

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bile acid receptor (Homo sapiens (Human)) | BDBM28529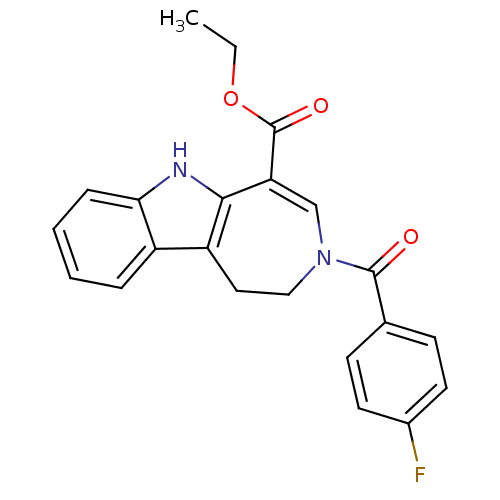 (Azepino[4,5-b]indole, 1 | ethyl 3-[(4-fluorophenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 600 | n/a | n/a | 7.0 | 22 |
Exelixis Inc. | Assay Description The assays were performed using CV-1 African Green Monkey Kidney cells transfected with the vectors harboring human FXR, RXR, and luciferase reporter... | J Med Chem 52: 904-7 (2009) Article DOI: 10.1021/jm8014124 BindingDB Entry DOI: 10.7270/Q2J38QVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM28531 (Azepino[4,5-b]indole, 6b | propyl 3-[(4-fluorophen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | 7.0 | 22 |
Exelixis Inc. | Assay Description The assays were performed using CV-1 African Green Monkey Kidney cells transfected with the vectors harboring human FXR, RXR, and luciferase reporter... | J Med Chem 52: 904-7 (2009) Article DOI: 10.1021/jm8014124 BindingDB Entry DOI: 10.7270/Q2J38QVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM28532 (Azepino[4,5-b]indole, 6c | butyl 3-[(4-fluoropheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | 7.0 | 22 |
Exelixis Inc. | Assay Description The assays were performed using CV-1 African Green Monkey Kidney cells transfected with the vectors harboring human FXR, RXR, and luciferase reporter... | J Med Chem 52: 904-7 (2009) Article DOI: 10.1021/jm8014124 BindingDB Entry DOI: 10.7270/Q2J38QVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM28533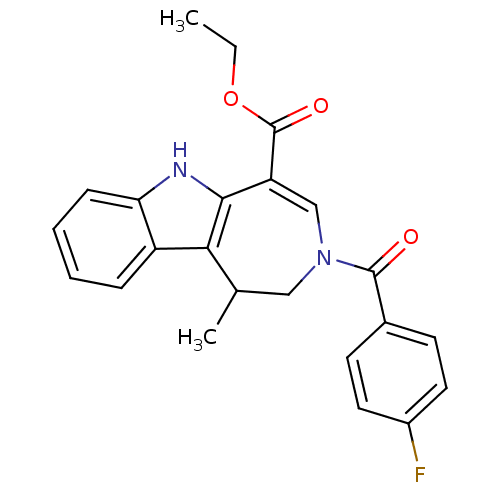 (Azepino[4,5-b]indole, 6d | ethyl 3-[(4-fluoropheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 57 | n/a | n/a | 7.0 | 22 |
Exelixis Inc. | Assay Description The assays were performed using CV-1 African Green Monkey Kidney cells transfected with the vectors harboring human FXR, RXR, and luciferase reporter... | J Med Chem 52: 904-7 (2009) Article DOI: 10.1021/jm8014124 BindingDB Entry DOI: 10.7270/Q2J38QVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM28534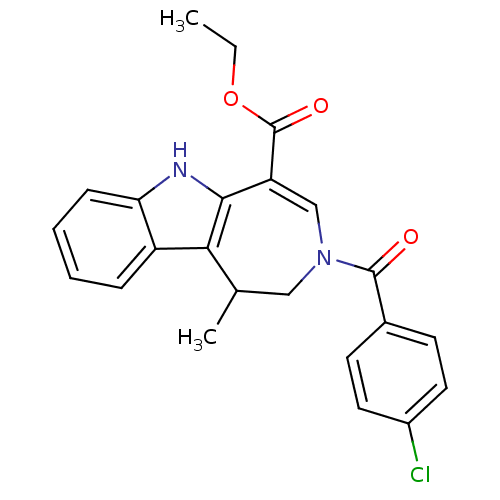 (Azepino[4,5-b]indole, 6e | ethyl 3-[(4-chloropheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 73 | n/a | n/a | 7.0 | 22 |
Exelixis Inc. | Assay Description The assays were performed using CV-1 African Green Monkey Kidney cells transfected with the vectors harboring human FXR, RXR, and luciferase reporter... | J Med Chem 52: 904-7 (2009) Article DOI: 10.1021/jm8014124 BindingDB Entry DOI: 10.7270/Q2J38QVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM28535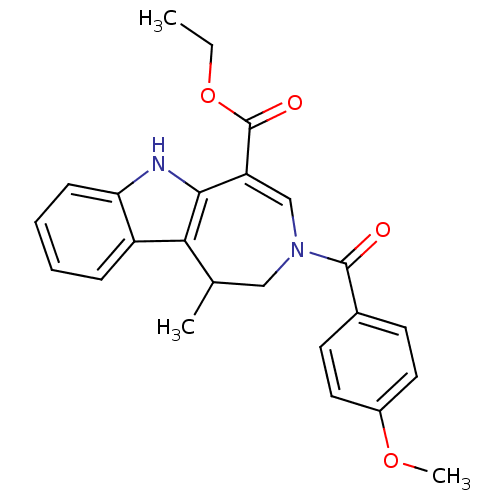 (Azepino[4,5-b]indole, 6f | ethyl 3-[(4-methoxyphen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | 7.0 | 22 |
Exelixis Inc. | Assay Description The assays were performed using CV-1 African Green Monkey Kidney cells transfected with the vectors harboring human FXR, RXR, and luciferase reporter... | J Med Chem 52: 904-7 (2009) Article DOI: 10.1021/jm8014124 BindingDB Entry DOI: 10.7270/Q2J38QVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM28536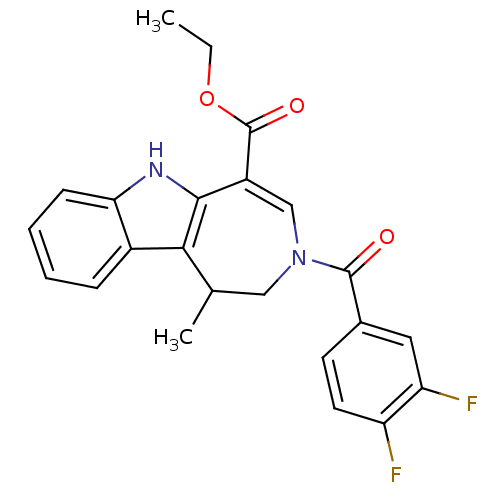 (Azepino[4,5-b]indole, 6g | ethyl 3-[(3,4-difluorop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 32 | n/a | n/a | 7.0 | 22 |
Exelixis Inc. | Assay Description The assays were performed using CV-1 African Green Monkey Kidney cells transfected with the vectors harboring human FXR, RXR, and luciferase reporter... | J Med Chem 52: 904-7 (2009) Article DOI: 10.1021/jm8014124 BindingDB Entry DOI: 10.7270/Q2J38QVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM28537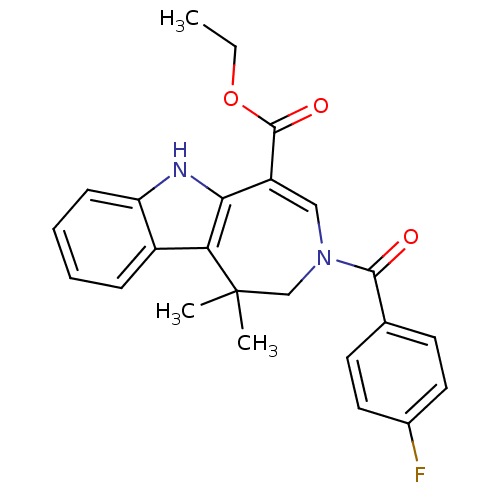 (Azepino[4,5-b]indole, 6h | ethyl 3-[(4-fluoropheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | 7.0 | 22 |
Exelixis Inc. | Assay Description The assays were performed using CV-1 African Green Monkey Kidney cells transfected with the vectors harboring human FXR, RXR, and luciferase reporter... | J Med Chem 52: 904-7 (2009) Article DOI: 10.1021/jm8014124 BindingDB Entry DOI: 10.7270/Q2J38QVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM28538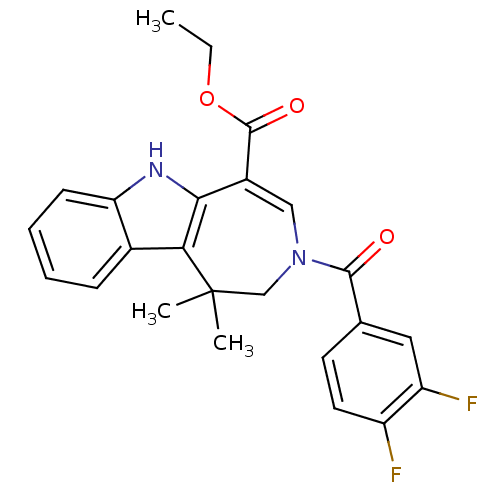 (Azepino[4,5-b]indole, 6i | FXR_5 | ethyl 3-[(3,4-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | 7.0 | 22 |
Exelixis Inc. | Assay Description The assays were performed using CV-1 African Green Monkey Kidney cells transfected with the vectors harboring human FXR, RXR, and luciferase reporter... | J Med Chem 52: 904-7 (2009) Article DOI: 10.1021/jm8014124 BindingDB Entry DOI: 10.7270/Q2J38QVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM28539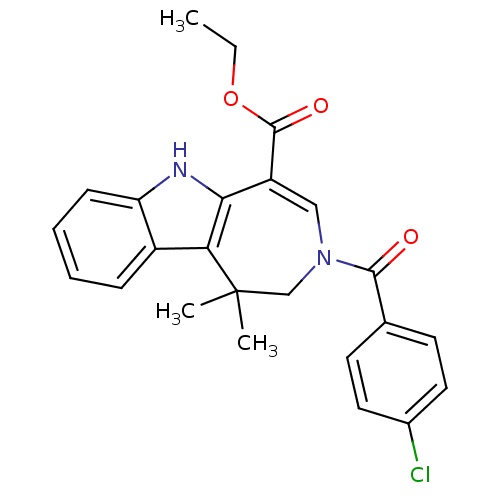 (Azepino[4,5-b]indole, 6j | ethyl 3-[(4-chloropheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | 7.0 | 22 |
Exelixis Inc. | Assay Description The assays were performed using CV-1 African Green Monkey Kidney cells transfected with the vectors harboring human FXR, RXR, and luciferase reporter... | J Med Chem 52: 904-7 (2009) Article DOI: 10.1021/jm8014124 BindingDB Entry DOI: 10.7270/Q2J38QVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM28540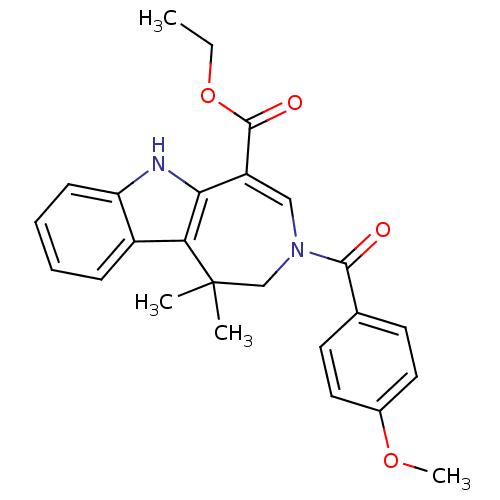 (Azepino[4,5-b]indole, 6k | ethyl 3-[(4-methoxyphen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 29 | n/a | n/a | 7.0 | 22 |
Exelixis Inc. | Assay Description The assays were performed using CV-1 African Green Monkey Kidney cells transfected with the vectors harboring human FXR, RXR, and luciferase reporter... | J Med Chem 52: 904-7 (2009) Article DOI: 10.1021/jm8014124 BindingDB Entry DOI: 10.7270/Q2J38QVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM28541 (Azepino[4,5-b]indole, 6l | propan-2-yl 3-[(4-fluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | 7.0 | 22 |
Exelixis Inc. | Assay Description The assays were performed using CV-1 African Green Monkey Kidney cells transfected with the vectors harboring human FXR, RXR, and luciferase reporter... | J Med Chem 52: 904-7 (2009) Article DOI: 10.1021/jm8014124 BindingDB Entry DOI: 10.7270/Q2J38QVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM28542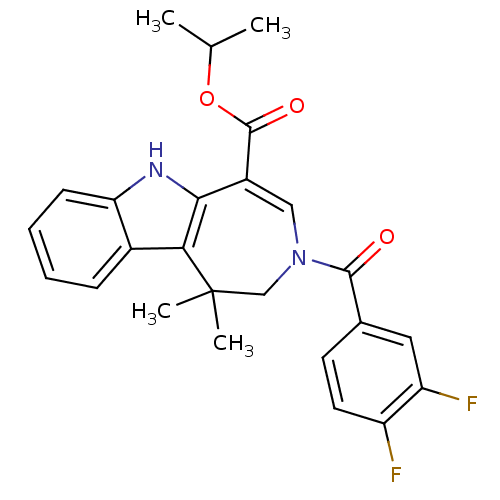 (WAY-362450 | XL335 | propan-2-yl 3-[(3,4-difluorop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | 7.0 | 22 |
Exelixis Inc. | Assay Description The assays were performed using CV-1 African Green Monkey Kidney cells transfected with the vectors harboring human FXR, RXR, and luciferase reporter... | J Med Chem 52: 904-7 (2009) Article DOI: 10.1021/jm8014124 BindingDB Entry DOI: 10.7270/Q2J38QVJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034775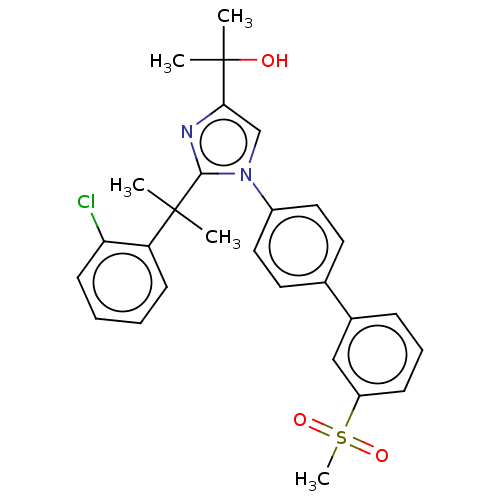 (CHEMBL3360975 | US10543183, Compound 38 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034776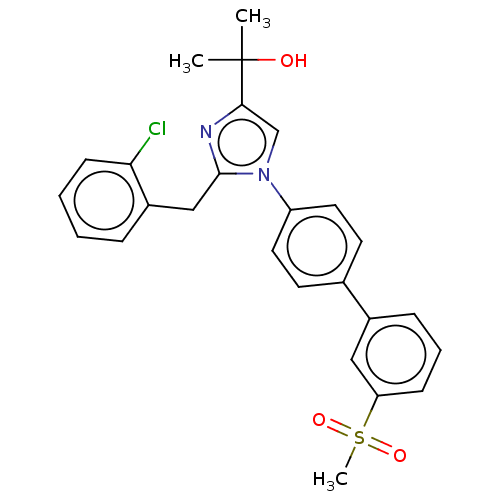 (CHEMBL3360974) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034777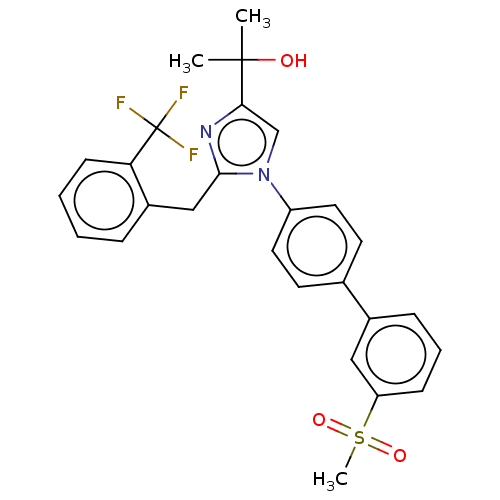 (CHEMBL3360973) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034778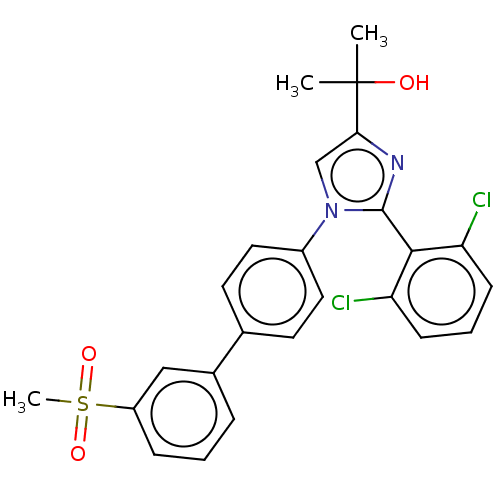 (CHEMBL3360972) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 670 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034779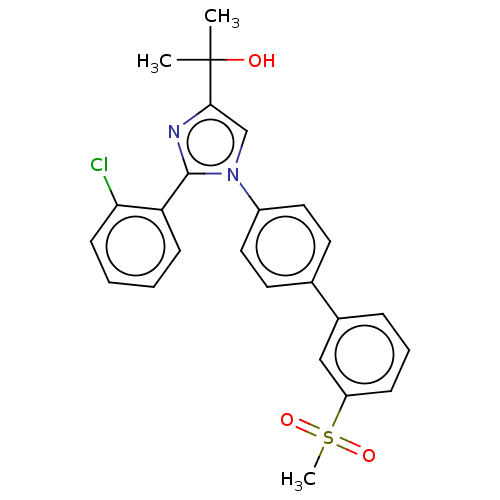 (CHEMBL3360971) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 600 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034780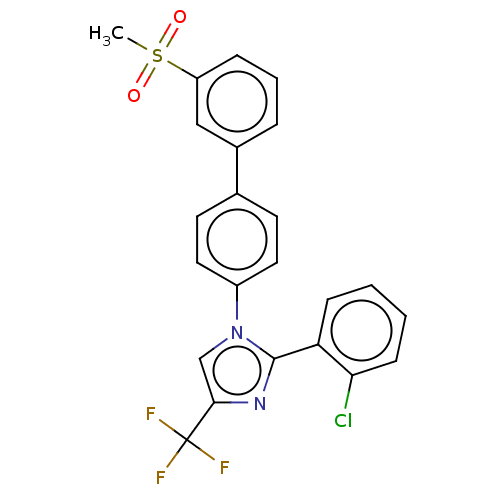 (CHEMBL3360970) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 61 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034781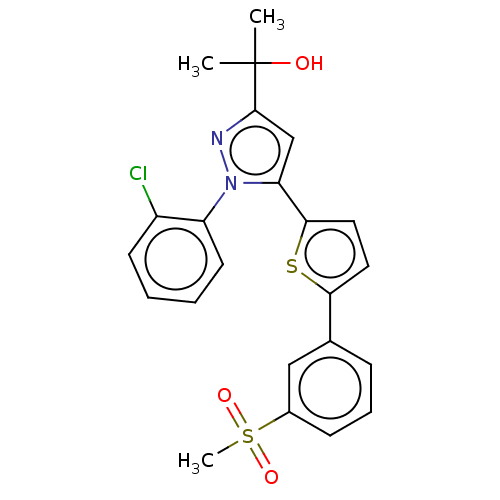 (CHEMBL3360969) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 620 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034782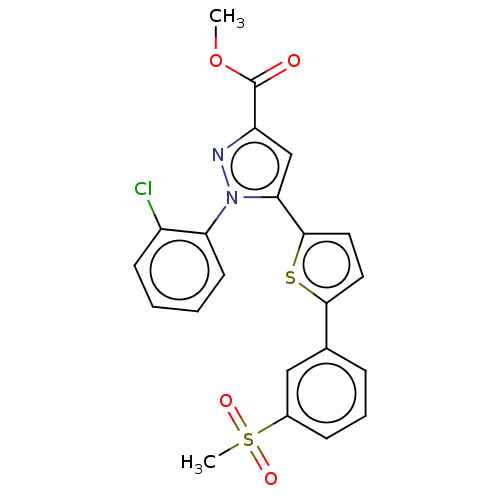 (CHEMBL3360968) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 64 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034783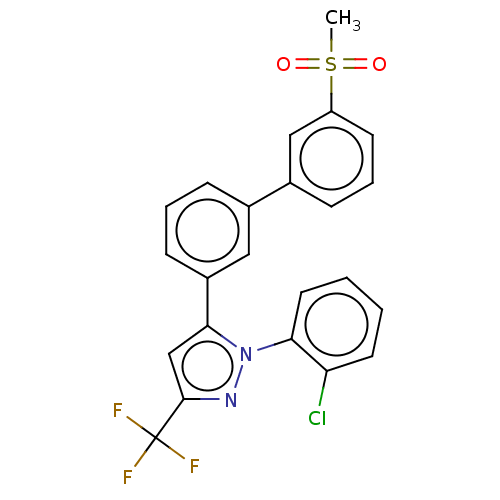 (CHEMBL3360967) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034784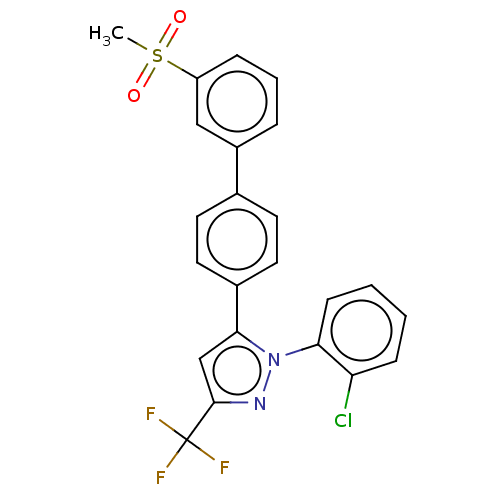 (CHEMBL3360966) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 49 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034785 (CHEMBL3360965) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 68 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034786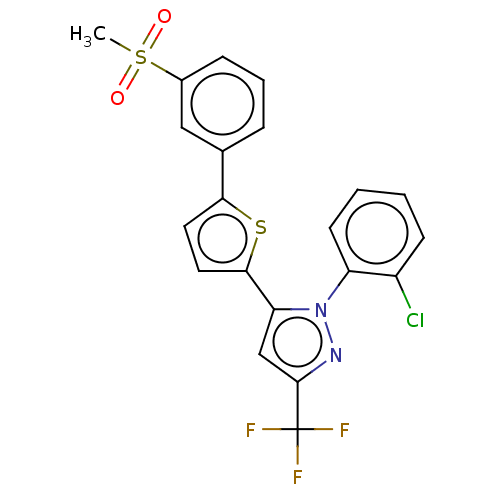 (CHEMBL3360964) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034788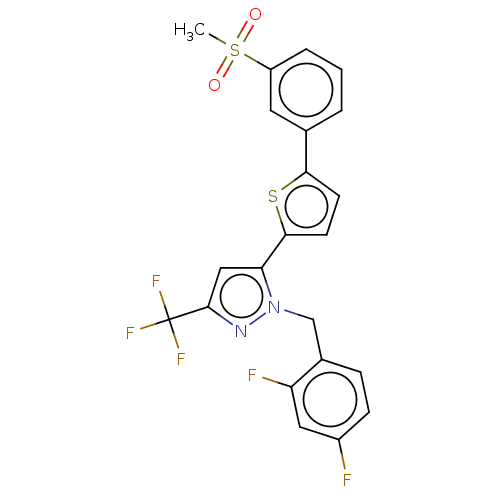 (CHEMBL3360963) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034789 (CHEMBL3360962) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034790 (CHEMBL3360961) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034791 (CHEMBL3360960) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034775 (CHEMBL3360975 | US10543183, Compound 38 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034776 (CHEMBL3360974) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 85 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034777 (CHEMBL3360973) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034778 (CHEMBL3360972) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034779 (CHEMBL3360971) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034780 (CHEMBL3360970) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034781 (CHEMBL3360969) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 540 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034782 (CHEMBL3360968) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034783 (CHEMBL3360967) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034784 (CHEMBL3360966) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034785 (CHEMBL3360965) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034786 (CHEMBL3360964) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034788 (CHEMBL3360963) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034789 (CHEMBL3360962) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 570 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034790 (CHEMBL3360961) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034791 (CHEMBL3360960) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM28530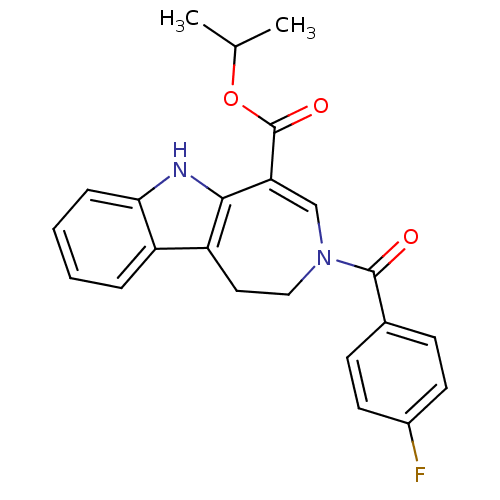 (Azepino[4,5-b]indole, 6a | propan-2-yl 3-[(4-fluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | 7.0 | 22 |
Exelixis Inc. | Assay Description The assays were performed using CV-1 African Green Monkey Kidney cells transfected with the vectors harboring human FXR, RXR, and luciferase reporter... | J Med Chem 52: 904-7 (2009) Article DOI: 10.1021/jm8014124 BindingDB Entry DOI: 10.7270/Q2J38QVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||