

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50296336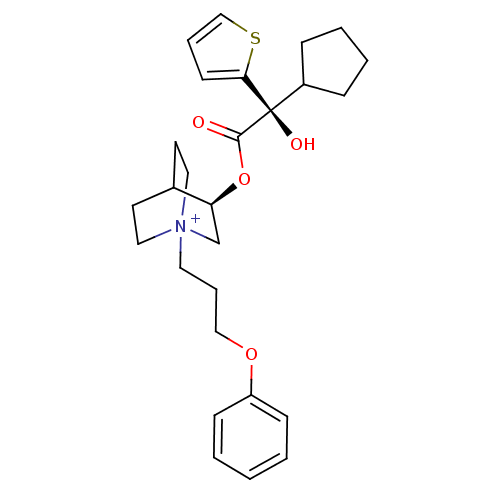 ((3R)-3-{[(2S)-2-Cyclopentyl-2-hydroxy-2-(2-thienyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296331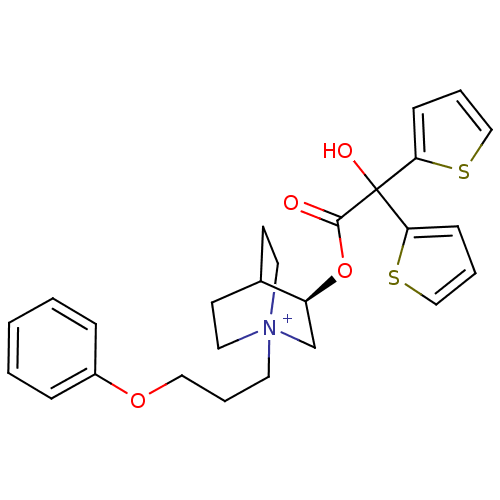 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M3 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296336 ((3R)-3-{[(2S)-2-Cyclopentyl-2-hydroxy-2-(2-thienyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M3 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296329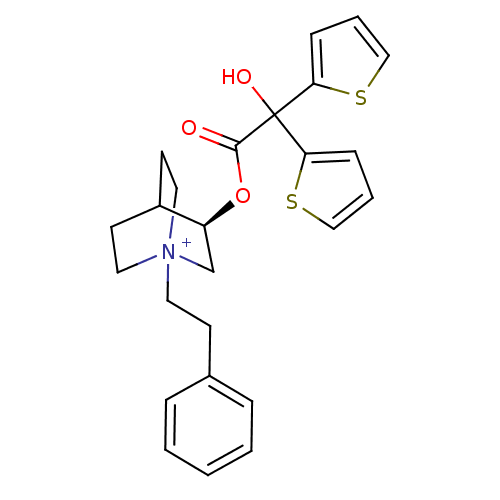 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(2-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M3 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50296331 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50296331 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50296329 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(2-phe...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50296329 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(2-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50296336 ((3R)-3-{[(2S)-2-Cyclopentyl-2-hydroxy-2-(2-thienyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M3 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50378083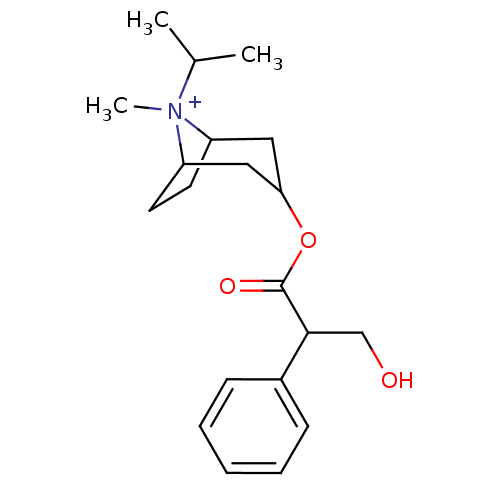 (Atrovent HFA | IPRATROPIUM BROMIDE) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M3 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50322229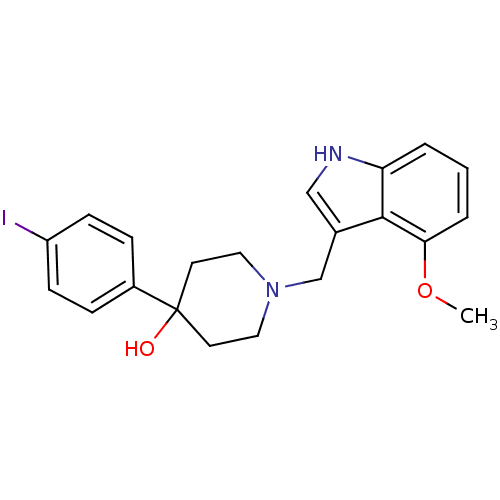 (4-(4-Iodophenyl)-1-((4-methoxy-1H-indol-3-yl)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human D2L receptor expressed in HEK cell membrane after 90 mins by scintillation counting analysis | J Med Chem 57: 3450-63 (2014) Article DOI: 10.1021/jm500126s BindingDB Entry DOI: 10.7270/Q2MW2JN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50116766 ((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from D3 receptor (unknown origin) measured after 90 mins by microbeta scintillation counting method | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50116766 ((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from human D3R expressed in HEK293 cell membranes measured after 90 mins in Tris buffer by topcount assay | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50378083 (Atrovent HFA | IPRATROPIUM BROMIDE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | 1.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50116766 ((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from human D3R expressed in HEK293 cell membranes measured after 90 mins in EBSS buffer by topcount assay | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human D2L receptor expressed in HEK cell membrane after 90 mins by scintillation counting analysis | J Med Chem 57: 3450-63 (2014) Article DOI: 10.1021/jm500126s BindingDB Entry DOI: 10.7270/Q2MW2JN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50378083 (Atrovent HFA | IPRATROPIUM BROMIDE) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | 1.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human D3 receptor expressed in HEK293 cell membrane after 90 mins by scintillation counting analysis | J Med Chem 57: 3450-63 (2014) Article DOI: 10.1021/jm500126s BindingDB Entry DOI: 10.7270/Q2MW2JN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50116766 ((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-methylspiperon from human D2RL expressed in HEK293 cell membranes measured after 90 mins by topcount assay | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50116766 ((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from human D3R expressed in HEK293 cell membranes measured after 90 mins in Tris buffer containing NaCl by topcount as... | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50400511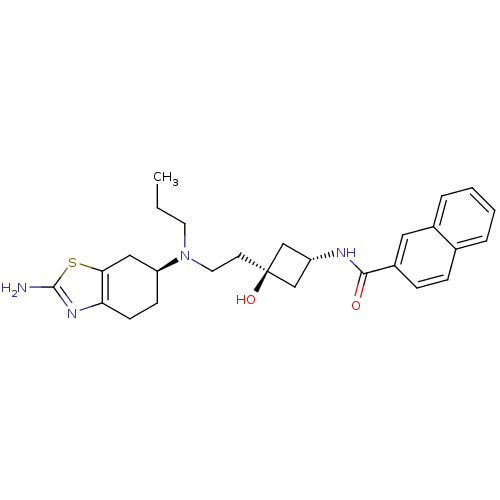 (CHEMBL2203406) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-methylspiperon from human D3R expressed in HEK293 cell membranes measured after 90 mins in EBSS buffer by topcount assay | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50050467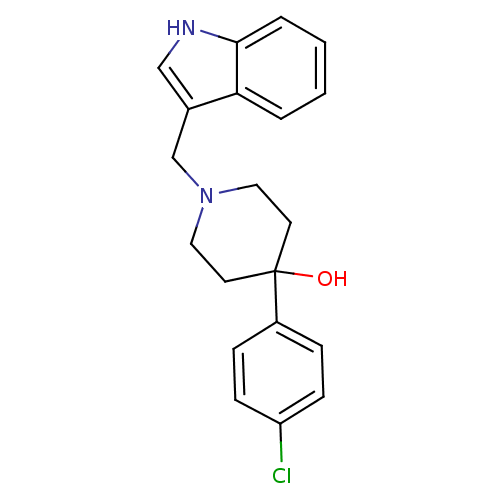 (1-((1H-indol-3-yl)methyl)-4-(4-chlorophenyl)piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human D2L receptor expressed in HEK cell membrane after 90 mins by scintillation counting analysis | J Med Chem 57: 3450-63 (2014) Article DOI: 10.1021/jm500126s BindingDB Entry DOI: 10.7270/Q2MW2JN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50116766 ((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-methylspiperon from human D3R expressed in HEK293 cell membranes measured after 90 mins in EBSS buffer by topcount assay | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50527972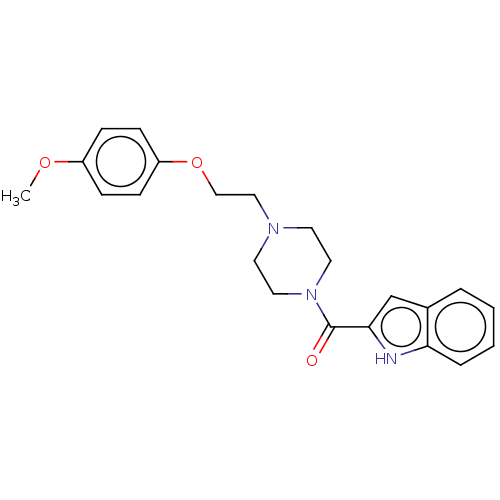 (CHEMBL4476784 | US11634404, Compound 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from human D3R expressed in HEK293 cell membranes measured after 90 mins in Tris buffer containing MgCl2 by topcount a... | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50116766 ((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-methylspiperon from human D3R expressed in HEK293 cell membranes measured after 90 mins in Tris buffer by topcount assay | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50116766 ((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-methylspiperon from human D3R expressed in HEK293 cell membranes measured after 90 mins in Tris buffer containing NaCl by topcou... | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50400511 (CHEMBL2203406) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-methylspiperon from human D2RL expressed in HEK293 cell membranes measured after 90 mins by topcount assay | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50527972 (CHEMBL4476784 | US11634404, Compound 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from human D3R expressed in HEK293 cell membranes measured after 90 mins in Tris buffer by topcount assay | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50003068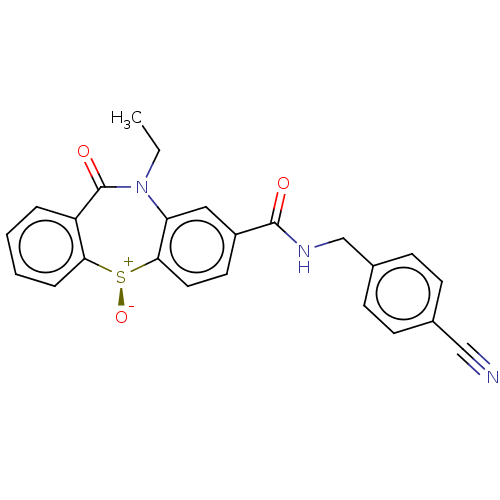 (CHEMBL3234537) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human D2L receptor expressed in HEK cell membrane after 90 mins by scintillation counting analysis | J Med Chem 57: 3450-63 (2014) Article DOI: 10.1021/jm500126s BindingDB Entry DOI: 10.7270/Q2MW2JN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50116766 ((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from recombinant human D4 receptor stably expressed in HEK cell membranes measured after 90 mins by microbeta ... | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50400511 (CHEMBL2203406) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from D3 receptor (unknown origin) measured after 90 mins by microbeta scintillation counting method | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50400511 (CHEMBL2203406) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from human SERT receptor expressed in stable HEK cell membranes after 90 mins by microbeta scintillation counting met... | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50400511 (CHEMBL2203406) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from recombinant human D4 receptor stably expressed in HEK cell membranes measured after 90 mins by microbeta ... | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50050467 (1-((1H-indol-3-yl)methyl)-4-(4-chlorophenyl)piperi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human D3 receptor expressed in HEK293 cell membrane after 90 mins by scintillation counting analysis | J Med Chem 57: 3450-63 (2014) Article DOI: 10.1021/jm500126s BindingDB Entry DOI: 10.7270/Q2MW2JN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50116766 ((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-rauwolscine from recombinant human alpha2C adrenergic receptor stably expressed in MDCK cell membranes measured after 90 mins by... | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50116766 ((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-rauwolscine from recombinant human alpha2B adrenergic receptor transiently expressed in HEKT cell membranes measured after 90 mi... | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50116766 ((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-rauwolscine from recombinant human alpha2A adrenergic receptor stably expressed in MDCK cell membranes measured after 90 mins by... | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50527972 (CHEMBL4476784 | US11634404, Compound 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [125I]-pindolol from recombinant human beta1 adrenergic receptor expressed in CHO Flp-In cell membranes measured after 90 mins by mic... | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50322229 (4-(4-Iodophenyl)-1-((4-methoxy-1H-indol-3-yl)methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human D3 receptor expressed in HEK293 cell membrane after 90 mins by scintillation counting analysis | J Med Chem 57: 3450-63 (2014) Article DOI: 10.1021/jm500126s BindingDB Entry DOI: 10.7270/Q2MW2JN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50003066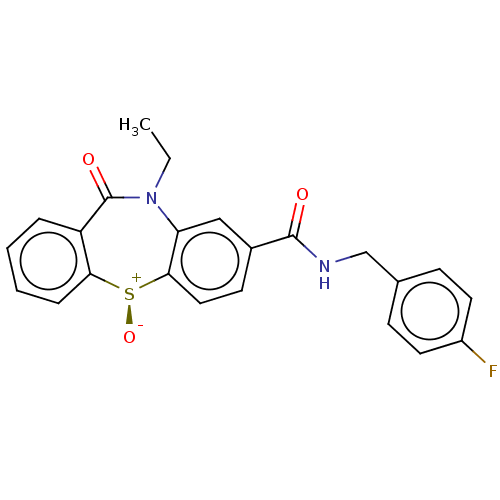 (CHEMBL3234536) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human D2L receptor expressed in HEK cell membrane after 90 mins by scintillation counting analysis | J Med Chem 57: 3450-63 (2014) Article DOI: 10.1021/jm500126s BindingDB Entry DOI: 10.7270/Q2MW2JN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50527972 (CHEMBL4476784 | US11634404, Compound 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from human D3R expressed in HEK293 cell membranes measured after 90 mins in Tris buffer containing NaCl by topcount as... | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50003074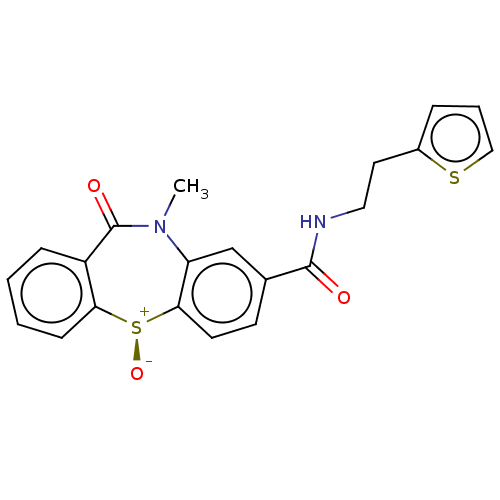 (CHEMBL3234544) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human D2L receptor expressed in HEK cell membrane after 90 mins by scintillation counting analysis | J Med Chem 57: 3450-63 (2014) Article DOI: 10.1021/jm500126s BindingDB Entry DOI: 10.7270/Q2MW2JN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50400511 (CHEMBL2203406) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-pyrilamine from human histamine H1 receptor expressed in stable HEK cell membranes after 90 mins by microbeta scintillation coun... | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50003068 (CHEMBL3234537) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human D3 receptor expressed in HEK293 cell membrane after 90 mins by scintillation counting analysis | J Med Chem 57: 3450-63 (2014) Article DOI: 10.1021/jm500126s BindingDB Entry DOI: 10.7270/Q2MW2JN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50003063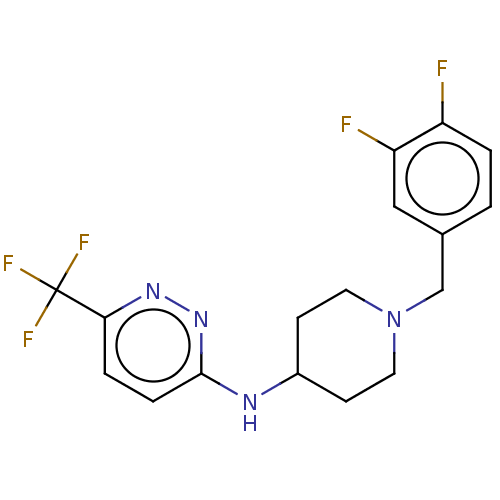 (CHEMBL3234237) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human D2L receptor expressed in HEK cell membrane after 90 mins by scintillation counting analysis | J Med Chem 57: 3450-63 (2014) Article DOI: 10.1021/jm500126s BindingDB Entry DOI: 10.7270/Q2MW2JN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50003065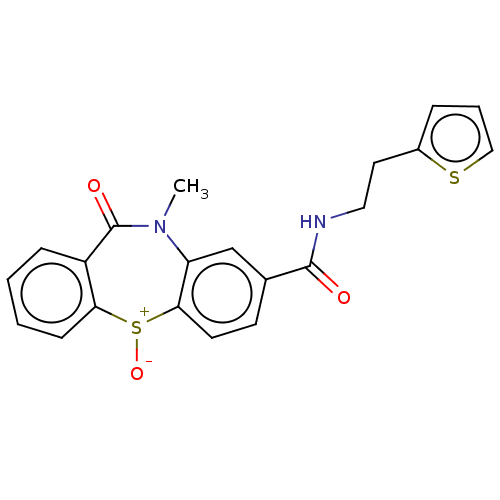 (CHEMBL3234522) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human D2L receptor expressed in HEK cell membrane after 90 mins by scintillation counting analysis | J Med Chem 57: 3450-63 (2014) Article DOI: 10.1021/jm500126s BindingDB Entry DOI: 10.7270/Q2MW2JN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50400511 (CHEMBL2203406) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-Win35428 from human DAT expressed in stable HEK cell membranes after 90 mins by microbeta scintillation counting method | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1847 total ) | Next | Last >> |