 Found 66 hits with Last Name = 'flores' and Initial = 'ba'
Found 66 hits with Last Name = 'flores' and Initial = 'ba' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135609

(US8846000, G-2)Show SMILES CC(C)Oc1cccc2C(=O)N(CCc3nc4cc(C)ccc4c(=O)n3-c3ccc4cnn(C)c4c3)C(=O)c12 Show InChI InChI=1S/C30H27N5O4/c1-17(2)39-25-7-5-6-22-27(25)30(38)34(28(22)36)13-12-26-32-23-14-18(3)8-11-21(23)29(37)35(26)20-10-9-19-16-31-33(4)24(19)15-20/h5-11,14-17H,12-13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125967

(CHEMBL3627846)Show SMILES Cc1ccc2c(c1)nc(CCN1C(=O)c3ccccc3C1=O)n(-c1ccc3n(C)ncc3c1)c2=O Show InChI InChI=1S/C27H21N5O3/c1-16-7-9-21-22(13-16)29-24(11-12-31-25(33)19-5-3-4-6-20(19)26(31)34)32(27(21)35)18-8-10-23-17(14-18)15-28-30(23)2/h3-10,13-15H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135597

(US8846000, 1-13)Show SMILES COc1ccc2c(c1)nc(CCN1C(=O)c3cccc(OC(C)C)c3C1=O)n(-c1ccc3cc[nH]c3c1)c2=O Show InChI InChI=1S/C30H26N4O5/c1-17(2)39-25-6-4-5-22-27(25)30(37)33(28(22)35)14-12-26-32-24-16-20(38-3)9-10-21(24)29(36)34(26)19-8-7-18-11-13-31-23(18)15-19/h4-11,13,15-17,31H,12,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135600
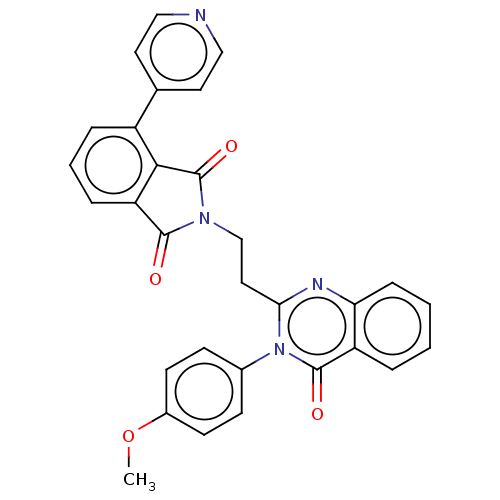
(US8846000, 1-16)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(c3C2=O)-c2ccncc2)nc2ccccc2c1=O Show InChI InChI=1S/C30H22N4O4/c1-38-21-11-9-20(10-12-21)34-26(32-25-8-3-2-5-23(25)29(34)36)15-18-33-28(35)24-7-4-6-22(27(24)30(33)37)19-13-16-31-17-14-19/h2-14,16-17H,15,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135600

(US8846000, 1-16)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(c3C2=O)-c2ccncc2)nc2ccccc2c1=O Show InChI InChI=1S/C30H22N4O4/c1-38-21-11-9-20(10-12-21)34-26(32-25-8-3-2-5-23(25)29(34)36)15-18-33-28(35)24-7-4-6-22(27(24)30(33)37)19-13-16-31-17-14-19/h2-14,16-17H,15,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135598

(US8846000, 1-14)Show SMILES COc1ccc2c(c1)nc(CCN1C(=O)c3cccc(OC(C)C)c3C1=O)n(-c1cccc(C)c1)c2=O Show InChI InChI=1S/C29H27N3O5/c1-17(2)37-24-10-6-9-22-26(24)29(35)31(27(22)33)14-13-25-30-23-16-20(36-4)11-12-21(23)28(34)32(25)19-8-5-7-18(3)15-19/h5-12,15-17H,13-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135608

(US8846000, G-1)Show SMILES CC(C)Oc1cccc2C(=O)N(CCc3nc4cc(C)ccc4c(=O)n3-c3ccc4cn[nH]c4c3)C(=O)c12 Show InChI InChI=1S/C29H25N5O4/c1-16(2)38-24-6-4-5-21-26(24)29(37)33(27(21)35)12-11-25-31-23-13-17(3)7-10-20(23)28(36)34(25)19-9-8-18-15-30-32-22(18)14-19/h4-10,13-16H,11-12H2,1-3H3,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135606

(US8846000, 1-24)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2cc(C)ccc2c1=O Show InChI InChI=1S/C29H27N3O5/c1-17(2)37-24-7-5-6-22-26(24)29(35)31(27(22)33)15-14-25-30-23-16-18(3)8-13-21(23)28(34)32(25)19-9-11-20(36-4)12-10-19/h5-13,16-17H,14-15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135614

(US8846000, D-7)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C28H25N3O5/c1-17(2)36-23-10-6-8-21-25(23)28(34)30(26(21)32)16-15-24-29-22-9-5-4-7-20(22)27(33)31(24)18-11-13-19(35-3)14-12-18/h4-14,17H,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135614

(US8846000, D-7)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C28H25N3O5/c1-17(2)36-23-10-6-8-21-25(23)28(34)30(26(21)32)16-15-24-29-22-9-5-4-7-20(22)27(33)31(24)18-11-13-19(35-3)14-12-18/h4-14,17H,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135596

(US8846000, 1-12)Show SMILES CC(C)Oc1cccc2C(=O)N(CCc3nc4ccccc4c(=O)n3-c3ccc(OCCF)cc3)C(=O)c12 Show InChI InChI=1S/C29H26FN3O5/c1-18(2)38-24-9-5-7-22-26(24)29(36)32(27(22)34)16-14-25-31-23-8-4-3-6-21(23)28(35)33(25)19-10-12-20(13-11-19)37-17-15-30/h3-13,18H,14-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135613
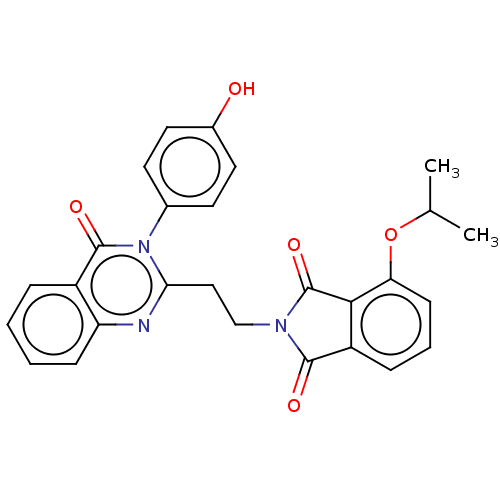
(US8846000, D-6)Show SMILES CC(C)Oc1cccc2C(=O)N(CCc3nc4ccccc4c(=O)n3-c3ccc(O)cc3)C(=O)c12 Show InChI InChI=1S/C27H23N3O5/c1-16(2)35-22-9-5-7-20-24(22)27(34)29(25(20)32)15-14-23-28-21-8-4-3-6-19(21)26(33)30(23)17-10-12-18(31)13-11-17/h3-13,16,31H,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135595
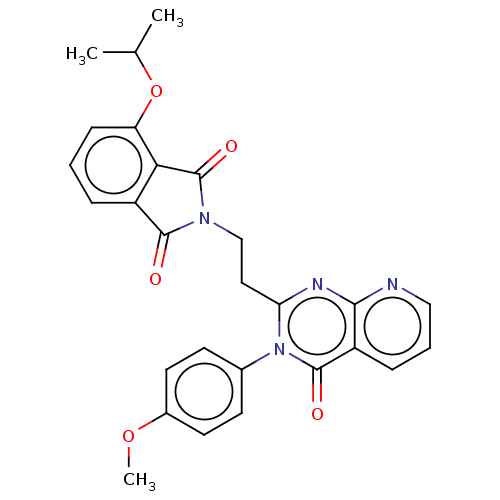
(US8846000, 1-11)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2ncccc2c1=O Show InChI InChI=1S/C27H24N4O5/c1-16(2)36-21-8-4-6-19-23(21)27(34)30(25(19)32)15-13-22-29-24-20(7-5-14-28-24)26(33)31(22)17-9-11-18(35-3)12-10-17/h4-12,14,16H,13,15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135599
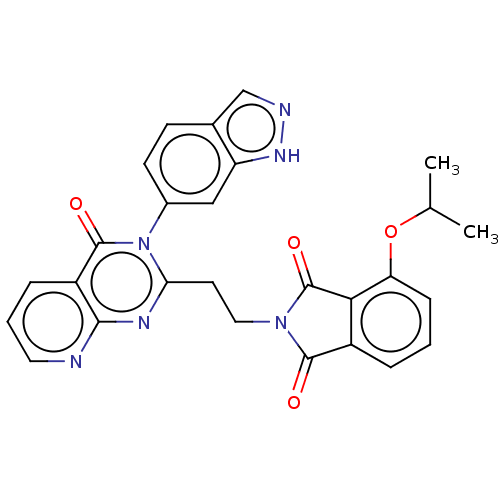
(US8846000, 1-15)Show SMILES CC(C)Oc1cccc2C(=O)N(CCc3nc4ncccc4c(=O)n3-c3ccc4cn[nH]c4c3)C(=O)c12 Show InChI InChI=1S/C27H22N6O4/c1-15(2)37-21-7-3-5-18-23(21)27(36)32(25(18)34)12-10-22-30-24-19(6-4-11-28-24)26(35)33(22)17-9-8-16-14-29-31-20(16)13-17/h3-9,11,13-15H,10,12H2,1-2H3,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135595

(US8846000, 1-11)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2ncccc2c1=O Show InChI InChI=1S/C27H24N4O5/c1-16(2)36-21-8-4-6-19-23(21)27(34)30(25(19)32)15-13-22-29-24-20(7-5-14-28-24)26(33)31(22)17-9-11-18(35-3)12-10-17/h4-12,14,16H,13,15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135591

(US8846000, 1-7)Show SMILES COc1ccc2c(c1)nc(CCN1C(=O)c3cccc(OC)c3C1=O)n(-c1cccc(C)c1)c2=O Show InChI InChI=1S/C27H23N3O5/c1-16-6-4-7-17(14-16)30-23(28-21-15-18(34-2)10-11-19(21)26(30)32)12-13-29-25(31)20-8-5-9-22(35-3)24(20)27(29)33/h4-11,14-15H,12-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135602

(US8846000, 1-19)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OCC(F)(F)F)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C27H20F3N3O5/c1-37-17-11-9-16(10-12-17)33-22(31-20-7-3-2-5-18(20)25(33)35)13-14-32-24(34)19-6-4-8-21(23(19)26(32)36)38-15-27(28,29)30/h2-12H,13-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135612
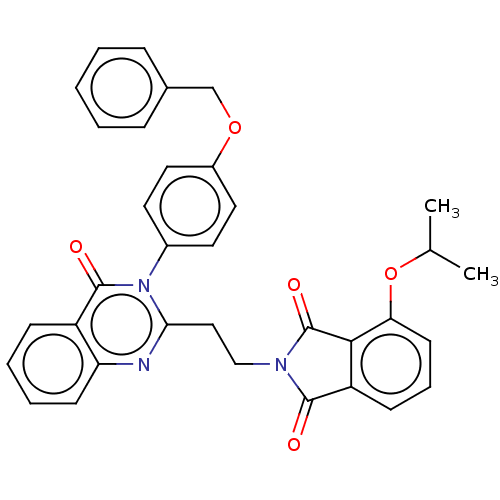
(US8846000, D-5)Show SMILES CC(C)Oc1cccc2C(=O)N(CCc3nc4ccccc4c(=O)n3-c3ccc(OCc4ccccc4)cc3)C(=O)c12 Show InChI InChI=1S/C34H29N3O5/c1-22(2)42-29-14-8-12-27-31(29)34(40)36(32(27)38)20-19-30-35-28-13-7-6-11-26(28)33(39)37(30)24-15-17-25(18-16-24)41-21-23-9-4-3-5-10-23/h3-18,22H,19-21H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398004
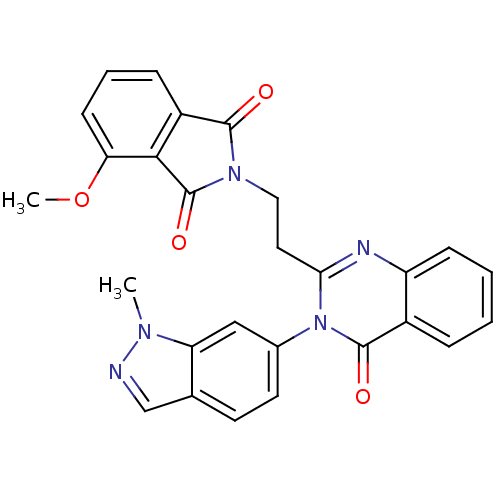
(CHEMBL2180401)Show SMILES COc1cccc2C(=O)N(CCc3nc4ccccc4c(=O)n3-c3ccc4cnn(C)c4c3)C(=O)c12 Show InChI InChI=1S/C27H21N5O4/c1-30-21-14-17(11-10-16(21)15-28-30)32-23(29-20-8-4-3-6-18(20)26(32)34)12-13-31-25(33)19-7-5-9-22(36-2)24(19)27(31)35/h3-11,14-15H,12-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135604

(US8846000, 1-21)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC4CCC4)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C29H25N3O5/c1-36-19-14-12-18(13-15-19)32-25(30-23-10-3-2-8-21(23)28(32)34)16-17-31-27(33)22-9-5-11-24(26(22)29(31)35)37-20-6-4-7-20/h2-3,5,8-15,20H,4,6-7,16-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50126108
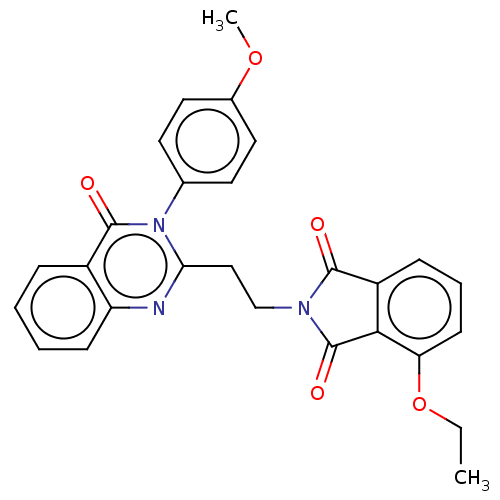
(CHEMBL3627840)Show SMILES CCOc1cccc2C(=O)N(CCc3nc4ccccc4c(=O)n3-c3ccc(OC)cc3)C(=O)c12 Show InChI InChI=1S/C27H23N3O5/c1-3-35-22-10-6-8-20-24(22)27(33)29(25(20)31)16-15-23-28-21-9-5-4-7-19(21)26(32)30(23)17-11-13-18(34-2)14-12-17/h4-14H,3,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135592

(US8846000, 1-8)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC)c3C2=O)nc2cc(C)ccc2c1=O Show InChI InChI=1S/C27H23N3O5/c1-16-7-12-19-21(15-16)28-23(30(26(19)32)17-8-10-18(34-2)11-9-17)13-14-29-25(31)20-5-4-6-22(35-3)24(20)27(29)33/h4-12,15H,13-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135603

(US8846000, 1-20)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OCC4CC4)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C29H25N3O5/c1-36-20-13-11-19(12-14-20)32-25(30-23-7-3-2-5-21(23)28(32)34)15-16-31-27(33)22-6-4-8-24(26(22)29(31)35)37-17-18-9-10-18/h2-8,11-14,18H,9-10,15-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135611
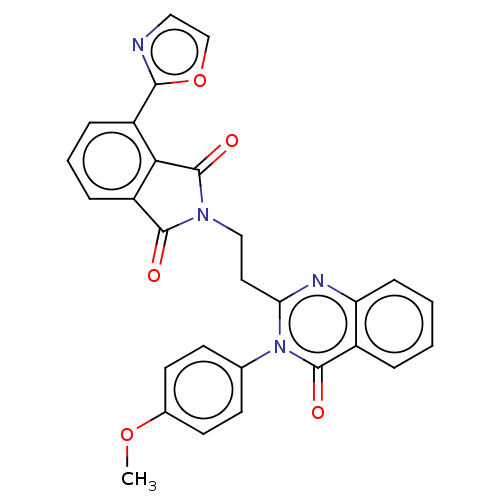
(US8846000, C-2)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(-c4ncco4)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C28H20N4O5/c1-36-18-11-9-17(10-12-18)32-23(30-22-8-3-2-5-19(22)27(32)34)13-15-31-26(33)21-7-4-6-20(24(21)28(31)35)25-29-14-16-37-25/h2-12,14,16H,13,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50126110
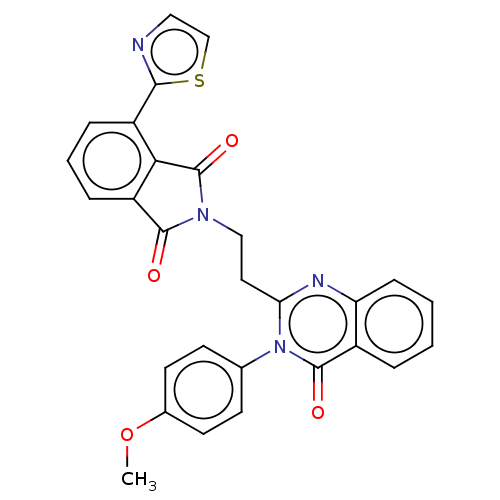
(CHEMBL3627843)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(-c4nccs4)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C28H20N4O4S/c1-36-18-11-9-17(10-12-18)32-23(30-22-8-3-2-5-19(22)27(32)34)13-15-31-26(33)21-7-4-6-20(24(21)28(31)35)25-29-14-16-37-25/h2-12,14,16H,13,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398008
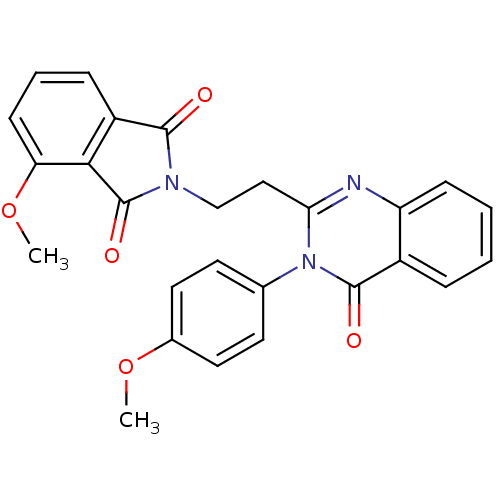
(CHEMBL2180426)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C26H21N3O5/c1-33-17-12-10-16(11-13-17)29-22(27-20-8-4-3-6-18(20)25(29)31)14-15-28-24(30)19-7-5-9-21(34-2)23(19)26(28)32/h3-13H,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398008

(CHEMBL2180426)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C26H21N3O5/c1-33-17-12-10-16(11-13-17)29-22(27-20-8-4-3-6-18(20)25(29)31)14-15-28-24(30)19-7-5-9-21(34-2)23(19)26(28)32/h3-13H,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135605
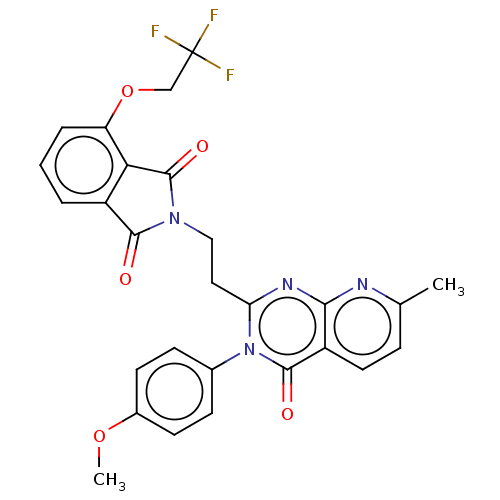
(US8846000, 1-22)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OCC(F)(F)F)c3C2=O)nc2nc(C)ccc2c1=O Show InChI InChI=1S/C27H21F3N4O5/c1-15-6-11-19-23(31-15)32-21(34(25(19)36)16-7-9-17(38-2)10-8-16)12-13-33-24(35)18-4-3-5-20(22(18)26(33)37)39-14-27(28,29)30/h3-11H,12-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135588
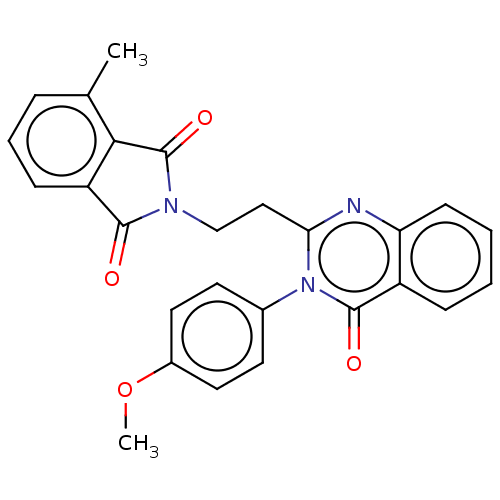
(US8846000, 1-4)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(C)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C26H21N3O4/c1-16-6-5-8-20-23(16)26(32)28(24(20)30)15-14-22-27-21-9-4-3-7-19(21)25(31)29(22)17-10-12-18(33-2)13-11-17/h3-13H,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50126107

(CHEMBL3627818)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3c(C2=O)c(Cl)ccc3Cl)nc2ccccc2c1=O Show InChI InChI=1S/C25H17Cl2N3O4/c1-34-15-8-6-14(7-9-15)30-20(28-19-5-3-2-4-16(19)23(30)31)12-13-29-24(32)21-17(26)10-11-18(27)22(21)25(29)33/h2-11H,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125995
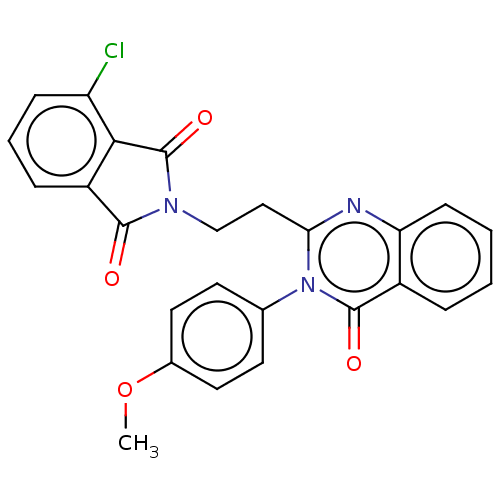
(CHEMBL3627816)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(Cl)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C25H18ClN3O4/c1-33-16-11-9-15(10-12-16)29-21(27-20-8-3-2-5-17(20)24(29)31)13-14-28-23(30)18-6-4-7-19(26)22(18)25(28)32/h2-12H,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50126109

(CHEMBL3627842)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(Br)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C25H18BrN3O4/c1-33-16-11-9-15(10-12-16)29-21(27-20-8-3-2-5-17(20)24(29)31)13-14-28-23(30)18-6-4-7-19(26)22(18)25(28)32/h2-12H,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135607
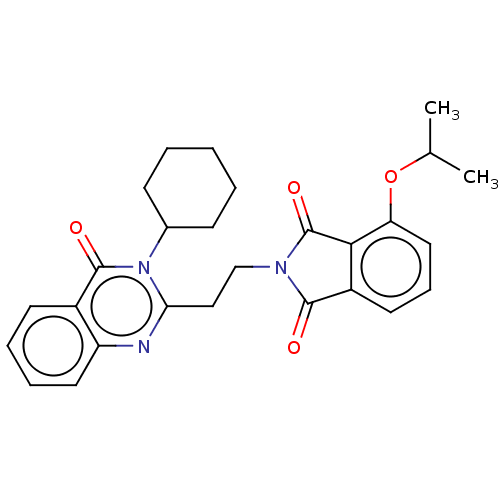
(US8846000, 1-25)Show SMILES CC(C)Oc1cccc2C(=O)N(CCc3nc4ccccc4c(=O)n3C3CCCCC3)C(=O)c12 Show InChI InChI=1S/C27H29N3O4/c1-17(2)34-22-14-8-12-20-24(22)27(33)29(25(20)31)16-15-23-28-21-13-7-6-11-19(21)26(32)30(23)18-9-4-3-5-10-18/h6-8,11-14,17-18H,3-5,9-10,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125966

(CHEMBL3627814)Show SMILES O=C1N(CCc2nc3ccccc3c(=O)n2-c2ccc3[nH]ccc3c2)C(=O)c2ccccc12 Show InChI InChI=1S/C26H18N4O3/c31-24-18-5-1-2-6-19(18)25(32)29(24)14-12-23-28-22-8-4-3-7-20(22)26(33)30(23)17-9-10-21-16(15-17)11-13-27-21/h1-11,13,15,27H,12,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
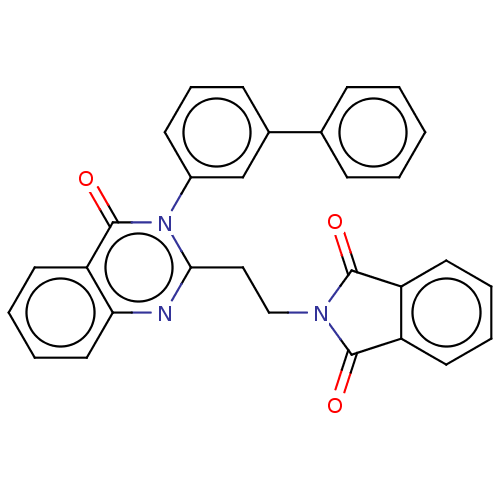
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135586
(US8846000, 1-2)Show SMILES O=C1N(CCc2nc3ccccc3c(=O)n2-c2cccc(c2)-c2ccccc2)C(=O)c2ccccc12 Show InChI InChI=1S/C30H21N3O3/c34-28-23-13-4-5-14-24(23)29(35)32(28)18-17-27-31-26-16-7-6-15-25(26)30(36)33(27)22-12-8-11-21(19-22)20-9-2-1-3-10-20/h1-16,19H,17-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
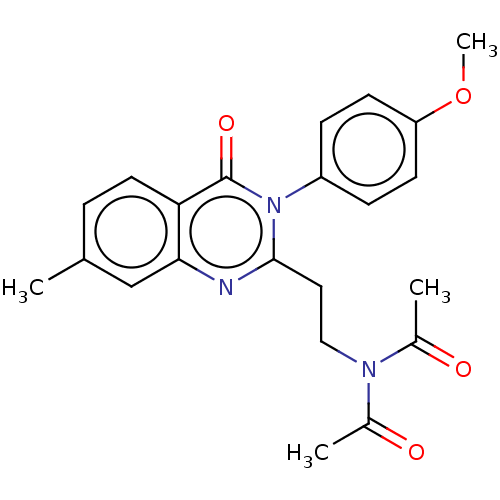
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125994
(CHEMBL3627813)Show SMILES COc1ccc(cc1)-n1c(CCN(C(C)=O)C(C)=O)nc2cc(C)ccc2c1=O Show InChI InChI=1S/C22H23N3O4/c1-14-5-10-19-20(13-14)23-21(11-12-24(15(2)26)16(3)27)25(22(19)28)17-6-8-18(29-4)9-7-17/h5-10,13H,11-12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135610

(US8846000, B-2)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(O)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C25H19N3O5/c1-33-16-11-9-15(10-12-16)28-21(26-19-7-3-2-5-17(19)24(28)31)13-14-27-23(30)18-6-4-8-20(29)22(18)25(27)32/h2-12,29H,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135610

(US8846000, B-2)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(O)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C25H19N3O5/c1-33-16-11-9-15(10-12-16)28-21(26-19-7-3-2-5-17(19)24(28)31)13-14-27-23(30)18-6-4-8-20(29)22(18)25(27)32/h2-12,29H,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135589
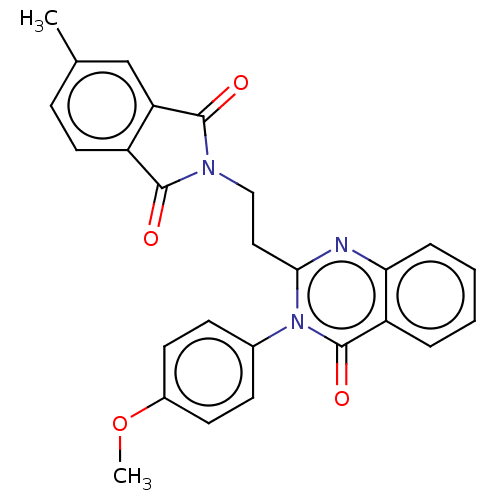
(US8846000, 1-5)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3ccc(C)cc3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C26H21N3O4/c1-16-7-12-19-21(15-16)25(31)28(24(19)30)14-13-23-27-22-6-4-3-5-20(22)26(32)29(23)17-8-10-18(33-2)11-9-17/h3-12,15H,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50126100

(CHEMBL3627817)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3ccc(Cl)cc3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C25H18ClN3O4/c1-33-17-9-7-16(8-10-17)29-22(27-21-5-3-2-4-19(21)25(29)32)12-13-28-23(30)18-11-6-15(26)14-20(18)24(28)31/h2-11,14H,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135590

(US8846000, 1-6)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(C#N)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C26H18N4O4/c1-34-18-11-9-17(10-12-18)30-22(28-21-8-3-2-6-19(21)25(30)32)13-14-29-24(31)20-7-4-5-16(15-27)23(20)26(29)33/h2-12H,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135593
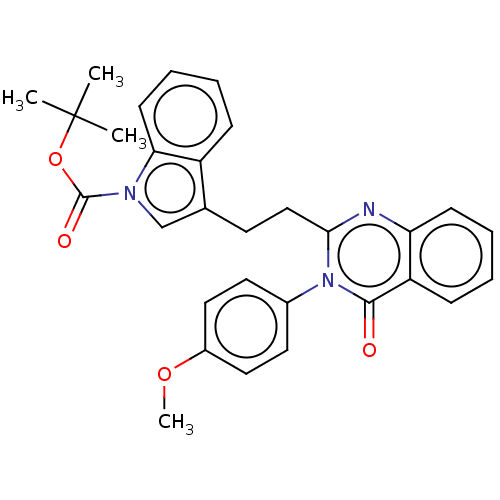
(US8846000, 1-9)Show SMILES COc1ccc(cc1)-n1c(CCc2cn(C(=O)OC(C)(C)C)c3ccccc23)nc2ccccc2c1=O Show InChI InChI=1S/C30H29N3O4/c1-30(2,3)37-29(35)32-19-20(23-9-6-8-12-26(23)32)13-18-27-31-25-11-7-5-10-24(25)28(34)33(27)21-14-16-22(36-4)17-15-21/h5-12,14-17,19H,13,18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125993
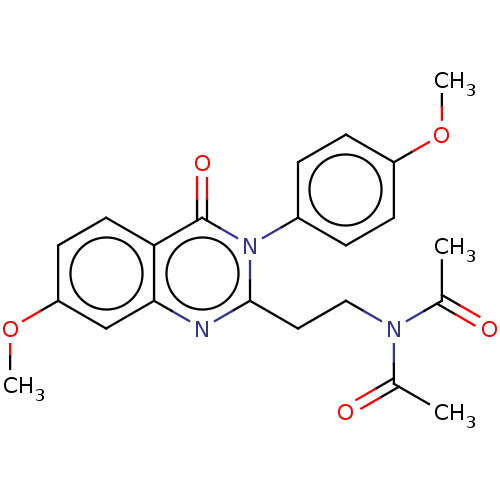
(CHEMBL3627812)Show SMILES COc1ccc(cc1)-n1c(CCN(C(C)=O)C(C)=O)nc2cc(OC)ccc2c1=O Show InChI InChI=1S/C22H23N3O5/c1-14(26)24(15(2)27)12-11-21-23-20-13-18(30-4)9-10-19(20)22(28)25(21)16-5-7-17(29-3)8-6-16/h5-10,13H,11-12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135585

(US8846000, 1-1)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3ccccc3C2=O)nc2ncccc2c1=O Show InChI InChI=1S/C24H18N4O4/c1-32-16-10-8-15(9-11-16)28-20(26-21-19(24(28)31)7-4-13-25-21)12-14-27-22(29)17-5-2-3-6-18(17)23(27)30/h2-11,13H,12,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125991
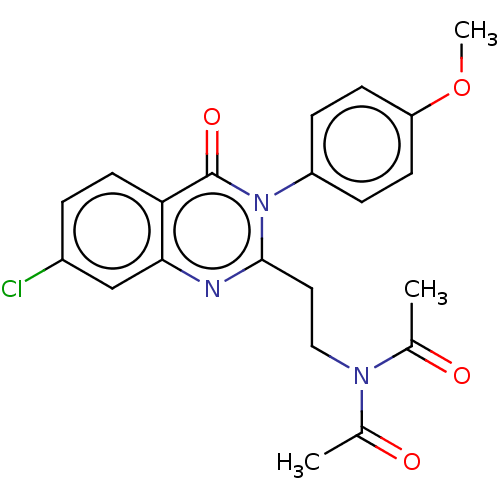
(CHEMBL3627810)Show SMILES COc1ccc(cc1)-n1c(CCN(C(C)=O)C(C)=O)nc2cc(Cl)ccc2c1=O Show InChI InChI=1S/C21H20ClN3O4/c1-13(26)24(14(2)27)11-10-20-23-19-12-15(22)4-9-18(19)21(28)25(20)16-5-7-17(29-3)8-6-16/h4-9,12H,10-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135594

(US8846000, 1-10)Show SMILES COc1ccc(cc1)-n1c(CCNC(=O)Nc2ccccc2-c2ccccc2)nc2ccccc2c1=O Show InChI InChI=1S/C30H26N4O3/c1-37-23-17-15-22(16-18-23)34-28(32-27-14-8-6-12-25(27)29(34)35)19-20-31-30(36)33-26-13-7-5-11-24(26)21-9-3-2-4-10-21/h2-18H,19-20H2,1H3,(H2,31,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125986
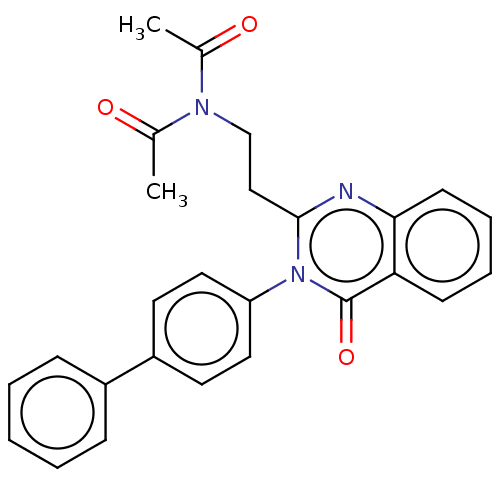
(CHEMBL3627805)Show SMILES CC(=O)N(CCc1nc2ccccc2c(=O)n1-c1ccc(cc1)-c1ccccc1)C(C)=O Show InChI InChI=1S/C26H23N3O3/c1-18(30)28(19(2)31)17-16-25-27-24-11-7-6-10-23(24)26(32)29(25)22-14-12-21(13-15-22)20-8-4-3-5-9-20/h3-15H,16-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135588

(US8846000, 1-4)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(C)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C26H21N3O4/c1-16-6-5-8-20-23(16)26(32)28(24(20)30)15-14-22-27-21-9-4-3-7-19(21)25(31)29(22)17-10-12-18(33-2)13-11-17/h3-13H,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125987
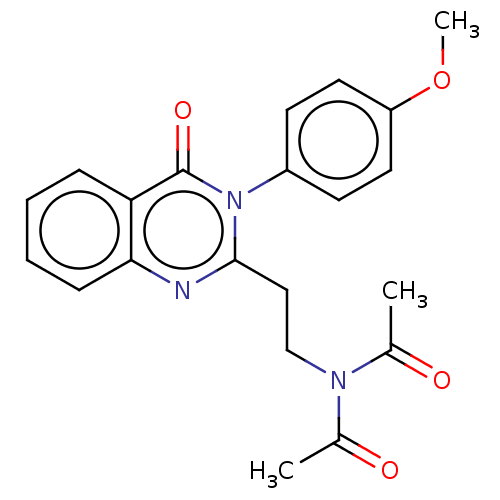
(CHEMBL3627806)Show SMILES COc1ccc(cc1)-n1c(CCN(C(C)=O)C(C)=O)nc2ccccc2c1=O Show InChI InChI=1S/C21H21N3O4/c1-14(25)23(15(2)26)13-12-20-22-19-7-5-4-6-18(19)21(27)24(20)16-8-10-17(28-3)11-9-16/h4-11H,12-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125988

(CHEMBL3627807)Show SMILES COc1ccc(cc1OC)-n1c(CCN(C(C)=O)C(C)=O)nc2ccccc2c1=O Show InChI InChI=1S/C22H23N3O5/c1-14(26)24(15(2)27)12-11-21-23-18-8-6-5-7-17(18)22(28)25(21)16-9-10-19(29-3)20(13-16)30-4/h5-10,13H,11-12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data