

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50048908 (CHEMBL415788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS1 receptor expressed in CHOK1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50048908 (CHEMBL415788) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS2 receptor expressed in human 1321N1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485783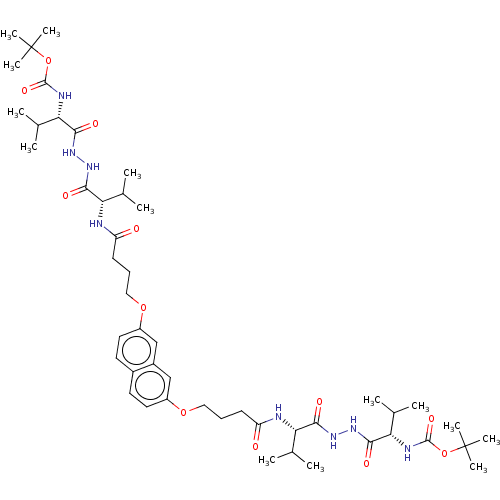 (CHEMBL2164408) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11 Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... | J Med Chem 55: 6762-75 (2012) Article DOI: 10.1021/jm300181j BindingDB Entry DOI: 10.7270/Q2125WJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50265841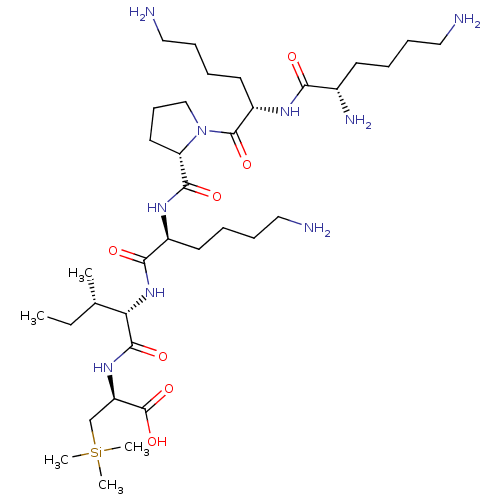 (CHEMBL4100456) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS2 receptor expressed in human 1321N1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485784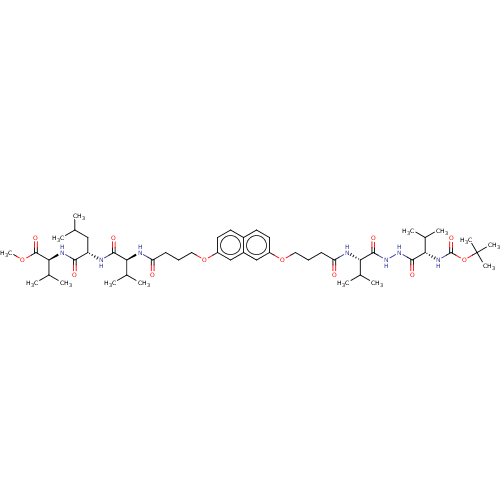 (CHEMBL2164407) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11 Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... | J Med Chem 55: 6762-75 (2012) Article DOI: 10.1021/jm300181j BindingDB Entry DOI: 10.7270/Q2125WJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485785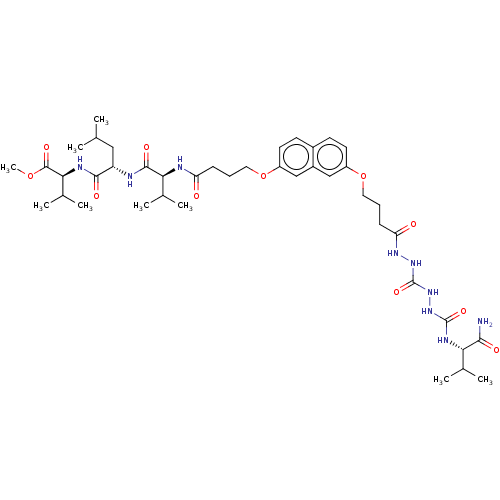 (CHEMBL2164409) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11 Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... | J Med Chem 55: 6762-75 (2012) Article DOI: 10.1021/jm300181j BindingDB Entry DOI: 10.7270/Q2125WJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50265836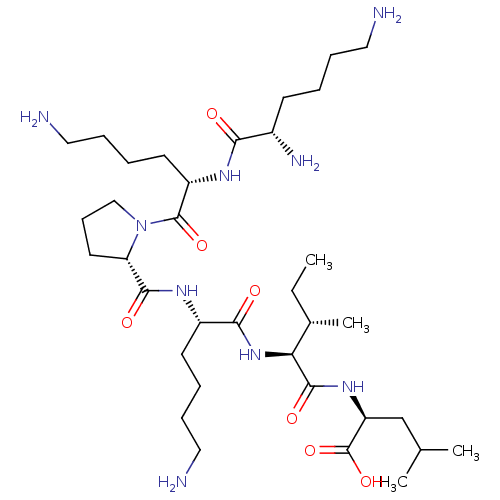 (CHEMBL4084378) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 262 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS2 receptor expressed in human 1321N1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50265835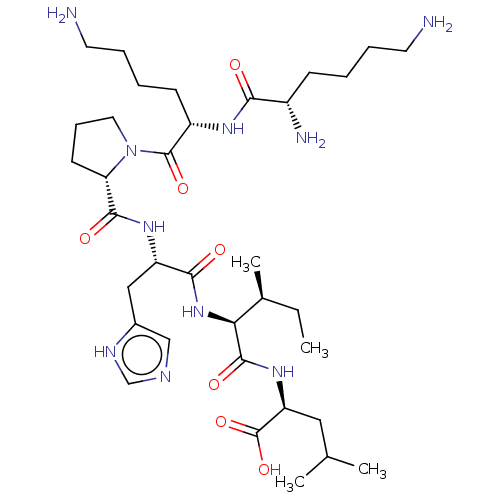 (CHEMBL4076517) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 456 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS1 receptor expressed in CHOK1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50265835 (CHEMBL4076517) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 474 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS2 receptor expressed in human 1321N1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50074687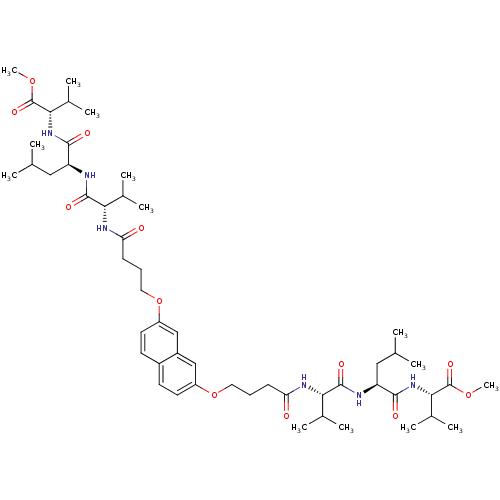 (2-[2-(2-{4-[7-(3-{1-[1-(1-Methoxycarbonyl-2-methyl...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11 Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... | J Med Chem 55: 6762-75 (2012) Article DOI: 10.1021/jm300181j BindingDB Entry DOI: 10.7270/Q2125WJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50265842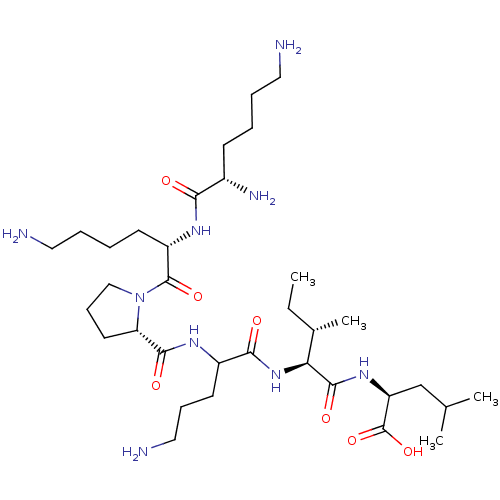 (CHEMBL4103884) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS2 receptor expressed in human 1321N1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50265841 (CHEMBL4100456) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 663 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS1 receptor expressed in CHOK1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485786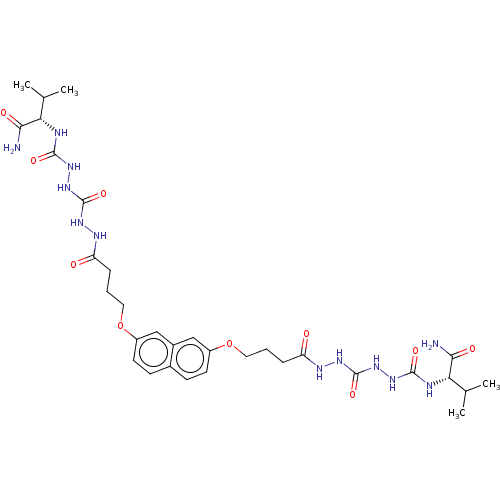 (CHEMBL2164074) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11 Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... | J Med Chem 55: 6762-75 (2012) Article DOI: 10.1021/jm300181j BindingDB Entry DOI: 10.7270/Q2125WJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50265833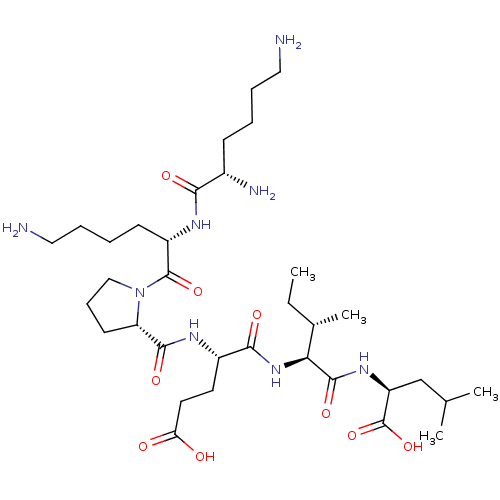 (CHEMBL4082440) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS2 receptor expressed in human 1321N1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50265834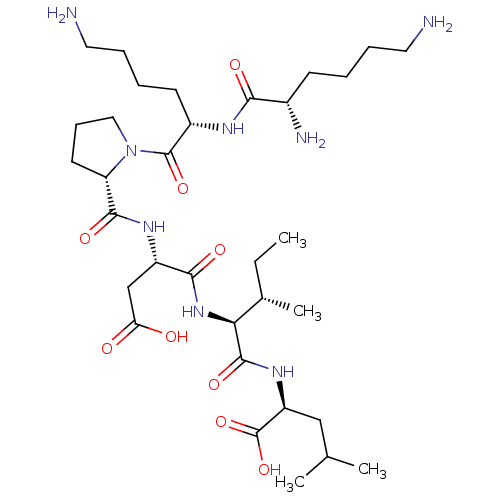 (CHEMBL4074624) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS2 receptor expressed in human 1321N1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50265836 (CHEMBL4084378) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS1 receptor expressed in CHOK1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50265833 (CHEMBL4082440) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS1 receptor expressed in CHOK1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50265842 (CHEMBL4103884) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS1 receptor expressed in CHOK1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50265834 (CHEMBL4074624) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS1 receptor expressed in CHOK1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50124132 (CHEMBL3622802) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr3-NT from human NTS1 receptor expressed in CHOK1 cell membranes incubated for 30 mins by gamma-counting based competitive r... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50124132 (CHEMBL3622802) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr3-NT from human NTS2 receptor expressed in 1321N1 cell membranes incubated for 30 mins by gamma-counting based competitive ... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50342242 ((S)-2-((2S,3S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS1 receptor | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50048908 (CHEMBL415788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr3-NT from human NTS1 receptor expressed in CHOK1 cell membranes incubated for 30 mins by gamma-counting based competitive r... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50342242 ((S)-2-((2S,3S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS2 receptor | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50048908 (CHEMBL415788) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr3-NT from human NTS2 receptor expressed in 1321N1 cell membranes incubated for 30 mins by gamma-counting based competitive ... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50124135 (CHEMBL3622803) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr3-NT from human NTS1 receptor expressed in CHOK1 cell membranes incubated for 30 mins by gamma-counting based competitive r... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50124143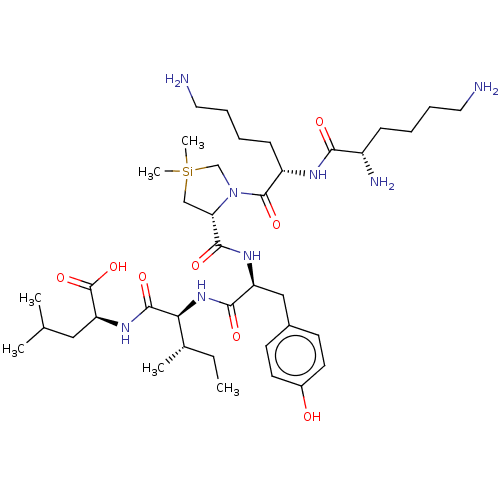 (CHEMBL3622806) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr3-NT from human NTS1 receptor expressed in CHOK1 cell membranes incubated for 30 mins by gamma-counting based competitive r... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50124143 (CHEMBL3622806) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr3-NT from human NTS2 receptor expressed in 1321N1 cell membranes incubated for 30 mins by gamma-counting based competitive ... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50124135 (CHEMBL3622803) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr3-NT from human NTS2 receptor expressed in 1321N1 cell membranes incubated for 30 mins by gamma-counting based competitive ... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50124141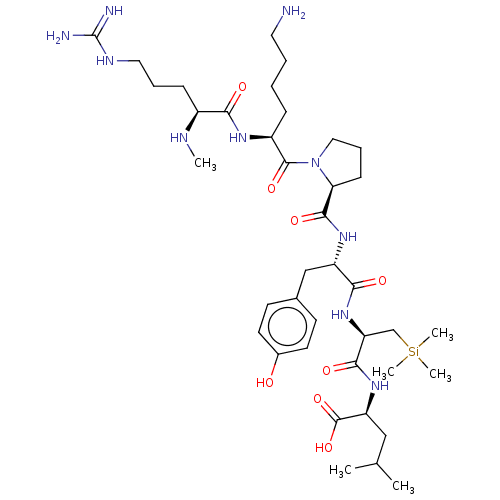 (CHEMBL3622805) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr3-NT from human NTS2 receptor expressed in 1321N1 cell membranes incubated for 30 mins by gamma-counting based competitive ... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50124146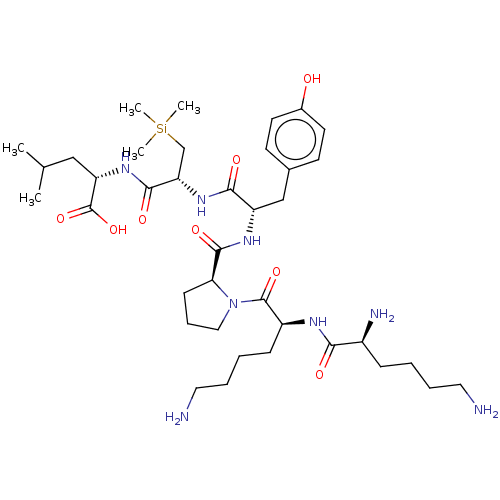 (CHEMBL3622801) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr3-NT from human NTS1 receptor expressed in CHOK1 cell membranes incubated for 30 mins by gamma-counting based competitive r... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50124141 (CHEMBL3622805) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr3-NT from human NTS1 receptor expressed in CHOK1 cell membranes incubated for 30 mins by gamma-counting based competitive r... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50124146 (CHEMBL3622801) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 405 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr3-NT from human NTS2 receptor expressed in 1321N1 cell membranes incubated for 30 mins by gamma-counting based competitive ... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50124138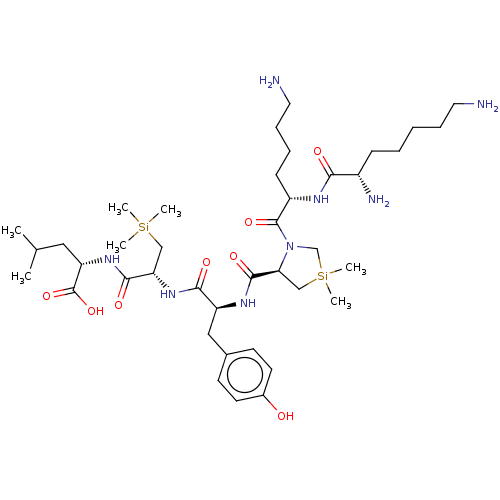 (CHEMBL3622804) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 968 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr3-NT from human NTS1 receptor expressed in CHOK1 cell membranes incubated for 30 mins by gamma-counting based competitive r... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50124138 (CHEMBL3622804) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr3-NT from human NTS2 receptor expressed in 1321N1 cell membranes incubated for 30 mins by gamma-counting based competitive ... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50124146 (CHEMBL3622801) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 receptor expressed in CHOK1 cells co-expressing hNTS1-GFP10/RlucII-beta-arrestin 2 assessed as beta-arrestin2 recruitm... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50048908 (CHEMBL415788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 receptor expressed in CHOK1 cells co-expressing hNTS1-GFP10/RlucII-beta-arrestin 2 assessed as beta-arrestin2 recruitm... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50124141 (CHEMBL3622805) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 204 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 receptor expressed in CHOK1 cells co-expressing Galphaq-RlucII(121)/Gbeta1/GFP10-Ggamma1 assessed as Galpha-q stimulat... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50124138 (CHEMBL3622804) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 receptor expressed in CHOK1 cells co-expressing Galphaq-RlucII(121)/Gbeta1/GFP10-Ggamma1 assessed as Galpha-q stimulat... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50124135 (CHEMBL3622803) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 receptor expressed in CHOK1 cells co-expressing Galphaq-RlucII(121)/Gbeta1/GFP10-Ggamma1 assessed as Galpha-q stimulat... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50124132 (CHEMBL3622802) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 receptor expressed in CHOK1 cells co-expressing Galphaq-RlucII(121)/Gbeta1/GFP10-Ggamma1 assessed as Galpha-q stimulat... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50124146 (CHEMBL3622801) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 169 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 receptor expressed in CHOK1 cells co-expressing Galphaq-RlucII(121)/Gbeta1/GFP10-Ggamma1 assessed as Galpha-q stimulat... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50048908 (CHEMBL415788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 receptor expressed in CHOK1 cells co-expressing Galphaq-RlucII(121)/Gbeta1/GFP10-Ggamma1 assessed as Galpha-q stimulat... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50124141 (CHEMBL3622805) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 in Sprague-Dawley rat ileum assessed as inhibition of carbachol-induced contraction | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50124135 (CHEMBL3622803) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 receptor expressed in CHOK1 cells co-expressing hNTS1-GFP10/RlucII-beta-arrestin 2 assessed as beta-arrestin2 recruitm... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50124138 (CHEMBL3622804) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 receptor expressed in CHOK1 cells co-expressing hNTS1-GFP10/RlucII-beta-arrestin 2 assessed as beta-arrestin2 recruitm... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50124141 (CHEMBL3622805) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 receptor expressed in CHOK1 cells co-expressing hNTS1-GFP10/RlucII-beta-arrestin 2 assessed as beta-arrestin2 recruitm... | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50048908 (CHEMBL415788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 in Sprague-Dawley rat ileum assessed as inhibition of carbachol-induced contraction | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50124146 (CHEMBL3622801) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 in Sprague-Dawley rat ileum assessed as inhibition of carbachol-induced contraction | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50124132 (CHEMBL3622802) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 in Sprague-Dawley rat ileum assessed as inhibition of carbachol-induced contraction | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 53 total ) | Next | Last >> |