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant HDAC3 (unknown origin)incubated for 15 mins before substrate addition and measured after 30 to 45 mins by HDAC-Glo substrat... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VH5SG7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50550344

(CHEMBL4798363)Show SMILES CCCCCCCC(=O)SCC\C=C\[C@@H]1CC(=O)NCc2cccc(n2)-c2cccc(n2)C(=O)N[C@@H](C(C)C)C(=O)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant HDAC2 (unknown origin)incubated for 15 mins before substrate addition and measured after 30 to 45 mins by HDAC-Glo substrat... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VH5SG7 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223734
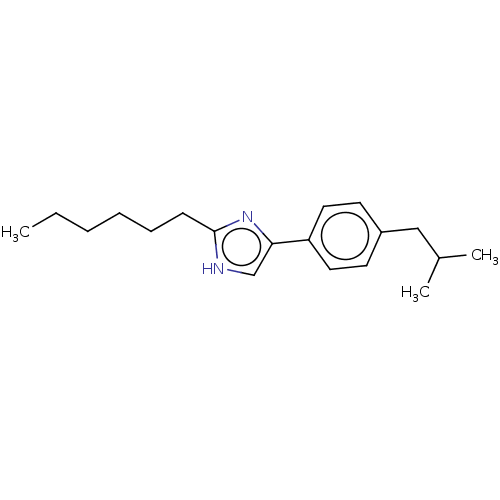
(CHEMBL331942)Show InChI InChI=1S/C19H28N2/c1-4-5-6-7-8-19-20-14-18(21-19)17-11-9-16(10-12-17)13-15(2)3/h9-12,14-15H,4-8,13H2,1-3H3,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223733
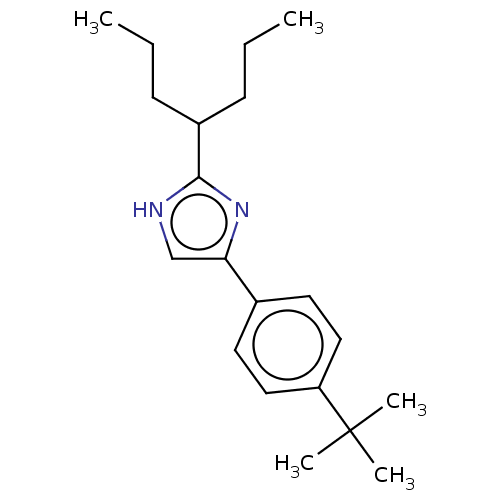
(CHEMBL120306)Show InChI InChI=1S/C20H30N2/c1-6-8-16(9-7-2)19-21-14-18(22-19)15-10-12-17(13-11-15)20(3,4)5/h10-14,16H,6-9H2,1-5H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50380399

(CHEMBL2018302 | Tubastatin A | US10227295, Compoun...)Show InChI InChI=1S/C20H21N3O2/c1-22-11-10-19-17(13-22)16-4-2-3-5-18(16)23(19)12-14-6-8-15(9-7-14)20(24)21-25/h2-9,25H,10-13H2,1H3,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50322422
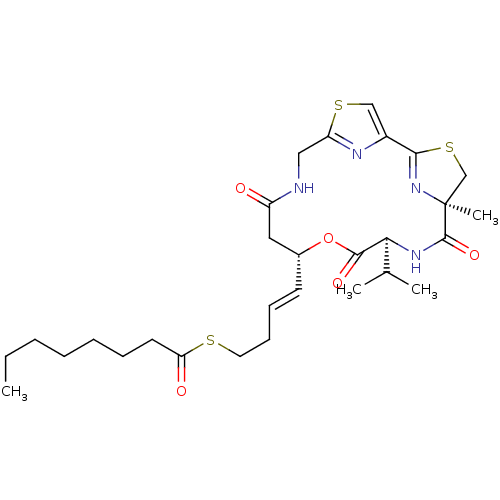
(CHEMBL1173445 | Largazole)Show SMILES CCCCCCCC(=O)SCC\C=C\[C@@H]1CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@@H](C(C)C)C(=O)O1 |r,t:26| Show InChI InChI=1S/C29H42N4O5S3/c1-5-6-7-8-9-13-24(35)39-14-11-10-12-20-15-22(34)30-16-23-31-21(17-40-23)26-33-29(4,18-41-26)28(37)32-25(19(2)3)27(36)38-20/h10,12,17,19-20,25H,5-9,11,13-16,18H2,1-4H3,(H,30,34)(H,32,37)/b12-10+/t20-,25+,29+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant HDAC3 (unknown origin)incubated for 15 mins before substrate addition and measured after 30 to 45 mins by HDAC-Glo substrat... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VH5SG7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50588566

(CHEMBL5209294) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50588560

(CHEMBL5172507) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50588560

(CHEMBL5172507) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50322422

(CHEMBL1173445 | Largazole)Show SMILES CCCCCCCC(=O)SCC\C=C\[C@@H]1CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@@H](C(C)C)C(=O)O1 |r,t:26| Show InChI InChI=1S/C29H42N4O5S3/c1-5-6-7-8-9-13-24(35)39-14-11-10-12-20-15-22(34)30-16-23-31-21(17-40-23)26-33-29(4,18-41-26)28(37)32-25(19(2)3)27(36)38-20/h10,12,17,19-20,25H,5-9,11,13-16,18H2,1-4H3,(H,30,34)(H,32,37)/b12-10+/t20-,25+,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant HDAC1 (unknown origin) incubated for 15 mins before substrate addition and measured after 30 to 45 mins by HDAC-Glo substra... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VH5SG7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50322422

(CHEMBL1173445 | Largazole)Show SMILES CCCCCCCC(=O)SCC\C=C\[C@@H]1CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@@H](C(C)C)C(=O)O1 |r,t:26| Show InChI InChI=1S/C29H42N4O5S3/c1-5-6-7-8-9-13-24(35)39-14-11-10-12-20-15-22(34)30-16-23-31-21(17-40-23)26-33-29(4,18-41-26)28(37)32-25(19(2)3)27(36)38-20/h10,12,17,19-20,25H,5-9,11,13-16,18H2,1-4H3,(H,30,34)(H,32,37)/b12-10+/t20-,25+,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant HDAC2 (unknown origin)incubated for 15 mins before substrate addition and measured after 30 to 45 mins by HDAC-Glo substrat... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VH5SG7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50588566

(CHEMBL5209294) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223722
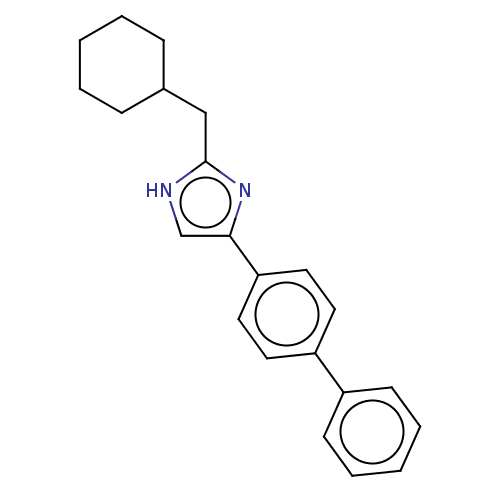
(CHEMBL334104)Show SMILES C(C1CCCCC1)c1nc(c[nH]1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H24N2/c1-3-7-17(8-4-1)15-22-23-16-21(24-22)20-13-11-19(12-14-20)18-9-5-2-6-10-18/h2,5-6,9-14,16-17H,1,3-4,7-8,15H2,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50588568

(CHEMBL5186798) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50588560

(CHEMBL5172507) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223723
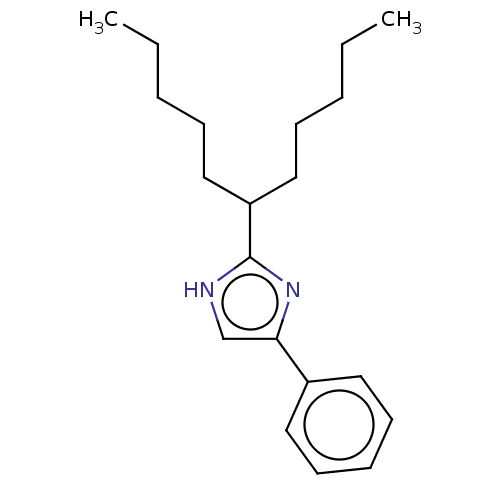
(CHEMBL119494)Show InChI InChI=1S/C20H30N2/c1-3-5-8-14-18(15-9-6-4-2)20-21-16-19(22-20)17-12-10-7-11-13-17/h7,10-13,16,18H,3-6,8-9,14-15H2,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50588568

(CHEMBL5186798) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50588566

(CHEMBL5209294) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223725
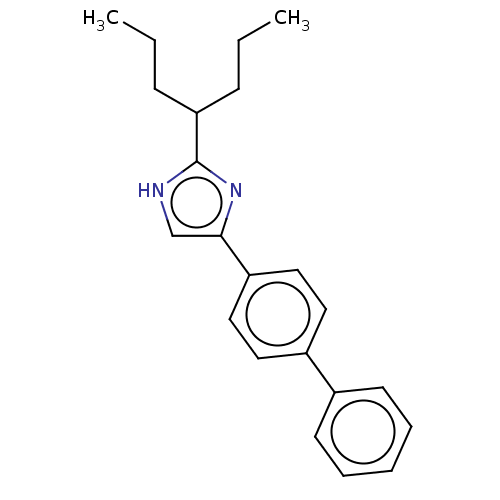
(CHEMBL118682)Show InChI InChI=1S/C22H26N2/c1-3-8-20(9-4-2)22-23-16-21(24-22)19-14-12-18(13-15-19)17-10-6-5-7-11-17/h5-7,10-16,20H,3-4,8-9H2,1-2H3,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50588568

(CHEMBL5186798) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50588567

(CHEMBL5181798) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223729
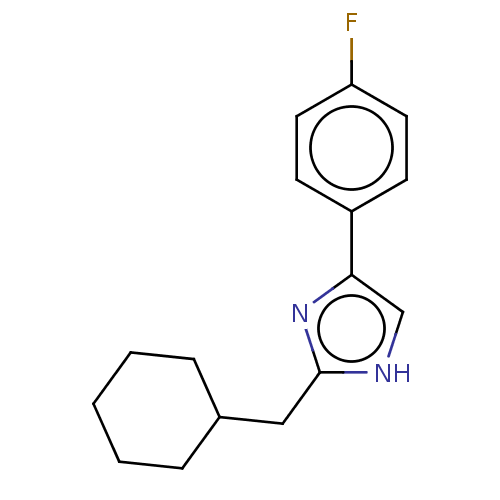
(CHEMBL418863)Show InChI InChI=1S/C16H19FN2/c17-14-8-6-13(7-9-14)15-11-18-16(19-15)10-12-4-2-1-3-5-12/h6-9,11-12H,1-5,10H2,(H,18,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588564
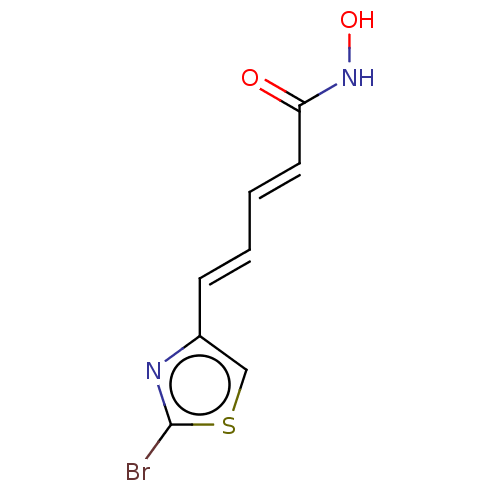
(CHEMBL5206154) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588569
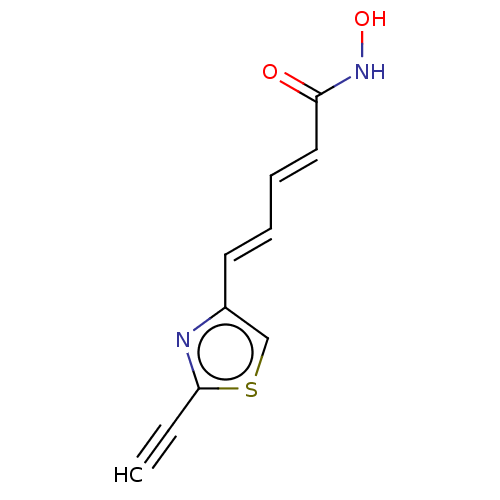
(CHEMBL5170140) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588570
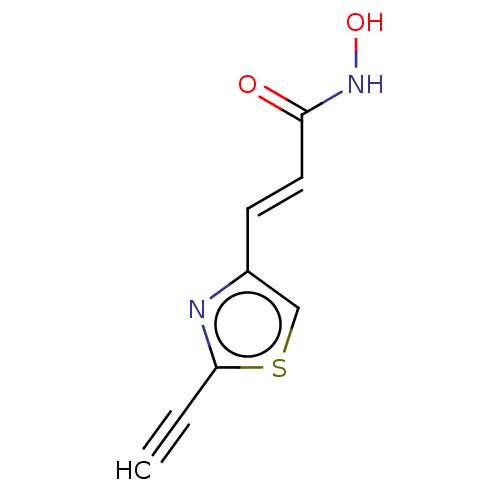
(CHEMBL5181479) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588565
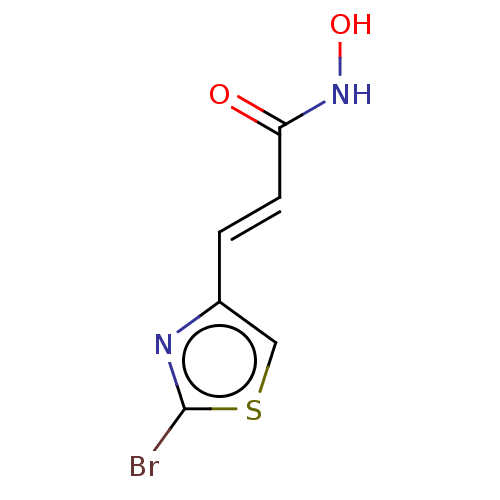
(CHEMBL5204550) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50588563

(CHEMBL5174162) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50588560

(CHEMBL5172507) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01276
BindingDB Entry DOI: 10.7270/Q2C53QTB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data