 Found 171 hits with Last Name = 'feoli' and Initial = 'a'
Found 171 hits with Last Name = 'feoli' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50526221
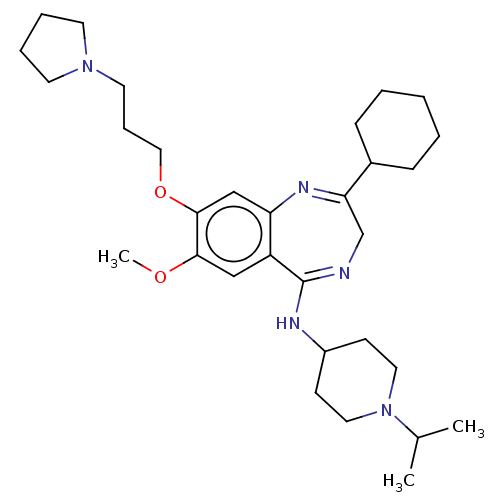
(CHEMBL4454542)Show SMILES COc1cc2c(cc1OCCCN1CCCC1)N=C(CN=C2NC1CCN(CC1)C(C)C)C1CCCCC1 |c:19,22| Show InChI InChI=1S/C31H49N5O2/c1-23(2)36-17-12-25(13-18-36)33-31-26-20-29(37-3)30(38-19-9-16-35-14-7-8-15-35)21-27(26)34-28(22-32-31)24-10-5-4-6-11-24/h20-21,23-25H,4-19,22H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human G9a using biotinylated-Histone H3 peptide (1 to 21 residues) as substrate after 30 mins in presence of va... |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50526221

(CHEMBL4454542)Show SMILES COc1cc2c(cc1OCCCN1CCCC1)N=C(CN=C2NC1CCN(CC1)C(C)C)C1CCCCC1 |c:19,22| Show InChI InChI=1S/C31H49N5O2/c1-23(2)36-17-12-25(13-18-36)33-31-26-20-29(37-3)30(38-19-9-16-35-14-7-8-15-35)21-27(26)34-28(22-32-31)24-10-5-4-6-11-24/h20-21,23-25H,4-19,22H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of recombinant human G9a using biotinylated-Histone H3 peptide (1 to 21 residues) as substrate at varying concentration af... |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50081125
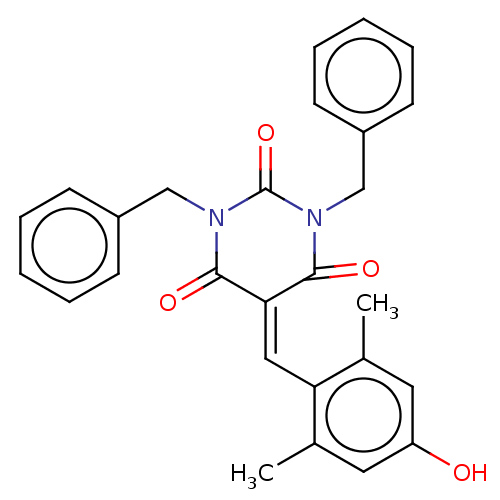
(CHEMBL3421961)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1\[#6]=[#6]-1/[#6](=O)-[#7](-[#6]-c2ccccc2)-[#6](=O)-[#7](-[#6]-c2ccccc2)-[#6]-1=O Show InChI InChI=1S/C27H24N2O4/c1-18-13-22(30)14-19(2)23(18)15-24-25(31)28(16-20-9-5-3-6-10-20)27(33)29(26(24)32)17-21-11-7-4-8-12-21/h3-15,30H,16-17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of P300 (unknown origin) using acetyl CoA as substrate after 15 mins by double reciprocal plot analysis |
J Med Chem 58: 2779-98 (2015)
Article DOI: 10.1021/jm5019687
BindingDB Entry DOI: 10.7270/Q2736SMP |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50081125

(CHEMBL3421961)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1\[#6]=[#6]-1/[#6](=O)-[#7](-[#6]-c2ccccc2)-[#6](=O)-[#7](-[#6]-c2ccccc2)-[#6]-1=O Show InChI InChI=1S/C27H24N2O4/c1-18-13-22(30)14-19(2)23(18)15-24-25(31)28(16-20-9-5-3-6-10-20)27(33)29(26(24)32)17-21-11-7-4-8-12-21/h3-15,30H,16-17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of P300 (unknown origin) using biotinylated H3 as substrate after 15 mins by double reciprocal plot analysis |
J Med Chem 58: 2779-98 (2015)
Article DOI: 10.1021/jm5019687
BindingDB Entry DOI: 10.7270/Q2736SMP |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50598022
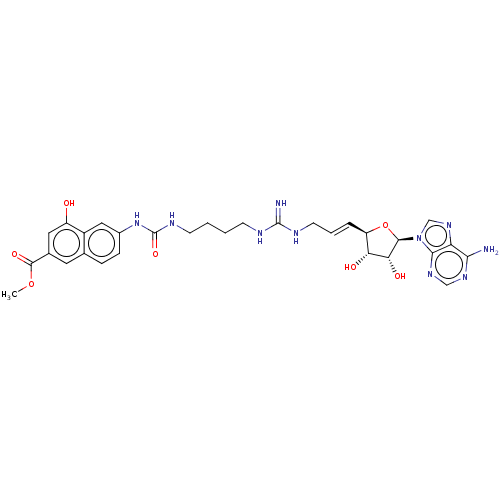
(CHEMBL5173678)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCCNC(=N)NC\C=C\[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)ccc2c1 |r| | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50598021

(CHEMBL5191769)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCCNC(=N)NCCC[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)ccc2c1 |r| | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50598020

(CHEMBL5202088)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCCNC(=N)NCC[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)ccc2c1 |r| | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8296
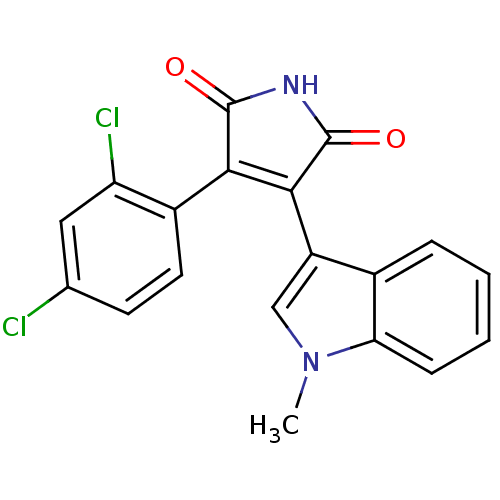
(3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2ccc(Cl)cc2Cl)c2ccccc12 |t:4| Show InChI InChI=1S/C19H12Cl2N2O2/c1-23-9-13(11-4-2-3-5-15(11)23)17-16(18(24)22-19(17)25)12-7-6-10(20)8-14(12)21/h2-9H,1H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) pre-incubated for 30 mins followed by ULight-GS (Ser641/pSer657) peptide substrate addition and measured afte... |
J Med Chem 61: 7640-7656 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00610
BindingDB Entry DOI: 10.7270/Q20Z75TR |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8296

(3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2ccc(Cl)cc2Cl)c2ccccc12 |t:4| Show InChI InChI=1S/C19H12Cl2N2O2/c1-23-9-13(11-4-2-3-5-15(11)23)17-16(18(24)22-19(17)25)12-7-6-10(20)8-14(12)21/h2-9H,1H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) pre-incubated for 30 mins followed by ULight-GS (Ser641/pSer657) peptide substrate addition and measured afte... |
Eur J Med Chem 163: 394-402 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.003
BindingDB Entry DOI: 10.7270/Q26W9FHZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8296

(3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2ccc(Cl)cc2Cl)c2ccccc12 |t:4| Show InChI InChI=1S/C19H12Cl2N2O2/c1-23-9-13(11-4-2-3-5-15(11)23)17-16(18(24)22-19(17)25)12-7-6-10(20)8-14(12)21/h2-9H,1H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) pre-incubated for 30 mins followed by ULight-GS (Ser641/pSer657) peptide substrate addition and measured afte... |
Eur J Med Chem 163: 394-402 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.003
BindingDB Entry DOI: 10.7270/Q26W9FHZ |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition G9a (unknown origin) |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT1
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of GLP (unknown origin) |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50526221

(CHEMBL4454542)Show SMILES COc1cc2c(cc1OCCCN1CCCC1)N=C(CN=C2NC1CCN(CC1)C(C)C)C1CCCCC1 |c:19,22| Show InChI InChI=1S/C31H49N5O2/c1-23(2)36-17-12-25(13-18-36)33-31-26-20-29(37-3)30(38-19-9-16-35-14-7-8-15-35)21-27(26)34-28(22-32-31)24-10-5-4-6-11-24/h20-21,23-25H,4-19,22H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human G9a using biotinylated-Histone H3 peptide (1 to 21 residues) after 30 mins in presence of SAM by AlphaLISA assay |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human G9a using biotinylated-Histone H3 peptide (1 to 21 residues) after 30 mins in presence of SAM by AlphaLISA assay |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50598000
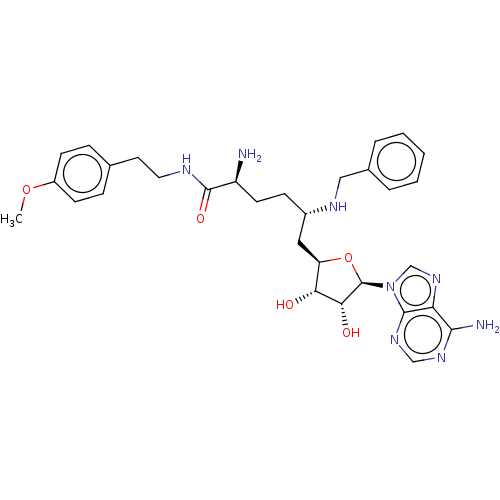
(CHEMBL5209447)Show SMILES COc1ccc(CCNC(=O)[C@@H](N)CC[C@@H](C[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)NCc2ccccc2)cc1 |r| | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50598018
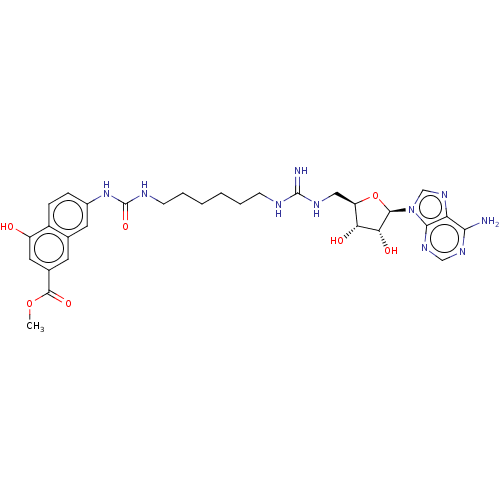
(CHEMBL5180108)Show SMILES COC(=O)c1cc(O)c2ccc(NC(=O)NCCCCCCNC(=N)NC[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)cc2c1 |r| | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT1
(Homo sapiens (Human)) | BDBM50396024
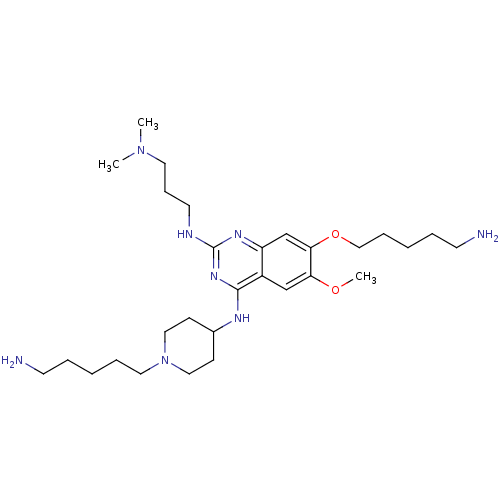
(CHEMBL1232453)Show SMILES COc1cc2c(NC3CCN(CCCCCN)CC3)nc(NCCCN(C)C)nc2cc1OCCCCCN Show InChI InChI=1S/C29H52N8O2/c1-36(2)16-10-15-32-29-34-25-22-27(39-20-9-5-7-14-31)26(38-3)21-24(25)28(35-29)33-23-11-18-37(19-12-23)17-8-4-6-13-30/h21-23H,4-20,30-31H2,1-3H3,(H2,32,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of GLP (unknown origin) |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50598020

(CHEMBL5202088)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCCNC(=N)NCC[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)ccc2c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50396024

(CHEMBL1232453)Show SMILES COc1cc2c(NC3CCN(CCCCCN)CC3)nc(NCCCN(C)C)nc2cc1OCCCCCN Show InChI InChI=1S/C29H52N8O2/c1-36(2)16-10-15-32-29-34-25-22-27(39-20-9-5-7-14-31)26(38-3)21-24(25)28(35-29)33-23-11-18-37(19-12-23)17-8-4-6-13-30/h21-23H,4-20,30-31H2,1-3H3,(H2,32,33,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition G9a (unknown origin) |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50598019

(CHEMBL5186617)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCCCCCNC(=N)NC[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)ccc2c1 |r| | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 7
(Homo sapiens (Human)) | BDBM50598017
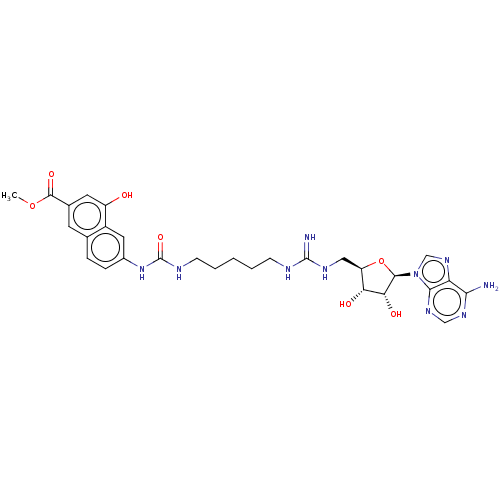
(CHEMBL5206272)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCCCNC(=N)NC[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)ccc2c1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 7
(Homo sapiens (Human)) | BDBM50598020

(CHEMBL5202088)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCCNC(=N)NCC[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)ccc2c1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 7
(Homo sapiens (Human)) | BDBM50598015

(CHEMBL5199380)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCNC(=N)NC[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)ccc2c1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1
(Homo sapiens) | BDBM50598011
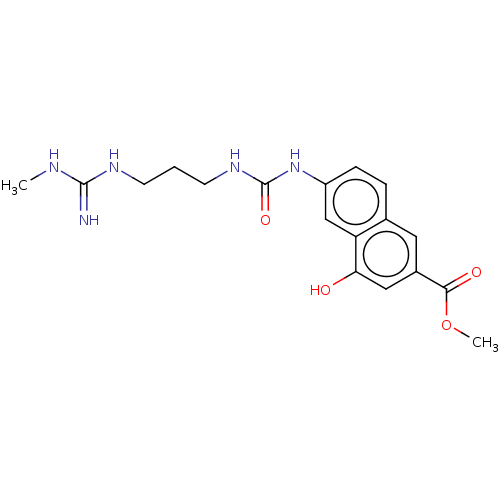
(CHEMBL5203516)Show SMILES CNC(=N)NCCCNC(=O)Nc1ccc2cc(cc(O)c2c1)C(=O)OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 7
(Homo sapiens (Human)) | BDBM50598018

(CHEMBL5180108)Show SMILES COC(=O)c1cc(O)c2ccc(NC(=O)NCCCCCCNC(=N)NC[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)cc2c1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50598017

(CHEMBL5206272)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCCCNC(=N)NC[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)ccc2c1 |r| | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein arginine N-methyltransferase 7
(Homo sapiens (Human)) | BDBM50598016

(CHEMBL5170047)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCCNC(=N)NC[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)ccc2c1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 7
(Homo sapiens (Human)) | BDBM50598019

(CHEMBL5186617)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCCCCCNC(=N)NC[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)ccc2c1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM7781
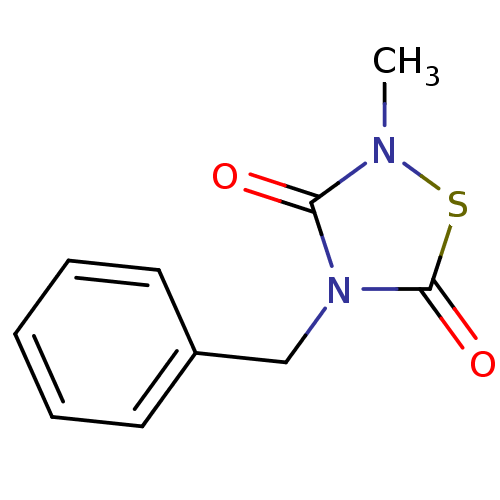
(4-Benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione ...)Show InChI InChI=1S/C10H10N2O2S/c1-11-9(13)12(10(14)15-11)7-8-5-3-2-4-6-8/h2-6H,7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) using GS-2 peptide substrate incubated for 30 mins by kinase-Glo luminescent assay |
J Med Chem 61: 7640-7656 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00610
BindingDB Entry DOI: 10.7270/Q20Z75TR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT1
(Homo sapiens (Human)) | BDBM50300028
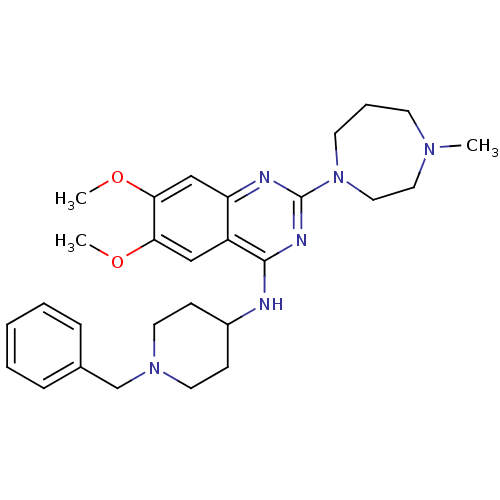
(CHEMBL569864 | N-(1-benzylpiperidin-4-yl)-6,7-dime...)Show SMILES COc1cc2nc(nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC)N1CCCN(C)CC1 Show InChI InChI=1S/C28H38N6O2/c1-32-12-7-13-34(17-16-32)28-30-24-19-26(36-3)25(35-2)18-23(24)27(31-28)29-22-10-14-33(15-11-22)20-21-8-5-4-6-9-21/h4-6,8-9,18-19,22H,7,10-17,20H2,1-3H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GLP (610 to 917 residues) using H3 peptide (1 to 20 residues) by mass spectrometry |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50598021

(CHEMBL5191769)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCCNC(=N)NCCC[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)ccc2c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 778 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1
(Homo sapiens) | BDBM50598022

(CHEMBL5173678)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCCNC(=N)NC\C=C\[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)ccc2c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 835 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1
(Homo sapiens) | BDBM50598018

(CHEMBL5180108)Show SMILES COC(=O)c1cc(O)c2ccc(NC(=O)NCCCCCCNC(=N)NC[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)cc2c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50368627
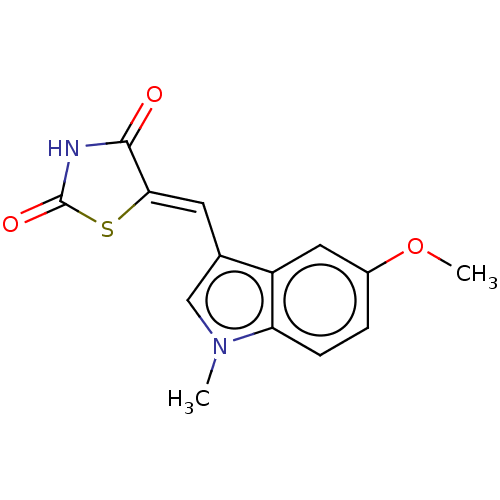
(CHEMBL4160653)Show InChI InChI=1S/C14H12N2O3S/c1-16-7-8(5-12-13(17)15-14(18)20-12)10-6-9(19-2)3-4-11(10)16/h3-7H,1-2H3,(H,15,17,18)/b12-5- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) using GS-2 peptide substrate incubated for 30 mins by kinase-Glo luminescent assay |
J Med Chem 61: 7640-7656 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00610
BindingDB Entry DOI: 10.7270/Q20Z75TR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50526220

(CHEMBL4539914)Show SMILES COc1cc2c(NC(CN=C2NC2CCN(CC2)C(C)C)C2CCCCC2)cc1OCCCN1CCCC1 |c:9| Show InChI InChI=1S/C31H51N5O2/c1-23(2)36-17-12-25(13-18-36)33-31-26-20-29(37-3)30(38-19-9-16-35-14-7-8-15-35)21-27(26)34-28(22-32-31)24-10-5-4-6-11-24/h20-21,23-25,28,34H,4-19,22H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 955 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human G9a using biotinylated-Histone H3 peptide (1 to 21 residues) after 30 mins in presence of SAM by AlphaLISA assay |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50526219
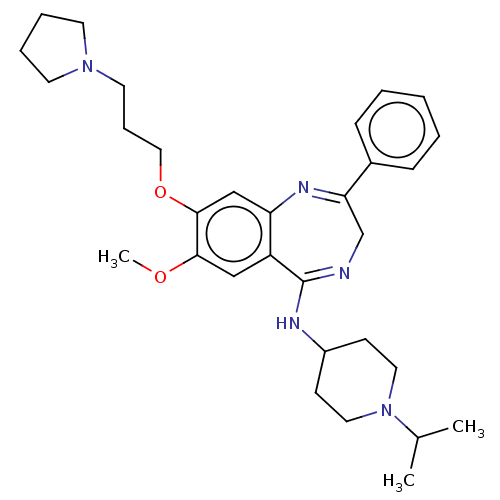
(CHEMBL4545884)Show SMILES COc1cc2c(cc1OCCCN1CCCC1)N=C(CN=C2NC1CCN(CC1)C(C)C)c1ccccc1 |c:19,22| Show InChI InChI=1S/C31H43N5O2/c1-23(2)36-17-12-25(13-18-36)33-31-26-20-29(37-3)30(38-19-9-16-35-14-7-8-15-35)21-27(26)34-28(22-32-31)24-10-5-4-6-11-24/h4-6,10-11,20-21,23,25H,7-9,12-19,22H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human G9a using biotinylated-Histone H3 peptide (1 to 21 residues) after 30 mins in presence of SAM by AlphaLISA assay |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50081125

(CHEMBL3421961)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1\[#6]=[#6]-1/[#6](=O)-[#7](-[#6]-c2ccccc2)-[#6](=O)-[#7](-[#6]-c2ccccc2)-[#6]-1=O Show InChI InChI=1S/C27H24N2O4/c1-18-13-22(30)14-19(2)23(18)15-24-25(31)28(16-20-9-5-3-6-10-20)27(33)29(26(24)32)17-21-11-7-4-8-12-21/h3-15,30H,16-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CBP (unknown origin) |
J Med Chem 58: 2779-98 (2015)
Article DOI: 10.1021/jm5019687
BindingDB Entry DOI: 10.7270/Q2736SMP |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1
(Homo sapiens) | BDBM50598010
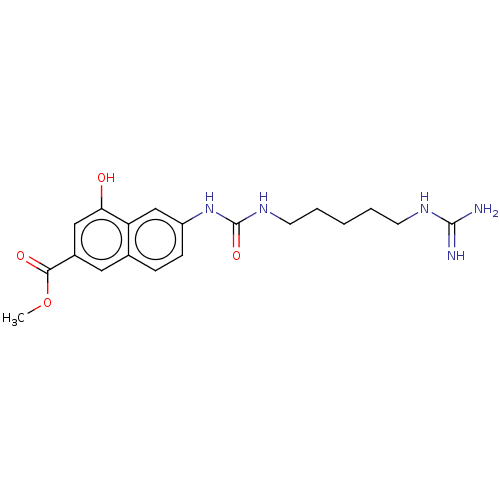
(CHEMBL5179077)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCCCNC(N)=N)ccc2c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 8
(Homo sapiens (Human)) | BDBM50598021

(CHEMBL5191769)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCCNC(=N)NCCC[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)ccc2c1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50198886
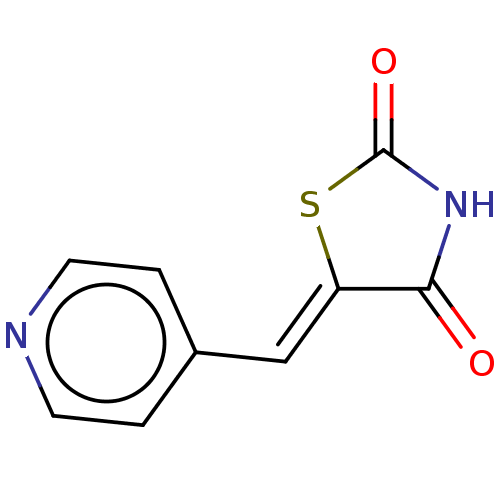
(CHEMBL3911800)Show InChI InChI=1S/C9H6N2O2S/c12-8-7(14-9(13)11-8)5-6-1-3-10-4-2-6/h1-5H,(H,11,12,13)/b7-5- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) using GS-2 peptide substrate incubated for 30 mins by kinase-Glo luminescent assay |
J Med Chem 61: 7640-7656 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00610
BindingDB Entry DOI: 10.7270/Q20Z75TR |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50598022

(CHEMBL5173678)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCCNC(=N)NC\C=C\[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)ccc2c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50081162
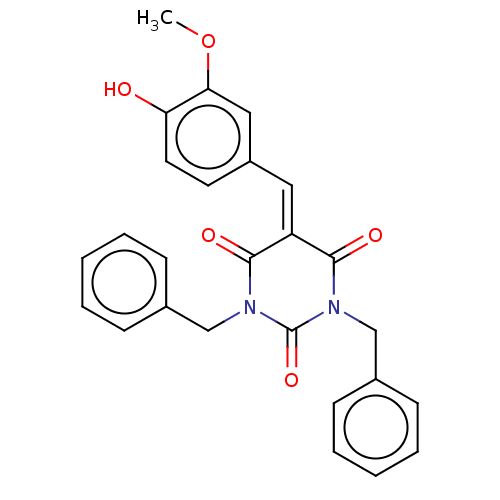
(CHEMBL3421943)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]-2\[#6](=O)-[#7](-[#6]-c3ccccc3)-[#6](=O)-[#7](-[#6]-c3ccccc3)-[#6]-2=O)ccc1-[#8] Show InChI InChI=1S/C26H22N2O5/c1-33-23-15-20(12-13-22(23)29)14-21-24(30)27(16-18-8-4-2-5-9-18)26(32)28(25(21)31)17-19-10-6-3-7-11-19/h2-15,29H,16-17H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of P300 (unknown origin) using histone H3/[acetyl-3H]-acetyl coenzyme A as substrate |
J Med Chem 58: 2779-98 (2015)
Article DOI: 10.1021/jm5019687
BindingDB Entry DOI: 10.7270/Q2736SMP |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50075052
(CHEMBL3414617)Show SMILES OC(=O)c1ccc2n(CCCc3ccccc3)c(NC(=O)c3ccccc3)nc2c1 Show InChI InChI=1S/C24H21N3O3/c28-22(18-11-5-2-6-12-18)26-24-25-20-16-19(23(29)30)13-14-21(20)27(24)15-7-10-17-8-3-1-4-9-17/h1-6,8-9,11-14,16H,7,10,15H2,(H,29,30)(H,25,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition G9a (unknown origin) |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50081160
(CHEMBL3421941)Show SMILES [#8]-c1ccc(\[#6]=[#6]-2\[#6](=O)-[#7](-[#6]-c3ccccc3)-[#6](=O)-[#7](-[#6]-c3ccccc3)-[#6]-2=O)cc1-[#8] Show InChI InChI=1S/C25H20N2O5/c28-21-12-11-19(14-22(21)29)13-20-23(30)26(15-17-7-3-1-4-8-17)25(32)27(24(20)31)16-18-9-5-2-6-10-18/h1-14,28-29H,15-16H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of P300 (unknown origin) using histone H3/[acetyl-3H]-acetyl coenzyme A as substrate |
J Med Chem 58: 2779-98 (2015)
Article DOI: 10.1021/jm5019687
BindingDB Entry DOI: 10.7270/Q2736SMP |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT1
(Homo sapiens (Human)) | BDBM50075052

(CHEMBL3414617)Show SMILES OC(=O)c1ccc2n(CCCc3ccccc3)c(NC(=O)c3ccccc3)nc2c1 Show InChI InChI=1S/C24H21N3O3/c28-22(18-11-5-2-6-12-18)26-24-25-20-16-19(23(29)30)13-14-21(20)27(24)15-7-10-17-8-3-1-4-9-17/h1-6,8-9,11-14,16H,7,10,15H2,(H,29,30)(H,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of GLP (unknown origin) |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50081170
(CHEMBL3421960)Show SMILES CC1=NN(C(=O)\C1=C/Cc1ccc(Cc2cc(C)c(C)cc2[N+]([O-])=O)o1)c1ccc(cc1)C(O)=O |t:1| Show InChI InChI=1S/C26H23N3O6/c1-15-12-19(24(29(33)34)13-16(15)2)14-22-9-8-21(35-22)10-11-23-17(3)27-28(25(23)30)20-6-4-18(5-7-20)26(31)32/h4-9,11-13H,10,14H2,1-3H3,(H,31,32)/b23-11- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of P300 (unknown origin) using histone H3/[acetyl-3H]-acetyl coenzyme A as substrate |
J Med Chem 58: 2779-98 (2015)
Article DOI: 10.1021/jm5019687
BindingDB Entry DOI: 10.7270/Q2736SMP |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1
(Homo sapiens) | BDBM50598008

(CHEMBL5199851)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCNC(N)=N)ccc2c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 7
(Homo sapiens (Human)) | BDBM50598022

(CHEMBL5173678)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCCNC(=N)NC\C=C\[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)ccc2c1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 6
(Homo sapiens (Human)) | BDBM50598022

(CHEMBL5173678)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCCNC(=N)NC\C=C\[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)ccc2c1 |r| | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1
(Homo sapiens) | BDBM50598021

(CHEMBL5191769)Show SMILES COC(=O)c1cc(O)c2cc(NC(=O)NCCCCNC(=N)NCCC[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)ccc2c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00252
BindingDB Entry DOI: 10.7270/Q2DF6W8F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data