

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50304627 ((2S,3S,4R,5R,6S)-6-methylpiperidine-2,3,4,5-tetrao...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of bovine kidney alpha-L-fucosidase by para-nitrophenolate release assay | Bioorg Med Chem 17: 8020-6 (2009) Article DOI: 10.1016/j.bmc.2009.10.010 BindingDB Entry DOI: 10.7270/Q2FB5307 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50048908 (CHEMBL415788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS1 receptor expressed in CHOK1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50065258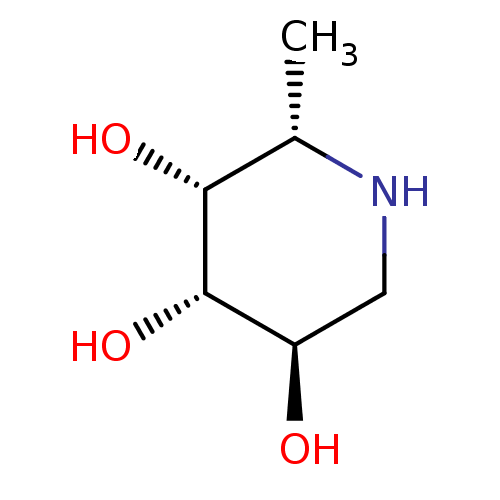 ((2S,3R,4S,5R)-2-Methyl-piperidine-3,4,5-triol | (2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of bovine kidney alpha-L-fucosidase by para-nitrophenolate release assay | Bioorg Med Chem 17: 8020-6 (2009) Article DOI: 10.1016/j.bmc.2009.10.010 BindingDB Entry DOI: 10.7270/Q2FB5307 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50048908 (CHEMBL415788) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS2 receptor expressed in human 1321N1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50304628 ((2S,3R,4S)-2-methyl-3,4-dihydro-2H-pyrrole-3,4-dio...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of bovine kidney alpha-L-fucosidase by para-nitrophenolate release assay | Bioorg Med Chem 17: 8020-6 (2009) Article DOI: 10.1016/j.bmc.2009.10.010 BindingDB Entry DOI: 10.7270/Q2FB5307 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50265841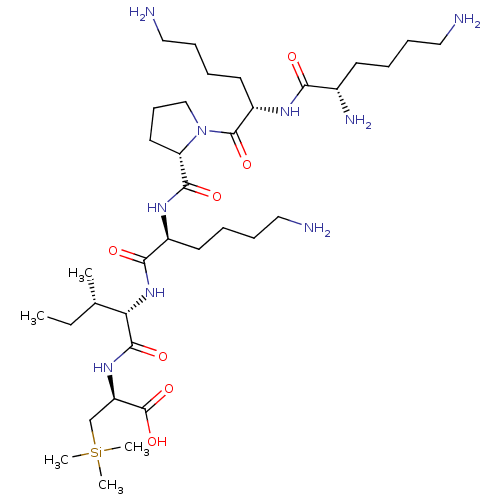 (CHEMBL4100456) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS2 receptor expressed in human 1321N1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50265836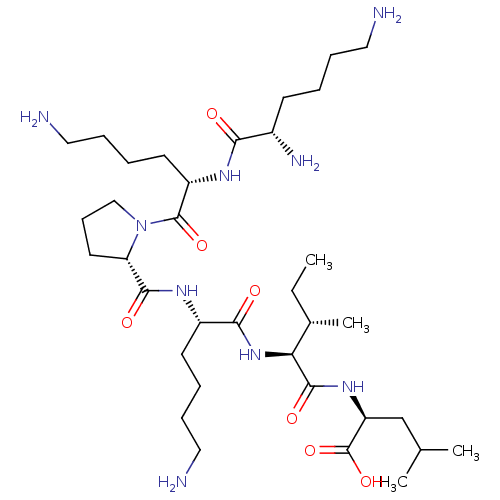 (CHEMBL4084378) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 262 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS2 receptor expressed in human 1321N1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50265835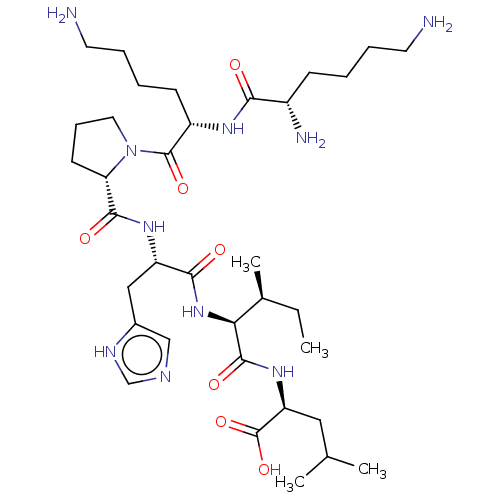 (CHEMBL4076517) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 456 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS1 receptor expressed in CHOK1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50265835 (CHEMBL4076517) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 474 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS2 receptor expressed in human 1321N1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50265842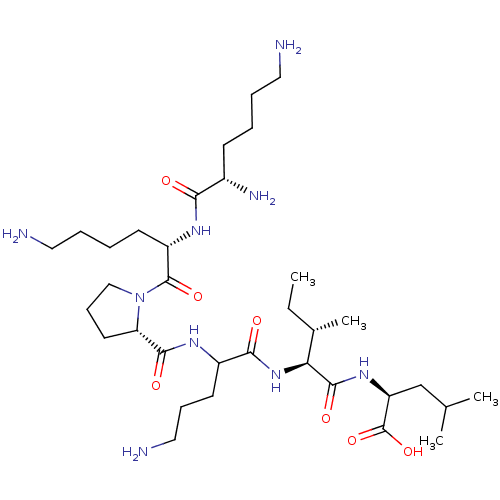 (CHEMBL4103884) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS2 receptor expressed in human 1321N1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50265841 (CHEMBL4100456) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 663 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS1 receptor expressed in CHOK1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50304626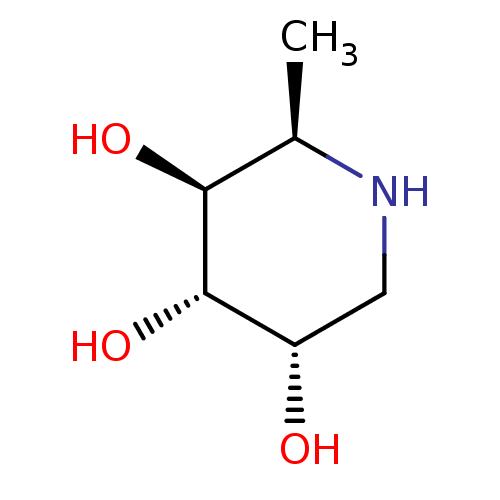 ((2R,3S,4S,5S)-2-methylpiperidine-3,4,5-triol | CHE...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of bovine kidney alpha-L-fucosidase by para-nitrophenolate release assay | Bioorg Med Chem 17: 8020-6 (2009) Article DOI: 10.1016/j.bmc.2009.10.010 BindingDB Entry DOI: 10.7270/Q2FB5307 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50265833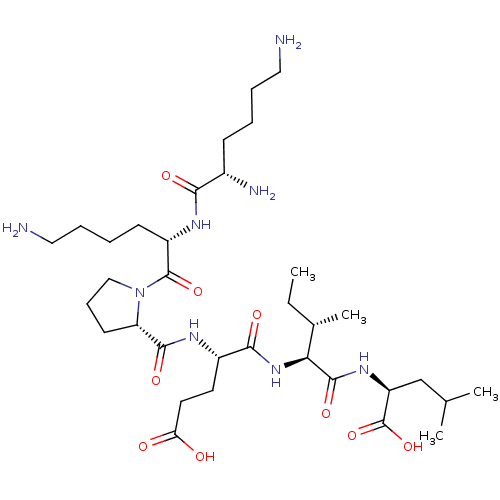 (CHEMBL4082440) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS2 receptor expressed in human 1321N1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50265834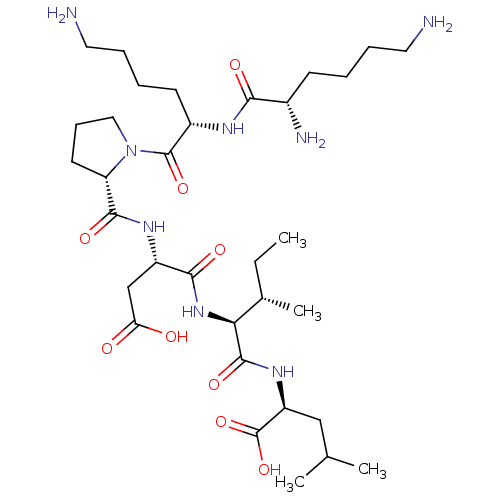 (CHEMBL4074624) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS2 receptor expressed in human 1321N1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50265836 (CHEMBL4084378) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS1 receptor expressed in CHOK1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50265833 (CHEMBL4082440) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS1 receptor expressed in CHOK1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50265834 (CHEMBL4074624) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS1 receptor expressed in CHOK1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50265842 (CHEMBL4103884) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS1 receptor expressed in CHOK1 cell membranes after 30 mins by gamma counting analysis | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50304629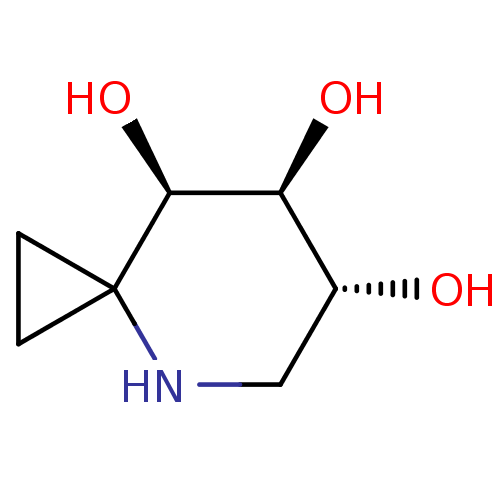 ((6R,7S,8R)-4-azaspiro[2.5]octane-6,7,8-triol | CHE...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of bovine kidney alpha-L-fucosidase by para-nitrophenolate release assay | Bioorg Med Chem 17: 8020-6 (2009) Article DOI: 10.1016/j.bmc.2009.10.010 BindingDB Entry DOI: 10.7270/Q2FB5307 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50342242 ((S)-2-((2S,3S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS1 receptor | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50342242 ((S)-2-((2S,3S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of 125I-[Tyr3]-NT from human NTS2 receptor | J Med Chem 60: 3303-3313 (2017) Article DOI: 10.1021/acs.jmedchem.6b01848 BindingDB Entry DOI: 10.7270/Q2377C6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||